Abstract
Purpose
Materials and Methods
Results
Electronic Supplementary Material
Notes
Ethical Statement
This study was performed in line with the principles of the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice. The study protocol received approval from the ethics committees of all participating centers, and written informed consent was obtained from all patients.
Author Contributions
Conceived and designed the analysis: Keam B, Ahn MJ.
Collected the data: Keam B, Kang EJ, Kim HR, Kwon JH, Yang Y, Kim MK, Yun T, Lee KH, Ahn MJ.
Contributed data or analysis tools: Kim Y, Keam B, Kim JS, Lee KW, Lee KE, Choi YH, Ji JH, Choi MY, Kim SB.
Performed the analysis: Kim Y, Keam B, Ahn MJ.
Wrote the paper: Kim Y, Keam B.
Conflicts of Interest
Bhumsuk Keam received research funding from MSD, AstraZeneca, and Ono Pharmaceutical Co., Ltd., and has served as an advisor for Handok, NeoImmuneTec, Trialinformatics, and ImmuneOncia, outside of the current work. This study was supported by BI. BI had no role in the design, analysis, or interpretation of the results in this study. BI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BI substances, as well as intellectual property considerations. Otherwise, the authors declare that they have no relevant conflicts of interest regarding the publication of this manuscript.
ACKNOWLEDGMENTS
References
Fig. 1.
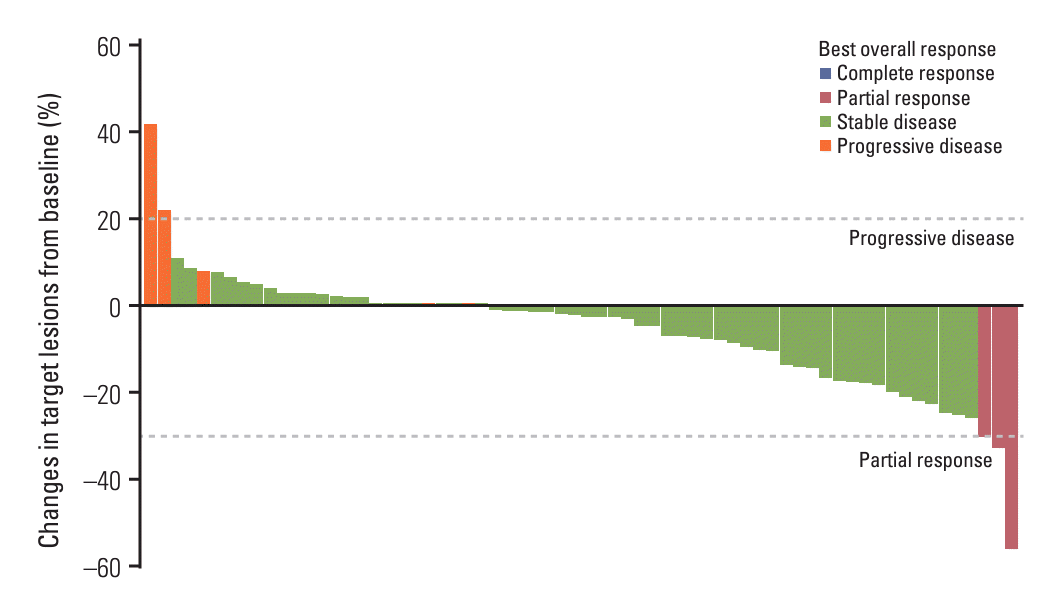
Fig. 2.
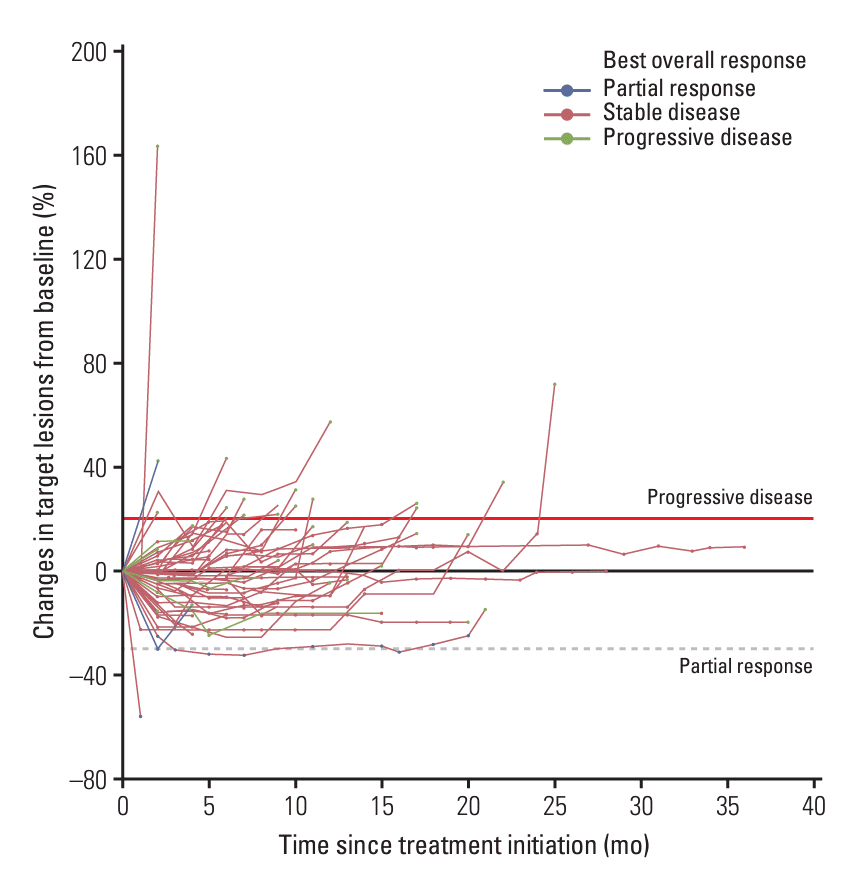
Fig. 3.
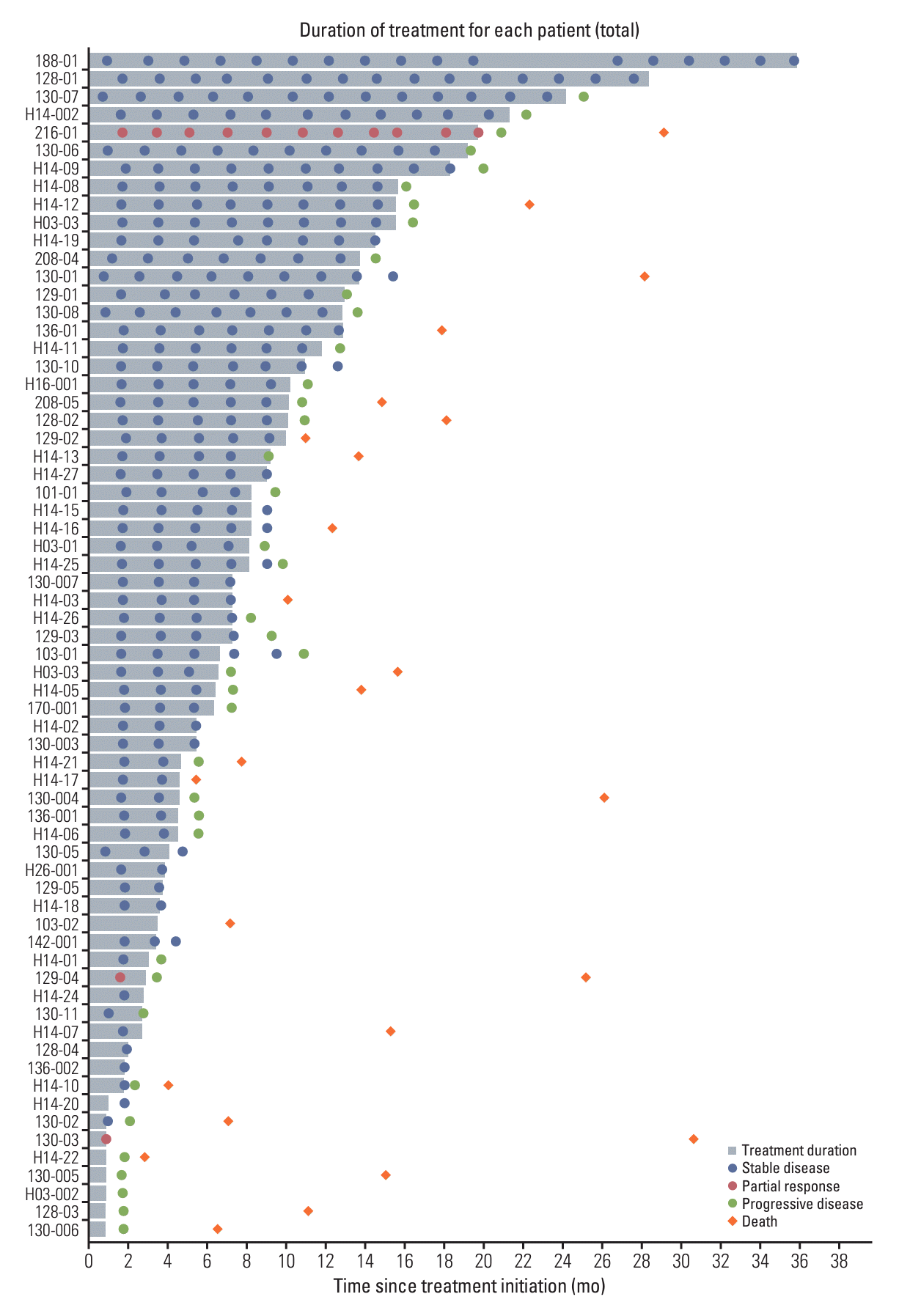
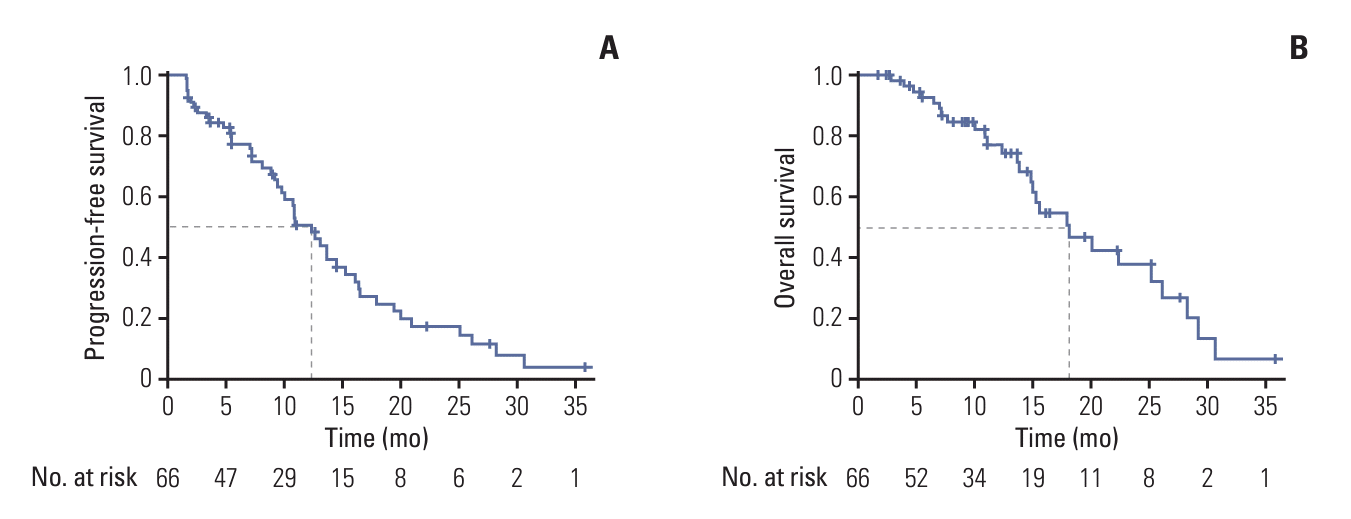
Fig. 4.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download