1. Kang MJ, Jung KW, Bang SH, Choi SH, Park EH, Yun EH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2020. Cancer Res Treat. 2023; 55:385–399.
2. Chon YE, Lee HA, Yoon JS, Park JY, Kim BH, Lee IJ, et al. Hepatocellular carcinoma in Korea between 2012 and 2014: an analysis of data from the Korean Nationwide Cancer Registry. J Liver Cancer. 2020; 20:135–147.
3. Singal AG, Kudo M, Bruix J. Breakthroughs in hepatocellular carcinoma therapies. Clin Gastroenterol Hepatol. 2023; 21:2135–2149.
4. Lee JS, Kim JM, Lee S, Choi JY, Cho W, Choi GS, et al. The prognosis in cases of hepatocellular carcinoma after hepatectomy: young patients versus older patients. Korean J Hepatobiliary Pancreat Surg. 2015; 19:154–160.
5. Park GC, Hwang S, Park YH, Choi JU; Korean Liver Cancer Study Group. Validation of prognostic impact of ADV score for resection of hepatocellular carcinoma: analysis using Korea Liver Cancer Registry database. Ann Surg Treat Res. 2020; 98:235–246.

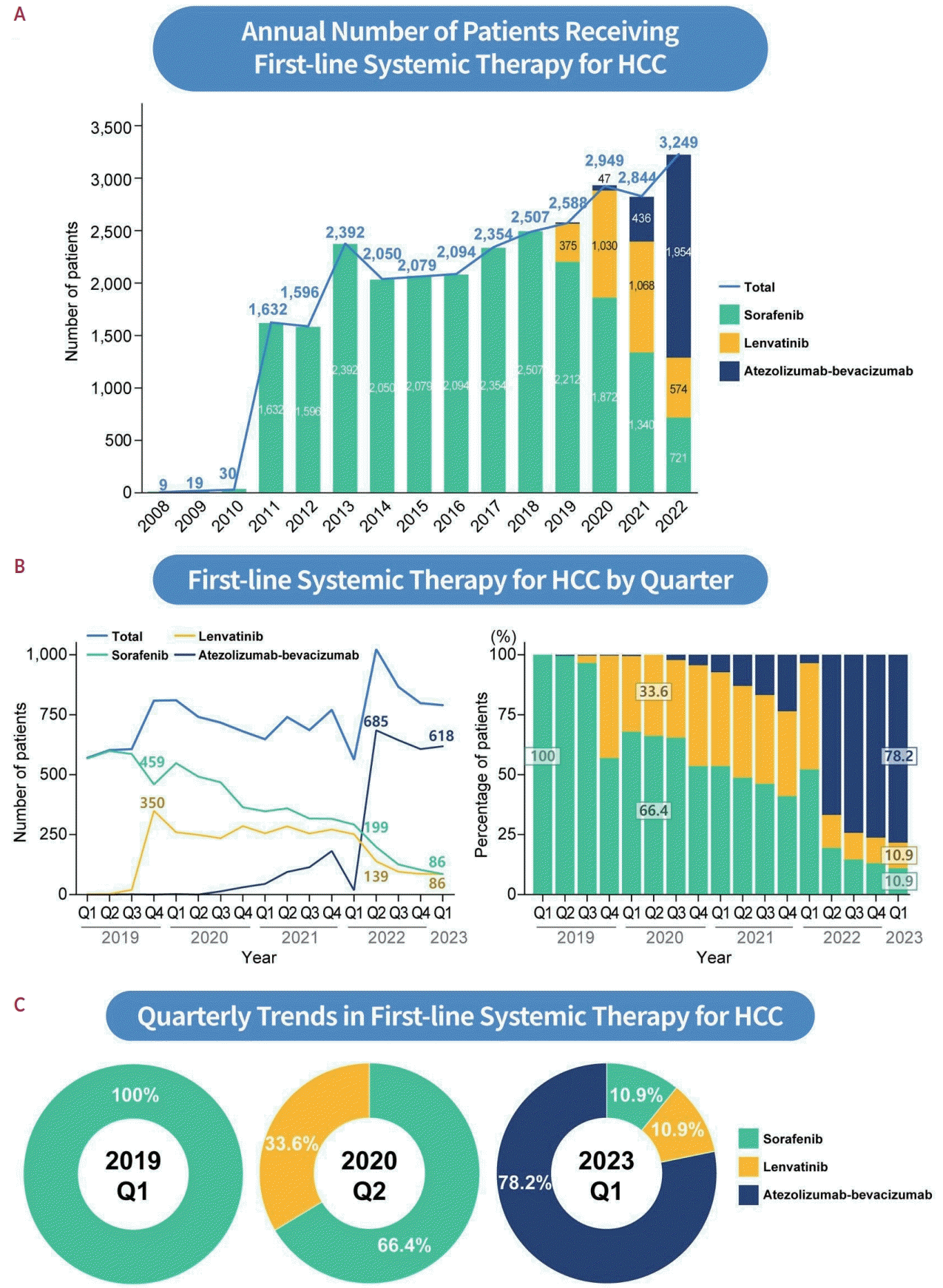
6. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.

7. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018; 391:1163–1173.

8. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022; 76:862–873.

9. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022; 1:EVIDoa2100070.
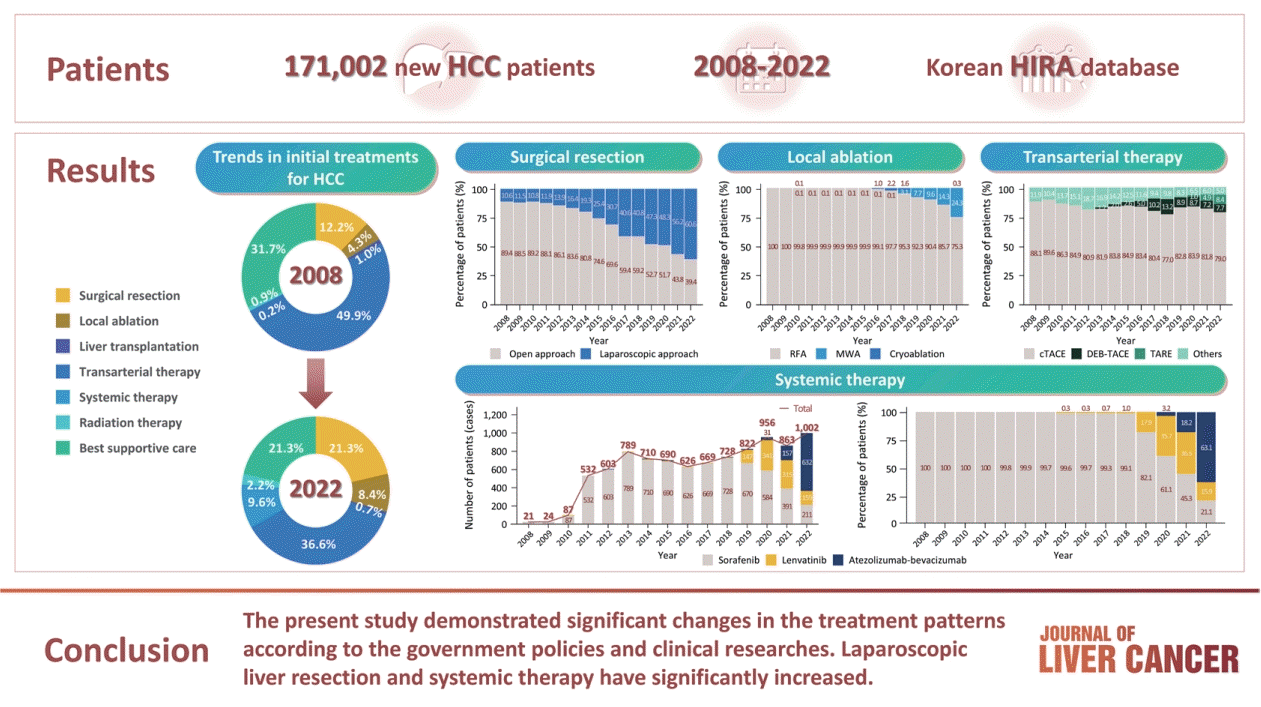
10. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.
11. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022; 76:681–693.

12. Chon YE, Jeong SW, Jun DW. Hepatocellular carcinoma statistics in South Korea. Clin Mol Hepatol. 2021; 27:512–514.

13. Kyoung DS, Kim HS. Understanding and utilizing claim data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) database for research. J Lipid Atheroscler. 2022; 11:103–110.

14. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017; 32:718–728.

15. Pinheiro PS, Jones PD, Medina H, Cranford HM, Koru-Sengul T, Bungum T, et al. Incidence of etiology-specific hepatocellular carcinoma: diverging trends and significant heterogeneity by race and ethnicity. Clin Gastroenterol Hepatol. 2024; 22:562–571.e8.
16. Park EH, Jung KW, Park NJ, Kang MJ, Yun EH, Kim HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2021. Cancer Res Treat. 2024; 56:357–371.

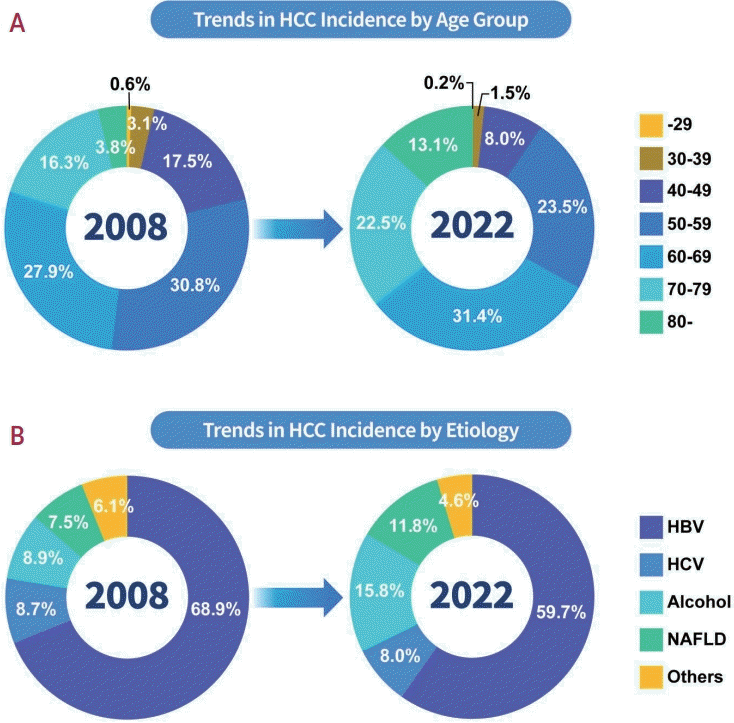
17. Cho Y, Kim BH, Park JW. The emerging age-pattern changes of patients with hepatocellular carcinoma in Korea. Clin Mol Hepatol. 2023; 29:99–101.

18. Kim YJ, Jang BK, Kim ES, Chung WJ, Park KS, Cho KB, et al. Hepatocellular carcinoma in the elderly: clinical characteristics, treatment, survival analysis in Korean patients older than 70 years. J Korean Med Sci. 2012; 27:1147–1154.

19. Torimura T, Iwamoto H. Optimizing the management of intermediatestage hepatocellular carcinoma: current trends and prospects. Clin Mol Hepatol. 2021; 27:236–245.

20. Hatanaka T, Kakizaki S, Hiraoka A, Kariyama K, Tsuji K, Ishikawa T, et al. The prognosis of elderly patients with hepatocellular carcinoma: a multicenter 19-year experience in Japan. Cancer Med. 2023; 12:345–357.
21. Kim BH, Lim YS, Kim EY, Kong HJ, Won YJ, Han S, et al. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J Gastroenterol Hepatol. 2018; 33:475–483.

22. Sohn W, Kang D, Kang M, Guallar E, Cho J, Paik YH. Impact of nationwide hepatocellular carcinoma surveillance on the prognosis in patients with chronic liver disease. Clin Mol Hepatol. 2022; 28:851–863.

23. Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila). 2012; 5:1124–1130.

24. Schneider CV, Schneider KM, Raptis A, Huang H, Trautwein C, Loomba R. Prevalence of at-risk MASH, MetALD and alcohol-associated steatotic liver disease in the general population. Aliment Pharmacol Ther. 2024; 59:1271–1281.

25. Bae H, Lee SA, Choi JW, Hwang SH, Park S, Park MS. Effectiveness of hepatocellular carcinoma surveillance and an optimal surveillance interval: nationwide cohort of Korea. Yonsei Med J. 2021; 62:758–766.
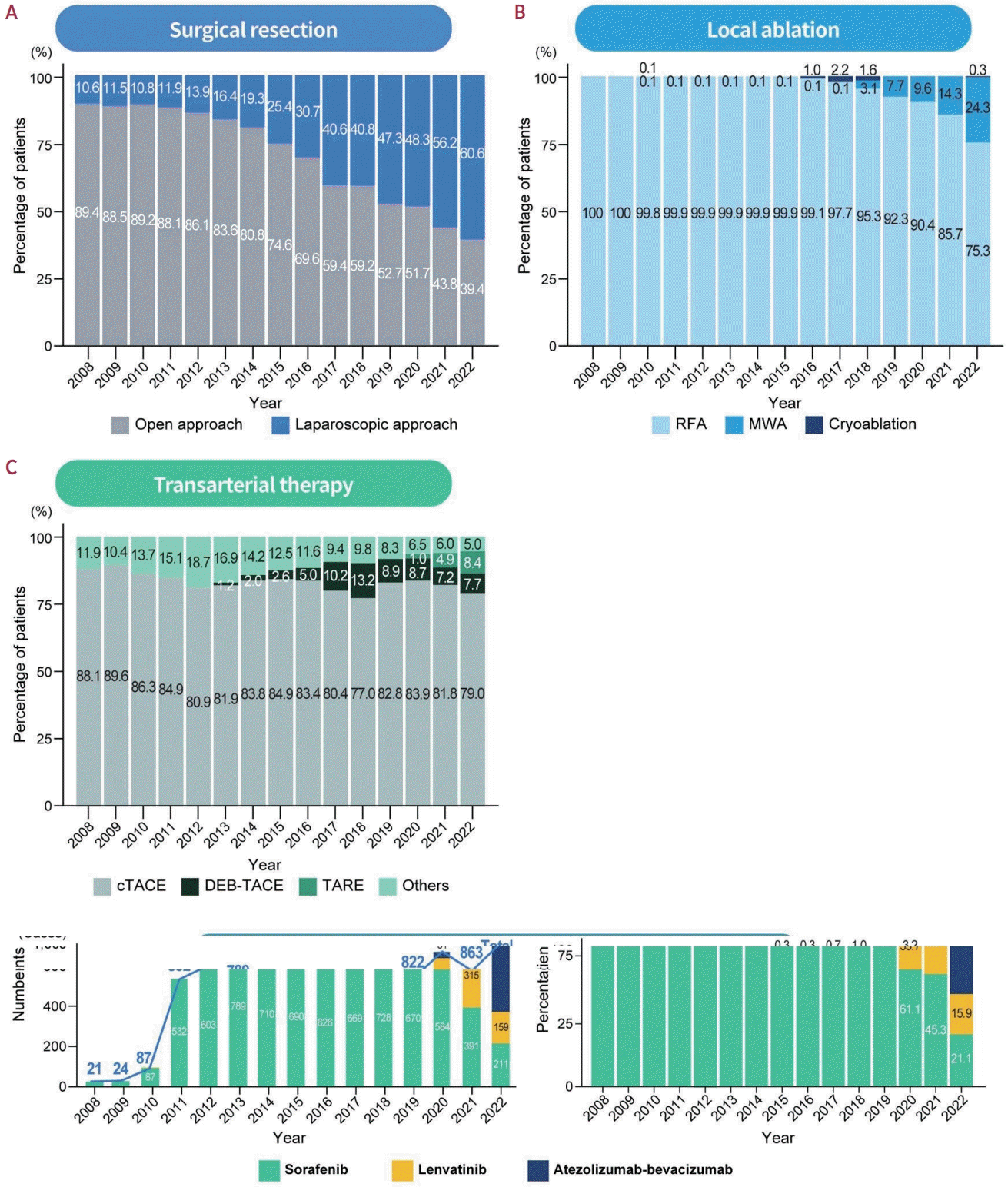
26. Chieh AKW, Chan A, Rotellar F, Kim KH. Laparoscopic major liver resections: current standards. Int J Surg. 2020; 82 Suppl:169–177.
27. Ciria R, Gomez-Luque I, Ocaña S, Cipriani F, Halls M, Briceño J, et al. A systematic review and meta-analysis comparing the short- and longterm outcomes for laparoscopic and open liver resections for hepatocellular carcinoma: updated results from the European guidelines meeting on laparoscopic liver surgery, Southampton, UK, 2017. Ann Surg Oncol. 2019; 26:252–263.

28. Witowski J, Rubinkiewicz M, Mizera M, Wysocki M, Gajewska N, Sitkowski M, et al. Meta-analysis of short- and long-term outcomes after pure laparoscopic versus open liver surgery in hepatocellular carcinoma patients. Surg Endosc. 2019; 33:1491–1507.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download