Abstract
Purpose
This study aimed to assess the long-term risks associated with a history of infectious mononucleosis (IM), primarily caused by the Epstein-Barr virus (EBV). Specifically analyzing the potential increase in developing nasopharyngeal cancer (NPC) and lymphoma in patients with a history of IM and exploring the prevalence of other EBV-associated conditions.
Materials and Methods
The Korean National Health Insurance Service (NHIS) database was utilized for a retrospective analysis, covering data from 2002 to 2021. A total of 25,582 IM patients and controls were included, with 1:1 propensity score matching. The study monitored outcomes, including lymphoma, NPC, gastric cancer, multiple sclerosis, and all-cause mortality.
Results
Patients with a history of IM demonstrated a significantly higher incidence of lymphoma (hazard ratio [HR], 5.320; 95% confidence interval [CI], 3.208 to 8.820; p < 0.001) and NPC (HR, 7.116; 95% CI, 1.617 to 31.314; p=0.009) during the follow-up period compared with the control group. Additionally, the IM group showed an increased rate of all-cause mortality (HR, 2.225; 95% CI, 1.858 to 2.663; p < 0.001).
Conclusion
This study suggests that individuals with a history of IM have an elevated risk of developing lymphoma and NPC in South Korea, emphasizing the importance of vigilant follow-up and monitoring. The results advocate for heightened awareness and potential national monitoring policies to address the long-term health implications of EBV infection and to implement preventive measures.
Infectious mononucleosis (IM) is a viral infection mainly caused by the Epstein-Barr virus (EBV). It is a contagious illness that primarily affects adolescents and young adults, although it can occur at any age. It is characterized by symptoms such as fever, sore throat, swollen lymph nodes, and fatigue, occasionally lasting for several weeks. Despite typically being considered a benign, self-limiting illness, it can cause serious and long-term sequelae such as splenic rupture, hepatitis, or neurologic complications [1]. Accordingly, primary care physicians and otolaryngologists are required to have a thorough understanding of its characteristics and to inform the diagnosis and management of patients.
EBV is a member of the herpesvirus family, and it is one of the most common viruses found in humans. With a high prevalence worldwide, most people will become infected throughout the course of their lives [2]. The virus is known for its ability to establish lifelong latent infections in its hosts and can enter a dormant phase wherein it remains in the body without causing symptoms. Besides IM, EBV has also been linked to other long-term consequent diseases, including autoimmune-related diseases, lymphoproliferative disorders, and various types of cancers [3-8]. Therefore, preventing EBV infection and monitoring individuals infected with EBV is crucial.
Nasopharyngeal cancer (NPC) and lymphoma in the head and neck are not uncommon for otolaryngologists, and both conditions are closely associated with EBV infection [9,10]. These related head and neck malignancies have rapid progression, and when discovered in the advanced stages, often have a poor prognosis. Early detection and diagnosis through meticulous physical examinations and endoscopic evaluations are therefore essential. Although not all EBV infections are associated with such serious conditions, individuals with a history of EBV-related diseases such as IM should be considered for active surveillance, given their increased potential for carcinogenesis associated with latent infection and lytic replication [11].
This study aimed to investigate whether the risk of developing NPC and lymphoma increases when patients with a history of IM are followed up and monitored. Additionally, we sought to determine whether the prevalence of other conditions associated with EBV also increases in patients with a history of IM, exploring the necessity for proactive monitoring of patients infected with EBV.
This study was conducted using the Korean National Health Insurance Service (NHIS) database (DB) (https://nhiss.nhis.or.kr, NHIS-2023-1-496). Data included sex, age, insurance subscriber type, insurance payment quintile, region, and medical records for the participants, ranging from 2002 to 2021. The NHIS DB contains highly accurate and reliable medical data for 97% of the Korean population [12,13]. The DB comprises an eligibility DB containing basic information on the individual, a medical information DB containing diagnosis and prescription codes, a birth and death DB, and a health examination DB containing physical examinations and patient history [14]. All diagnosis codes used International Classification of Diseases 10th revision (ICD-10).
Between 2002 and 2011, 42,672 participants were diagnosed with IM (ICD-10 code: B27) according to the NHIS DB. Diagnostic criteria of IM include main diagnosis and sub diagnosis in an outpatient or inpatient setting. Those without claim records of an EBV test (n=15,723) during the same period were excluded. This was done to prevent errors in estimating diagnoses based solely on clinical manifestations of IM or incorrect input of the B27.0 disease code, which could compromise the reliability of the diagnosed subjects. After excluding 98 individuals without socio-demographic information such as year of birth, sex, and area of residence and 1,280 subjects aged ≥ 45 years old comprising the top 5% in terms of age distribution, 25,582 participants were finally classified as the IM group. For the control group, 430,089 individuals who had not been diagnosed with IM between 2002 and 2021 among the population covered by health insurance as of 2006 were selected by random sampling. Among the control group, 1,102 individuals with missing data such as socio-demographic information and 1,280 subjects aged ≥ 45 years old comprising the top 5% in terms of age distribution were excluded. Finally, 430,089 participants were included in the analysis. One-to-one propensity score matching (PSM) was then performed given the different baseline characteristics such as age, sex, and medical history variables including endocrine disease (E10), liver disease (K75.4), immunologic disease (M03-06, M30-36, M45), and malignancy (C00-97). Finally, 25,582 individuals in each group were analyzed (Table 1, S1 Table).
We used the NHIS eligibility information database to determine date of birth, sex, and region of residence of all participants. For residential areas, Seoul, Gyeonggi-do, and metropolitan cities were classified as urban, and other areas were classified as rural. The primary outcomes, including lymphoma (ICD-10 code: C81-88), NPC (ICD-10 code: C11), gastric cancer (ICD-10 code: C16), and multiple sclerosis (MS) (ICD-10 code: G35) were confirmed by diagnosis through individual medical records. In the case of cancer incidence, we aimed to enhance diagnostic reliability by restricting the analysis to individuals for whom the diagnostic codes for the respective diseases were concurrently confirmed with the special code for cancer claims V193 or V194 meaning the expanding benefit coverage for cancer patients in South Korea. Death information was observed using the death date in the Statistics Korea database.
The index date was defined as the date of EBV diagnosis, and the date of health insurance eligibility registration in 2006 for the control group. After indexing, we noted whether lymphoma, NPC, gastric cancer, and MS occurred for each individual until 2021. During the follow-up period, individuals newly diagnosed with IM were not included in the cohort. Additionally, outcomes were defined as occurrences of the specified diseases only if they were diagnosed at least 1 month after the date of IM diagnosis. The follow-up end date was the earliest of the event date, death date, or December 31, 2021.
Statistical analyses were performed with SAS Enterprise Guide ver. 7.1 (SAS Institute Inc., Cary, NC) and RStudio version 4.0.3 (RStudio: Integrated Development for R. RStudio, PBC, Boston, MA; http://www.rstudio.com/). PSM was conducted using the nearest method in the ‘MatchIt’ package in the R software, and the caliper was set to 0.25. After the PSM, the standardized mean difference (SMD) was estimated for the balance between IM group and non-IM group. When the absolute value of the predicted SMD is less than 0.1, it is considered balanced. Frequency analysis was performed to show the baseline characteristics of the participants, and p-values were calculated through the chi-square test. Continuous variables such as age and number of diseases were presented as mean±standard deviation, and Student’s t-test was performed to evaluate the statistical difference between significant factors. To compare the occurrence of diseases as the main outcome of the two groups, Cox regression analysis was conducted to investigate hazard ratios (HR) and 95% confidence intervals (CI), and p-values of < 0.05 were considered to indicate statistical significance. Cumulative incidence probability plots were created using the ‘survminer’ and ‘ggcompetingrisks’ packages of the R software.
Table 1 presents the demographic data of the participants included in the analysis. After participants were matched in a 1:1 ratio between the IM and control groups, adjustment for potential confounding factors was performed, resulting in 25,582 individuals in each group. The estimated SMD were showed that there were no statistically significant differences in age, sex, medical history, or other demographics between the two groups (SMD < 0.1). The IM group had a slightly shorter follow-up observation period (180.7±36.1 months vs. 190.9±9.9 months, p < 0.001).
During the follow-up period in both groups, newly diagnosed cases of lymphoma and NPC were observed in 0.4% and 0.1% of the IM group, respectively, which were significantly higher than the rates in the control group (0.1% and 0%, respectively; all p < 0.05). Furthermore, the IM group exhibited significantly higher incidence rate of other malignancy (1.5% vs. 1.1%, p < 0.001) and all-cause mortality (1.5% vs. 0.7%, p < 0.001). We also obtained survival curves and cumulative incidence probability plots through competing risk analysis, and similar to the results in Table 1, we confirmed a significantly higher probability of developing lymphoma and NPC in the IM group compared with the control group (all p < 0.001) (Fig. 1, S2 Fig.). Collectively, individuals with a history of IM showed an increased risk of developing lymphoma, NPC, and other types of cancers, even with all-cause mortality.
We aimed to investigate whether a history of IM could be linked with various illnesses associated with EBV infection and their relative risks. We selected gastric cancer and MS among EBV-related diseases, but the incidence rates in the IM group were not significantly higher than those in the control group (all p > 0.05) (Table 2). In contrast, the incidence rate per 100,000 person-years for lymphoma differed significantly, with 24.1 cases in the IM group and 4.4 cases in the control group, showing an HR of 5.320 (95% CI, 3.208 to 8.820; p < 0.001). Similarly, for NPC, the incidence rate per 100,000 person-years was significantly higher in the IM group at 3.6 cases compared with 0.5 cases in the control group, with a significant HR of 7.116 (95% CI, 1.617 to 31.314; p=0.009). Additionally, the risk of death in the IM group was 2.225 times higher than in the control group (95% CI, 1.858 to 2.663; p < 0.001). In summary, gastric cancer and MS were not significantly associated with a history of IM in our cohort; however, a history of IM was a risk factor for lymphoma, NPC, and mortality.
EBV is primarily transmitted through droplets or saliva and enters through the upper respiratory tract, causing various diseases. Even after the acute infection period has passed, a form of a latent infection in B lymphocytes remains. With reduced immunity, the infection undergoes lytic replication or, even if immunity is normal, can cause lymphoma, NPC, and stomach cancer through a chronic reactivation process through the immune escape mechanism [11,15]. The World Health Organization and many countries has designated oncoviruses, such as hepatitis virus and human papillomavirus, as targets of health care management at the national level, and have encouraged the preparation of infection prevention measures and the development of vaccines through multinational pharmaceutical companies [16,17]. However, such efforts have been insufficient to control EBV. Therefore, social interest and awareness will be necessary for the health and medical community aim to prevent infection spread and manage infected patients.
IM is a representative disease associated with EBV, which primarily occurs in young patients and is often dismissed as a severe upper respiratory tract infection. However, considering the disease-related risk of EBV and possibility of latent reactivation, we planned this study to determine if patients with a history of IM require monitoring after symptoms disappear, in contrast to current medical practices. We extracted a large sample of participants with a history of IM using domestic NHIS data and analyzed the risk of disease morbidity over a long follow-up period. Surprisingly, individuals with a history of IM had more than a 5-fold higher risk of developing lymphoma and NPC than healthy controls. They also had an increased mortality rate from all causes. In 2003, Hjalgrim et al. [18] already reported a significantly elevated risk of EBV-associated Hodgkin lymphoma among patients confirmed with positive EBV tests for IM in a population-based Danish cohort and also indicated that the onset of lymphoma merely occurs within an average of 4 years. Consequently, there is a need to closely monitor these patients over long-term follow-up for the development of lymphoma, NPC, and other events of mortality rather than dismissing IM as a one-off, mild disease.
A potential mechanistic hypothesis for the increased incidence of lymphoma and NPC following IM onset could be speculated as follows. Firstly, as mentioned earlier, it is possible that the EBV infection acquired during IM persists and induces mutations in B cells and nasopharyngeal lymphoid tissue, thereby promoting carcinogenesis. Secondly, alterations in immune function within the human body following IM illness could weaken immune surveillance against malignant cells and lead to immune tolerance, resulting in diminished anti-tumor immune responses. Additionally, there may exist shared yet unknown carcinogenesis pathways between IM and lymphoma/NPC, which could arise not only from direct effects on the immune system by EBV but also from more complex molecular interactions at the biological level. Ultimately, molecular biological evidence of causality between IM and the occurrence of the EBV-mediated malignancies will be necessary through future basic experiments and translational research.
In EBV-endemic areas such as Taiwan, South China, the UK and some European regions, sero-epidemiologic surveys and monitoring for EBV have been conducted considering the risk of the infection and its associated diseases. Efforts are also underway to develop vaccines against EBV [19-22]. As a country adjacent to an EBV-endemic area, South Korea should be aware of EBV infection and a resulting disease outbreak and establish a national-level system to manage it. Furthermore, clinicians should have awareness and vigilance regarding EBV-mediated diseases, and they should pay more careful observation and attention when treating patients with related conditions.
To ensure temporal reliability of newly occurring EBV-associated conditions following IM diagnosis, we selectively identified patients with recorded disease codes at least one month after IM diagnosis. However, as observed in Fig. 1 and S2 Fig., we noted a somewhat rapid increase in the incidence rates of NPC and lymphoma within the initial 1-2 years following IM diagnosis. A plausible explanation for this could be the similarity in symptoms between IM and conditions such as fever, cervical lymphadenopathy, or tonsillar enlargement, which can also occur in patients with NPC and lymphoma, leading to confusion and overlap in short-term diagnoses. Additionally, patients diagnosed with IM may undergo tissue biopsy within a short period due to lack of improvement, resulting in subsequent diagnoses of NPC or lymphoma. Such occurrences may introduce errors and limitations in the sequential identification of EBV-associated conditions other than IM.
Our study defined co-morbidity using ICD-10 diagnosis codes; therefore, we cannot rule out the possibility of excluding patients who were actually omitted or whose diagnosis codes were entered incorrectly. When analyzing inflammatory bowel disease or immunologic disease, which are associated with EBV infection, the incidence was slightly higher than usual, so these were excluded from the analysis. There may be a potential limitation due to residual confounding effects from unpredictable variables on PSM in the present study. Factors such as body mass index or obesity status, and other carcinogen exposures may influence the incidence of lymphoma and NPC. The final outcomes, as well as the covariate values of the study, were derived from NHIS database sources, and consequently, these potential confounders may not have been evaluated in the present study. Careful interpretation of the main conclusions in the present study is advised. However, we tried to increase the reliability of IM diagnosis by considering the prescription code for EBV testing and the number of hospital visits in addition to the IM disease code. This study determined that patients with IM have a high risk of developing dangerous cancers such as lymphoma and NPC and is the first report of its kind in South Korea. Therefore, the results of our study may be helpful in establishing a health care system in the future to identify and follow-up IM patients with symptomatic EBV infection and monitor EBV-associated severe disease development through early intervention.
In our study, when patients in South Korea with a history of IM were followed up, the risk of developing lymphoma and NPC increased, and the risk of developing other types of cancers and mortality from all causes was also high. Patients with IM should therefore be closely followed up, and future work should look to establish a national monitoring policy to determine whether serious EBV-related diseases occur within these patients, and to prepare measures to prevent EBV infection and spread.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB No. S2022-2067) of Asan Medical Center. Informed consent from the participants was waived due to the study nature.
Author Contributions
Conceived and designed the analysis: Kang SH, Lee YH, Myong JP, Kwon M.
Collected the data: Kang SH, Lee YH, Myong JP, Kwon M.
Contributed data or analysis tools: Kang SH, Lee YH, Myong JP, Kwon M.
Performed the analysis: Kang SH, Lee YH, Myong JP, Kwon M.
Wrote the paper: Kang SH, Lee YH, Myong JP, Kwon M.
ACKNOWLEDGMENTS
This work was supported by the Korean Society of Otorhinolaryngology-Head and Neck Surgery grant (2023) and National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (RS-2023-00208625) of Korea.
References
1. Sylvester JE, Buchanan BK, Silva TW. Infectious mononucleosis: rapid evidence review. Am Fam Physician. 2023; 107:71–78.
2. Dunmire SK, Verghese PS, Balfour HH Jr. Primary Epstein-Barr virus infection. J Clin Virol. 2018; 102:84–92.

3. Houen G, Trier NH. Epstein-Barr virus and systemic autoimmune diseases. Front Immunol. 2020; 11:587380.

4. Soldan SS, Lieberman PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. 2023; 21:51–64.

5. Marques-Piubelli ML, Salas YI, Pachas C, Becker-Hecker R, Vega F, Miranda RN. Epstein-Barr virus-associated B-cell lymphoproliferative disorders and lymphomas: a review. Pathology. 2020; 52:40–52.

6. Hue SS, Oon ML, Wang S, Tan SY, Ng SB. Epstein-Barr virus-associated T- and NK-cell lymphoproliferative diseases: an pdate and diagnostic approach. Pathology. 2020; 52:111–127.

7. Lindsay J, Othman J, Heldman MR, Slavin MA. Epstein-Barr virus posttransplant lymphoproliferative disorder: update on management and outcomes. Curr Opin Infect Dis. 2021; 34:635–645.

9. Vasudevan HN, Yom SS. Nasopharyngeal carcinoma and its association with Epstein-Barr virus. Hematol Oncol Clin North Am. 2021; 35:963–971.

10. Murray PG, Young LS. An etiological role for the Epstein-Barr virus in the pathogenesis of classical Hodgkin lymphoma. Blood. 2019; 134:591–596.

11. Munz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol. 2019; 17:691–700.

12. Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data configuration and publication trends for the Korean National Health Insurance and Health Insurance Review & Assessment Database. Diabetes Metab J. 2020; 44:671–678.

13. Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction: a scoping review. Precis Future Med. 2022; 6:9–31.

14. Choi EK. Cardiovascular research using the Korean National Health Information Database. Korean Circ J. 2020; 50:754–772.

15. Kerr JR. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol. 2019; 72:651–658.

16. Stasi C, Silvestri C, Voller F, Cipriani F. The epidemiological changes of HCV and HBV infections in the era of new antiviral therapies and the anti-HBV vaccine. J Infect Public Health. 2016; 9:389–395.

17. Prabhu M, Eckert LO. Development of World Health Organization (WHO) recommendations for appropriate clinical trial endpoints for next-generation human papillomavirus (HPV) vaccines. Papillomavirus Res. 2016; 2:185–189.

18. Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med. 2003; 349:1324–1332.

19. Chen CY, Huang KY, Shen JH, Tsao KC, Huang YC. A large-scale seroprevalence of Epstein-Barr virus in Taiwan. PLoS One. 2015; 10:e0115836.

20. Shi J, Ma W, Li W. Epidemiologic features of children with Epstein-Barr virus associated diseases in Hangzhou, China. J Med Virol. 2020; 92:1277–1282.

Fig. 1.
The cumulative incidence probabilities using survival curves of lymphoma (A) and nasopharyngeal cancer (B) occurrence according to the history of infectious mononucleosis (IM).
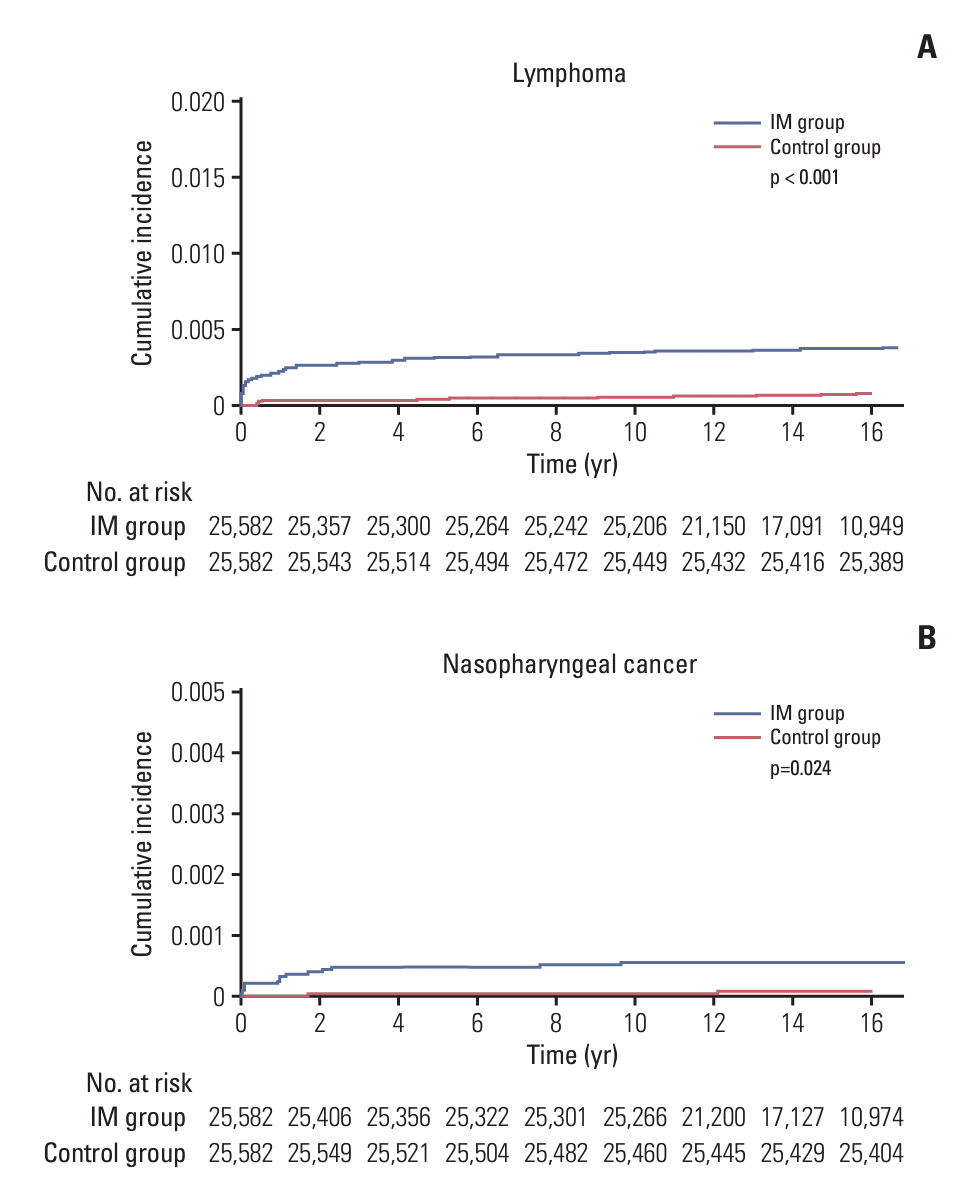
Table 1.
Comparison of clinical characteristics between groups based on a history of infectious mononucleosis
| IM group (n=25,582) | Control group (n=25,582) | SMD | p-value | |
|---|---|---|---|---|
| Age (yr) | 6 (1-44) | 6 (3-44) | 0.001 | |
| Sex (male/female) | 14,922 (58.3)/10,660 (41.7) | 14,901 (58.2)/10,681 (41.8) | 0.002 | |
| Comorbidities, present | ||||
| Endocrine disease | 22 (0.1) | 16 (0.1) | 0.009 | |
| Liver disease | 10 | 8 | 0.004 | |
| Immunologic disease | 596 (2.3) | 605 (2.4) | 0.002 | |
| Malignancy | 284 (1.1) | 273 (1.1) | 0.004 | |
| Residence, urban/rural | 18,014 (70.4)/7,568 (29.6) | 17,701 (69.2)/7,881 (30.8) | 0.027 | |
| Follow-up informationa) | ||||
| Duration (mo) | 180.7±36.1 | 190.9±9.9 | < 0.001 | |
| Lymphoma | 93 (0.4) | 18 (0.1) | < 0.001 | |
| NPC | 14 (0.1) | 2 (0.0) | 0.006 | |
| Other malignancies | 389 (1.5) | 271 (1.1) | < 0.001 | |
| Death | 380 (1.5) | 177 (0.7) | < 0.001 |
Table 2.
Comparison of disease occurrence frequency and risk ratio by group according to a history of infectious mononucleosis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download