Abstract
Purpose
Numerous patients experience long-term complications after hematopoietic stem cell transplantation (HSCT). This study aimed to identify the frequency and risk factors for psychiatric and endocrine complications following HSCT through big data analyses.
Materials and Methods
We established a cohort of patients with hematologic disease who underwent HSCT in Korea between 2010 and 2012 using the Health Insurance Review & Assessment Service data. A total of 3,636 patients were identified, and insurance claims were tracked using psychiatric and endocrine diagnostic International Classification of Diseases, 10th Revision codes for the ensuing decade. We identified the incidence rates of long-term complications based on the baseline disease and HSCT type. Prognostic factors for each complication were scrutinized using logistic regression analysis.
Results
A total of 1,879 patients underwent allogeneic HSCT and 1,757 patients received autologous HSCT. Post-HSCT, 506 patients were diagnosed with depression, 465 with anxiety disorders, and 659 with diabetes. The highest incidence of long-term complications occurred within the first year post-HSCT (12.2%), subsequently decreasing over time. Risk factors for depressive disorders after allogeneic HSCT included female sex, a total body irradiation–based conditioning regimen, and cyclosporine. Identified risk factors for diabetes mellitus comprised old age, total body irradiation–based conditioning regimen, and non-antithymocyte globulin protocol. Regarding autologous HSCT, only female sex was identified as a risk factor for depressive disorders, whereas elderly patients and those with multiple myeloma were identified as poor prognostic factors for diabetes mellitus.
Hematopoietic stem cell transplantation (HSCT) is a potential curative treatment for various malignant or nonmalignant hematologic diseases, including acute leukemia, myelodysplastic syndrome, myeloproliferative neoplasm, lymphoma, multiple myeloma, and aplastic anemia [1]. The global incidence of HSCT has demonstrated a consistent upward trajectory [2-4]. Recent advancements, such as the utilization of post-transplant cyclophosphamide-based regimens for effective graft versus host disease (GVHD) control, diverse conditioning regimens, and improved supportive care, have led to a notable increase in HSCT using alternative donors and transplantations for elderly patients [5-9]. Nevertheless, HSCT remains associated with significant treatment-related mortality, ranging from 0%-5% for autologous HSCT and 7%-27% for allogeneic HSCT [10]. Relapse, GVHD, and infections are associated with early mortality in HSCT [11]. Additionally, various long-term complications can manifest, including chronic GHVD, secondary malignancies, cardiovascular, pulmonary, renal, and neurologic toxicity, immune dysfunction, endocrine dysfunction, and psychological disorders [12]. As the frequency of HSCT increases, the rate of long-term survival after transplantation also increases. However, due to the impact of these long-term complications, even patients who survive without relapse after HSCT exhibit a relatively shorter life expectancy than the general population [13,14].
Therefore, comprehensively understanding the occurrence rates and risk factors of long-term complications associated with transplantation is a growing imperative. Although some studies have reported long-term complications following hematopoietic stem cell transplantation, no large-scale research has been conducted in Korea.
In this study, using the Korean National Health Insurance (KNHI) and the Health Insurance Review and Assessment (HIRA) databases, we explored long-term psychiatric and endocrine/rheumatoid complications following autologous or allogeneic hematopoietic stem cell transplantation.
We used the KNHI Claims Database from the HIRA. In Korea, enrollment in the KNHI program is mandatory for nearly 97% of the population, with the exception of the remaining 3% covered under the Medical Aid Program. Healthcare providers are required to permit HIRA to review all medical costs covered by the KNHI for this population. Thus, HIRA has a huge database that contains information on all medical claims for nearly all Koreans, excluding details on procedures or drugs not covered by the national health insurance. Moreover, upon approval, HIRA makes this database available for various studies conducted by Korean researchers. According to the Act on the Protection of Personal Information Maintained by Public Agencies, this database consists of concealed data on individuals’ identities using unidentifiable codes.
We established a cohort of patients with hematologic disease who received HSCT in Korea between 2010 and 2012, utilizing data from the HIRA. The data of patients who had the International Classification of Diseases, 10th Revision (ICD-10) for hematologic diseases and claims codes for HSCT procedures between 2010 and 2012 were retrieved. To detect all procedure codes for HSCT, we employed the X5131, X5133, and X5135 codes for allogeneic HSCT and the X5132, X5134, and X5136 codes for autologous HSCT.
Following the identification of patients, all medical claims that occurred between the date of HSCT and December 31, 2018, were captured to identify patients diagnosed with psychiatric or endocrine/rheumatoid diseases during this period. To obtain information on newly diagnosed psychiatric and endocrine/rheumatoid diseases, we applied a minimum 1-year washout window period before detecting the ICD-10 code for psychiatric and endocrine/rheumatoid diseases. Therefore, individuals with an ICD-10 code for these conditions between January 1, 2009, and the HSCT date were not regarded as newly diagnosed patients and were not assigned to the group with psychiatric and endocrine/rheumatoid diseases. We used the ICD-10 codes F10-19, F32-33, F40-41, F43, and F45 for psychiatric diseases, whereas codes E10, E11, E03, M05, and M05 for endocrine/rheumatoid diseases. Relevant information, including age, sex, stem cell source for HSCT, conditioning method, and immunosuppressant was obtained from the HIRA database.
All patients with missing data were excluded from the statistical analysis. A multivariate logistic regression model was used to analyze the risk factors for psychiatric diseases. Statistical significance was established at p < 0.05. All statistical analyses were performed using the SAS software ver. 9.4 (SAS Institute Inc., Cary, NC) and R statistical software ver. 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria; http://cran.r-project.org/).
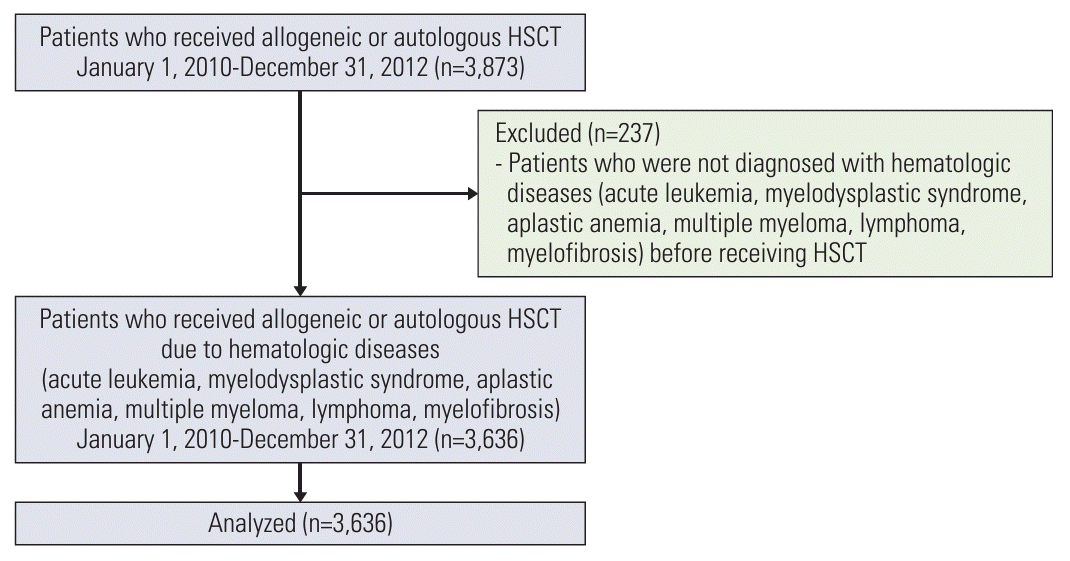
Between January 2010 and December 2012, a total of 3,873 patients were registered under the HSCT procedures code. Among them, we excluded 237 patients who were not diagnosed with hematologic diseases, including acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, aplastic anemia, multiple myeloma, lymphoma, and myelofibrosis; subsequently, 3,636 patients were enrolled in this study (Fig. 1).
A total of 1,879 patients received allogeneic HSCT and 1,757 patients received autologous HSCT. Patients who underwent allogeneic HSCT were younger than those who underwent autologous HSCT. Among patients who underwent allogeneic HSCT, 65.7% were under 50 years and 34.0% were 50-65 years of age; in contrast, among patients who underwent autologous HSCT, 39.3% were under 50 years and 60.1% were 50-65 years of age. Additionally, allogeneic HSCT was mostly performed in patients with acute leukemia (71.5%; 52.3% with acute myeloid leukemia and 19.3% with acute lymphoid leukemia) and myelodysplastic syndrome (18.0%). Meanwhile, autologous transplantation was predominantly performed in patients with multiple myeloma (48.3%) and lymphoma (43.5%). Patients who underwent allogeneic HSCT mostly required immunosuppressant, whereas those who underwent autologous HSCT did not require such drugs (Table 1).
After HSCT, 506 patients (13.9%) were diagnosed with depression, 465 (12.8%) with anxiety disorders, 81 (2.2%) with adjustment disorders, and 77 (2.1%) with somatoform disorders. Additionally, 659 patients had diabetes (18.1%), 213 had thyroid disease (5.9%), and 33 had rheumatoid disease (0.9%). Overall, no significant differences were observed between patients who received allogeneic HSCT and those who received autologous HSCT (Table 2). The incidence of long-term psychiatric and endocrine/rheumatoid complications was the highest at 12.2% and 13.1%, respectively, within one year of HSCT, decreasing over time (5.2% and 4.2% at 2nd year and 3.6% and 2.3% at 3rd year) (Fig. 2).
Risk factors for depressive disorder, the most common psychiatric complication following allogeneic HSCT, included female sex (odds ratio [OR], 1.364; 95% confidence interval [CI], 1.088 to 1.712; p=0.007), underlying dyslipidemia (OR, 1.363; 95% CI, 1.044 to 1.780; p=0.023), total body irradiation (TBI)–based conditioning regimen (OR, 1.592; CI, 1.194 to 2.120; p=0.002), and use of cyclosporine (OR, 1.383; 95% CI, 1.056 to 1.811; p=0.019). Underlying hematological diseases were not significantly associated with the occurrence of depressive disorders (p=0.618). Risk factors for diabetes mellitus, the most common endocrine complication, were identified as older age (≥ 50; OR, 1.658; 95% CI, 1.303 to 2.110; p < 0.001), TBI-based conditioning regimen (OR, 1.529; 95% CI, 1.168 to 2.003; p=0.002), and non–antithymocyte globulin (ATG) protocol (OR, 0.554; 95% CI, 0.432 to 0.711; p < 0.001) (Table 3). For autologous HSCT, only female sex (OR, 1.302; 95% CI, 1.040 to 1.630; p=0.021) was identified as a risk factor for psychiatric complications. Old age (≥ 50; OR, 2.235; 95% CI, 1.721 to 2.904; p < 0.001) and a diagnosis of multiple myeloma (OR, 2.178; 95% CI, 1.169 to 4.058; p=0.038) were identified as poor prognostic factors for diabetes mellitus (Table 4).
Our study incorporated a substantial volume of real-world medical data from adult patients who underwent HSCT with a follow-up period of at least seven years. The findings revealed that approximately 31.9% of patients experienced psychiatric disorders post-HSCT, with depression and anxiety disorders being the most prevalent, along with adjustment and somatoform disorders. Considering that the prevalence of major depression in the general population of Korea is reported to be approximately 5% [15], our results suggest a significant occurrence of psychiatric disorders among HSCT patients. Previous studies have reported depression and/or anxiety rates of up to 40% within a few years after HSCT, gradually decreasing after the first year [16]. In our study, psychiatric complications were most prevalent during the first year of HSCT, showing a gradual decline thereafter.
Cheon et al. [17] reported that female survivors showed higher psychiatric distress. Consistent with this finding, we also observed that female recipients experienced more depressive disorders after both allogeneic and autologous HSCT. Therefore, emphasizing psychological counseling during the initial 1-2 years after HSCT is crucial, especially for female recipients.
A previous study reported that patients with allogeneic HSCT might be at a greater risk of psychiatric distress than patients with autologous HSCT because of their decreased physical function [18]; however, in our study, no significant difference was observed between patients who received allogeneic HSCT and those who received autologous HSCT.
Sun et al. [19] reported that patients exposed to TBI, those with chronic GVHD, and those exposed to prednisone had a significantly increased risk of psychological problems. In our study, depressive disorder was more prevalent in the TBI group than those who did not undergo TBI conditioning. Previous studies also suggested that factors, including burden of disease, pain, decreased physical function, concerns about long-term health, familial or social role, and financial issues were associated with the occurrence of psychiatric disorders in HSCT survivors [12,18,20]. However, our study solely analyzed diagnoses based on ICD codes, limiting our ability to assess the consequences of accounting for patients’ symptoms or their socioeconomic circumstances.
Several studies have reported that patients who have undergone transplantation experienced an elevated risk of insulin resistance and glucose intolerance [21-24]. Baker et al. [25] compared HSCT survivors with their siblings to assess the occurrence of late complications after HSCT. They reported that survivors of allogeneic HSCT had a three-fold higher risk of diabetes even after adjusting for age, sex, and body mass index [25]. Additionally, they reported that older age increased the risk of diabetes; however, the odds of developing diabetes decreased over time post-HSCT [25]. Similarly, our study demonstrated that the incidence of diabetes increased in both allogeneic and autologous HSCT recipients. The highest incidence of endocrine complications occurred within the first year post-HSCT, gradually decreased over time following HSCT.
Our study also identified a TBI-based conditioning regimen as a risk factor for the development of diabetes after HSCT. Consistent with our findings, several previous studies on pediatric patients have reported an association between TBI conditioning and the development of insulin resistance and diabetes [21,26,27]. Although evidence is limited, abdominal radiation exposure may be associated with the development of diabetes. Administration of chemotherapy agent, immunosuppressive agents, and excessive inflammation during the transplantation process have also been suggested as risk factors for post-transplantation diabetes [21,23-25]. Most patients who underwent allogeneic HSCT were administered calcineurin inhibitors, such as cyclosporine or tacrolimus, as GVHD prophylaxis. Calcineurin inhibitors affect glucose metabolism and insulin secretion and are associated with post-transplant diabetes [28]. However, tacrolimus and cyclosporine differ in their impact on glucose metabolism, with a higher incidence of diabetes associated with tacrolimus use compared to cyclosporine in solid organ transplantation [28-30]. Consistent with solid organ transplantation study findings, our study demonstrated that in allogeneic HSCT, the use of tacrolimus was associated with a higher risk of diabetes. Therefore, switching from tacrolimus to cyclosporine may improve glycemic control under certain circumstances. Additionally, the group that did not receive ATG had a higher risk of developing diabetes. This could be attributed to the relatively higher incidence of GVHD in the ATG nonuse group, leading to a longer exposure period to steroids or immunosuppressive agents.
After autologous HSCT, diabetes is most likely to occur in patients with multiple myeloma. This association might be attributed to the fact that multiple myeloma often relapses even after HSCT, and patients continue to receive chemotherapy, including high-dose steroids, after relapse.
The primary limitation of our study is that we lacked clear information about each patient’s disease course, precise medication duration including immunosuppressant and steroid, the presence of GVHD, physical functions, and financial difficulties, as the HIRA data primarily consists of medical claims data. Consequently, there were limitations in assessing how each of risk factors precisely affects long-term complications. Nevertheless, since there has been a paucity of large-scale research about long-term complications after HSCT, our study has strengths as the large-scale study using real-world medical data from adult patients with a sufficient follow-up period.
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Korea University Guro Hospital (IRB No. 2020GR0373) and also approved by HIRA (M20200813712). Consent to participate in the research was waived because we used anonymized data for retrospective analysis.
References
1. Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015; 21:1863–9.
2. Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Camara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021; 56:1651–64.

3. D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020; 26:e177–82.
4. Iida M, Kodera Y, Dodds A, Ho AYL, Nivison-Smith I, Akter MR, et al. Advances in hematopoietic stem cell transplantation in the Asia-Pacific region: the second report from APBMT 2005-2015. Bone Marrow Transplant. 2019; 54:1973–86.

5. Maurer K, Ho VT, Inyang E, Cutler C, Koreth J, Shapiro RM, et al. Posttransplant cyclophosphamide vs tacrolimus-based GVHD prophylaxis: lower incidence of relapse and chronic GVHD. Blood Adv. 2023; 7:3903–15.

6. Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022; 107:1045–53.

7. Gagelmann N, Kroger N. Dose intensity for conditioning in allogeneic hematopoietic cell transplantation: can we recommend “when and for whom” in 2021? Haematologica. 2021; 106:1794–804.

8. Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017; 130:1156–64.

9. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017; 52:811–7.

10. Wauben B, van Yperen NC, van der Poel MW, Kohler S, van Greevenbroek MM, Schouten HC. Assessing long-term effects after stem cell transplantation: design of the MOSA study. J Clin Epidemiol. 2022; 148:10–6.

11. Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005; 36:757–69.

12. Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2017; 10:220–7.

13. Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011; 29:2230–9.

14. Martin PJ, Counts GW Jr, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010; 28:1011–6.

15. Kim GE, Jo MW, Shin YW. Increased prevalence of depression in South Korea from 2002 to 2013. Sci Rep. 2020; 10:16979.

16. Hjermstad MJ, Loge JH, Evensen SA, Kvaloy SO, Fayers PM, Kaasa S. The course of anxiety and depression during the first year after allogeneic or autologous stem cell transplantation. Bone Marrow Transplant. 1999; 24:1219–28.

17. Cheon J, Lee YJ, Jo JC, Kweon K, Koh S, Min YJ, et al. Late complications and quality of life assessment for survivors receiving allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2021; 29:975–86.

18. Rueda-Lara M, Lopez-Patton MR. Psychiatric and psychosocial challenges in patients undergoing haematopoietic stem cell transplants. Int Rev Psychiatry. 2014; 26:74–86.

19. Sun CL, Francisco L, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS). Blood. 2011; 118:4723–31.

20. Nakamura ZM, Nash RP, Quillen LJ, Richardson DR, McCall RC, Park EM. Psychiatric care in hematopoietic stem cell transplantation. Psychosomatics. 2019; 60:227–37.

21. Hirabayashi K, Nakazawa Y, Matsuura H, Hara Y, Kurata T, Hirabayashi K, et al. Risk factors for diabetes mellitus and impaired glucose tolerance following allogeneic hematopoietic stem cell transplantation in pediatric patients with hematological malignancies. Int J Hematol. 2014; 99:477–86.

22. Atilla E, Atilla PA, Toprak SK, Demirer T. A review of late complications of allogeneic hematopoietic stem cell transplantations. Clin Transplant. 2017; 31:e13062.

23. Chemaitilly W, Sklar CA. Endocrine complications of hematopoietic stem cell transplantation. Endocrinol Metab Clin North Am. 2007; 36:983–98.

24. Griffith ML, Jagasia M, Jagasia SM. Diabetes mellitus after hematopoietic stem cell transplantation. Endocr Pract. 2010; 16:699–706.

25. Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007; 109:1765–72.

26. Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006; 91:4401–7.

27. Bonanomi S, Gaiero A, Masera N, Rovelli A, Uderzo C, Fichera G, et al. Distinctive characteristics of diabetes mellitus after hematopoietic cell transplantation during childhood. Pediatr Transplant. 2006; 10:461–5.

28. Subramanian S, Trence DL. Immunosuppressive agents: effects on glucose and lipid metabolism. Endocrinol Metab Clin North Am. 2007; 36:891–905.
Fig. 2.
The time of diagnosis of psychiatric and endocrine/rheumatoid complications after hematopoietic stem cell transplantation (HSCT). (A) The time of diagnosis of psychiatric complication after HSCT. (B) The time of diagnosis of endocrine/rheumatoid complication after HSCT.
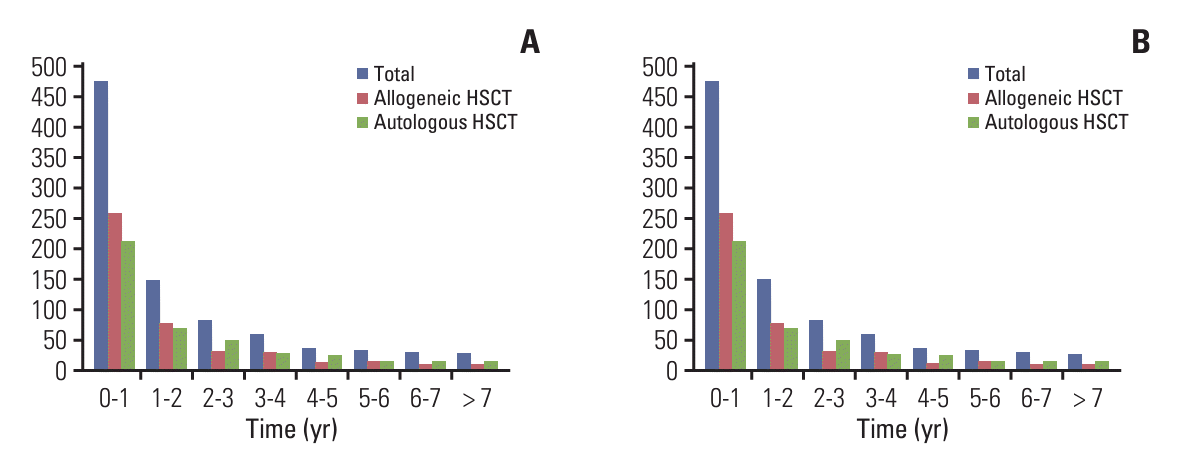
Table 1.
Baseline characteristics of the study population
Table 2.
Incidences of psychiatric and endocrine/rheumatoid complications following transplantation
Table 3.
Risk factors associated with long-term complications following allogeneic hematopoietic stem cell transplantation
Table 4.
Risk factors associated with long-term complications following autologous hematopoietic stem cell transplantation
| Variable |
Depressive disordera) |
Diabetes mellitusb) |
||||
|---|---|---|---|---|---|---|
| Incidence, n (%) | OR | p-value | Incidence, n (%) | OR | p-value | |
| Age (yr) | 0.494 | < 0.001 | ||||
| < 50 (n=680) | 155 (22.8) | 1 | 98 (14.4) | 1 | ||
| ≥ 50 (n=1,052) | 261 (24.8) | 0.916 (0.712-1.178) | 307 (29.2) | 2.235 (1.721-2.904) | ||
| Sex | 0.021 | 0.315 | ||||
| Male (n=1,020) | 223 (21.9) | 1 | 228 (22.4) | 1 | ||
| Female (n=712) | 193 (27.1) | 1.302 (1.040-1.630) | 177 (24.9) | 1.125 (0.894-1.415) | ||
| Comorbidity | ||||||
| Hypertension (n=509) | 134 (26.3) | 1.060 (0.819-1.372) | 0.660 | |||
| Diabetes (n=340) | 93 (27.4) | 1.213 (0.917-1.606) | 0.176 | |||
| Dyslipidemia (n=434) | 111 (25.6) | 1.024 (0.789-1.330) | 0.858 | |||
| Baseline disease | 0.066 | 0.038 | ||||
| Myeloid leukemia (n=119) | 24 (20.2) | 1 | 14 (11.8) | 1 | ||
| MM (n=849) | 243 (28.6) | 1.534 (0.918-2.565) | 232 (27.3) | 2.178 (1.169-4.058) | ||
| Lymphoma (n=764) | 149 (19.5) | 0.997 (0.600-1.658) | 159 (20.8) | 1.578 (0.859-2.896) | ||
| Conditioning | 0.694 | 0.347 | ||||
| Busulfan based (n=736) | 144 (19.6) | 1 | 155 (21.1) | 1 | ||
| Non-busulfan based (n=996) | 272 (27.3) | 1.079 (0.740-1.573) | 250 (25.1) | 0.826 (0.553-1.231) | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download