Abstract
The objective of this study was to investigate the protective effect and potential mechanism of action of hemin on bleomycin-induced pulmonary fibrosis in mice. Male C57BL/6 mice were randomly divided into control, bleomycin and bleomycin + hemin groups. Mice in the bleomycin and bleomycin + hemin groups were injected intratracheally with bleomycin to establish the pulmonary fibrosis model. The bleomycin + hemin group mice were injected intraperitoneally with hemin starting 7 days before modeling until the end of Day 21 after modeling. Pathological changes in lung tissue were assessed by HE and Masson staining. Malondialdehyde (MDA), superoxide dismutase (SOD) and catalase (CAT) levels were determined in lung tissue. Immunohistochemistry was performed to assess the expression of α-SMA and collagen I. The serum levels of IL-6 and TNF-α were measured via ELISA. Western blotting was used to determine the expression of TGF-β1, SIRT1, PGC-1α and HO-1 and the phosphorylation levels of p38, ERK1/2, JNK, AMPK and NF-κB p65 in lung tissue. Hemin significantly reduced lung indices, increased terminal body weight. It also significantly increased SOD and CAT activities; decreased MDA, IL-6 and TNF-α levels; reduced the levels of α-SMA and collagen I-positive cells; upregulated SIRT1, PGC-1α and HO-1 expression; promoted AMPK phosphorylation; and downregulated TGF-β1 expression and p38, ERK1/2, JNK and NF-κB p65 phosphorylation. Hemin might attenuate oxidative damage and inflammatory responses and reduces extracellular matrix deposition by regulating the expression and phosphorylation of proteins associated with the TGF-β1/MAPK and AMPK/SIRT1/PGC-1α/HO-1/NF-κB pathways, thereby alleviating bleomycin-induced pulmonary fibrosis.
Pulmonary fibrosis (PF) is a progressive, age-related lung disease that can lead to structural remodeling, impaired gas exchange, respiratory failure and death [1]. The causes of PF are complex, and the median survival after diagnosis is extremely short [2]. Currently, the only agents recommended by evidence-based guidelines are pirfenidone and nintedanib, but they cannot effectively improve patients' quality of life and inhibit disease progression [3-7]. Therefore, further research into the mechanisms of PF and effective therapies is particularly important.
Oxidative stress (OS) is involved in the process of PF through three main pathways: directly causing damage to alveolar epithelial cells and promoting apoptosis; increasing the aggregation of inflammatory cells, overexpressing tumor necrosis factor-α (TNF-α), interleukin (IL) and nuclear transcription factor-κB (NF-κB) and releasing large amounts of inflammatory factors; and directly stimulating the production of transforming growth factor-β1 (TGF-β1) [8-11]. TGF-β1 is a key inducer of PF progression and stimulates the production of reactive oxygen species (ROS) through the activation of the Smad 2/3 and mitogen-activated protein kinase (MAPK) pathways [12]. In turn, high levels of ROS promote the expression of TGF-β1, which induces the transformation of fibroblasts into myofibroblasts and accelerates the development of PF [13]. MAPKs are members of the serine-threonine kinase superfamily and consist of three MAPK subfamilies: the extracellular signal-regulated kinase (ERK), p38 MAPK and c-Jun-n-terminal kinase (JNK) [14]. Chien et al. [15] showed that the phosphorylation levels of p38, ERK and JNK were significantly elevated in the lung tissue of mice with bleomycin-induced PF, suggesting that p38, ERK and JNK are involved in the progression of PF. Wang et al. [16] reported that peptide DR8 ameliorated bleomycin-induced PF through inhibition of TGF-β1/MAPK pathway activation and attenuation of oxidative damage. Therefore, we suggest that inhibiting TGF-β1/MAPK pathway activation may be an effective treatment for PF.
TGF-β1 also induces alveolar epithelial cell injury by inhibiting adenosine monophosphate-activated protein kinase (AMPK) activity. AMPK is a 'switch' that regulates energy metabolic homeostasis in the body and is one of the key factors contributing to many fibrotic diseases, including PF [12,17]. AMPK has an inhibitory effect on the inflammatory response in lung diseases, and its mechanism may be related to the regulation of the expression of downstream genes, such as silent information regulator 1 (sirtuin, SIRT1), peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), and p53 [18]. Heme oxygenase-1 (HO-1) is a rate-limiting enzyme that catalyzes the degradation of heme, and several studies have confirmed the protective role of heme-induced HO-1 in acute lung injury [19], pulmonary hypertension [20] and chronic obstructive pulmonary disease [21]. AMPK/HO-1 activation plays a critical role in several physiological processes, including anti-inflammatory, antioxidant, antiapoptotic and vasculoprotective effects [22,23]. In addition, Yang et al. [24] reported that hemin-induced HO-1 ameliorated carbon tetrachloride-induced liver fibrosis in rats by regulating peroxisome proliferation-activated receptor (PPARγ) and NF-κB expression. Cho et al. [25] reported that rosiglitazone-induced HO-1 inhibits lipopolysaccharide-mediated lung inflammation by modulating the PKCα/AMPKα/p38MAPKα/SIRT1/PPARγ pathway. Therefore, in the present study, hemin was used to induce HO-1, and the expression and phosphorylation levels of proteins related to the TGF-β1/MAPK and AMPK/SIRT1/PGC-1α/HO-1/NF-κB pathways were detected in the lung tissue of mice with bleomycin-induced PF. Additionally, the protective effect of hemin on PF and the possible underlying mechanisms were investigated.
Male C57BL/6 mice (weight 20–25 g) were purchased from Changsha Tianqin Biotechnology [Certificate number SCXK (XIANG) 2019-0014]. The animals were maintained on a 12-h day/12-h night cycle at a temperature of 20°C–24°C. Mice were allowed to eat and drink ad libitum. All the experimental procedures were approved by the Animal Experimental Ethics Committee of Wannan Medical College (LISC-2020-157).
Male C57BL/6 mice were randomized into control, bleomycin and bleomycin + hemin groups, with 15 mice in each group. In accordance with the methods of Ma et al. [26], mice were anesthetized, 3 mg/kg bleomycin was injected intratracheally into the trachea of mice in the bleomycin and bleomycin + hemin groups to establish the PF model, and saline was administered to the control group. Mice in the bleomycin + hemin group received intraperitoneal injections of hemin at a dose of 30 mg/kg (once every other day) for 7 days prior to bleomycin stimulation and were continuously treated with hemin for 21 days after bleomycin injection. The preparation, dosage and method of administration of hemin were based on previous studies [19,27,28]. After the last administration of the drug, the mice in each group were weighed, blood was collected from the eyeballs, the animals were sacrificed, the lung tissue was weighed, and the lung coefficient was calculated (lung weight/body weight; mg/g).
Bleomycin sulfate (9041-93-4, purity ≥ 90%) and hemin chloride (16009-13-5, purity ≥ 97%) were purchased from Hefei Bomei Biotechnology. The α-SMA antibody was purchased from Beijing Bioworld Technology; the GAPDH antibody was purchased from Shanghai Beyotime Biotechnology; the collagen I antibody was purchased from Affinity Biosciences; and TGF-β1, AMPK, p-AMPK, SIRT1 and PGC-1α antibodies were purchased from Abcam; and p38, p-p38, ERK1/2, p-ERK1/2, JNK, p-JNK, NF-κB p65, p-NF-κB p65 and HO-1 antibodies were purchased from Cell Signaling Technology.
Fresh lung tissues were fixed in 4% paraformaldehyde for 48 h and then dehydrated in gradient ethanol before being processed into wax blocks. The wax blocks were cut into 5 μm thick sections, and the sections were deparaffinized in xylene and then placed in gradient ethanol and double-distilled water and stained according to the instructions of the HE (Fuzhou Phygene Science and Technology) and Masson (Hefei Bomei Biotechnology) staining kits. ImageJ software was used for image analysis of Masson's staining results (Broken Symmetry Software).
An appropriate amount of lung tissue was removed, saline or hydrolysate was added, and the mixture was centrifuged (4°C, 3,000 rpm, 10 min). The supernatant was extracted, after which the MDA, SOD and CAT kits (Nanjing Jiancheng Bioengineering Institute) were used to detect the activity or content of MDA, SOD and CAT in the lung tissue.
The serum of mice in each group was collected, and the levels of IL-6 and TNF-α in the serum of mice in each group were detected by using ELISA (IL-6 and TNF-α; Wuhan Elabscience Biotechnology) kits, which were operated strictly according to the instructions.
Sections were deparaffinized in xylene and then placed in gradient ethanol, and double distilled water, and immunohistochemistry experiments were performed using a concentrated SABC-POD (rabbit IgG) kit (Wuhan Boster Biological Technology) according to Huang et al. [29]. The sections were immersed in sodium citrate solution for antigen retrieval (heat in a microwave oven to 98°C–100°C and then on a low heat for 10 min). The sections were washed with ultrapure water, followed by 3% hydrogen peroxide to inactivate peroxidase (room temperature, 10 min). Then, the goat serum (37°C, 1 h) was added, and α-SMA and collagen I primary antibodies (1:150) were subsequently added, after which the sections were placed in a refrigerator at 4°C overnight. The sections were then removed and placed in an oven (37°C, 1 h) to allow the temperature to recover, after which secondary antibodies were added to the sections dropwise (37°C, 1 h), followed by SABC added dropwise (37°C, 1 h) and staining using an HRP-DAB kit (Beijing Tiangen Biochemical Technology). The images were analysed and processed using ImageJ software.
Appropriate amounts of lung tissue were removed, RIPA lysis buffer was added, homogenized and allowed to stand for 2 h, followed by centrifugation (4°C, 12,000 rpm, 10 min). The supernatant was extracted, and the protein concentration was determined using a BCA kit (Shanghai Beyotime Biotechnology). A 10% SDS polyacrylamide gel was prepared, followed by sample loading, electrophoresis (80-mV constant pressure electrophoresis until the target proteins were separated). The proteins were subsequently transferred to a PVDF membrane (a constant current of 200 mA, 1–2 h), after which the membranes were immersed in 5% skim milk or BSA (4°C, 1 h). The membranes were subsequently incubated with TGF-β1, p38, p-p38, ERK1/2, p-ERK1/2, JNK, p-JNK, AMPK, p-AMPK, HO-1, PGC-1α, SIRT1, NF-κB p65, p-NF-κB p65 and GAPDH primary antibodies (1:1,000, 4°C, overnight), and the next day, the membranes were incubated with the corresponding secondary antibodies (4°C, 2 h), washed with TBST buffer, incubated with fluorescent liquid and exposed. The bands were analyzed using ImageJ software.
The data are presented as the means ± standard deviations. Statistical analysis was performed using GraphPad Prism 8 software (version 8.0; GraphPad Software, Inc.). One-way analysis of variance (ANOVA) was performed for multiple comparisons between groups. The Tukey’s test was used for pairwise comparisons. The survival rates were recorded, estimated by the Kaplan–Meier method and were compared by a log-rank test. p < 0.05 was considered to indicate statistical significance.
Compared with those in the control group, the mice in the bleomycin group had significantly lower survival rates (p < 0.01) (Fig. 1A) and terminal body weights (p < 0.01) (Fig. 1B) and a significantly greater lung index (p < 0.01) (Fig. 1C). The difference in survival among the mice in the bleomycin + hemin group was not statistically significant (p > 0.05), although there was an increase in survival. Pretreatment with hemin resulted in a marked increase in the final body weight (p < 0.01) and a marked decrease in the lung indices of the mice (p < 0.01).
As shown in Fig. 2A, the lung tissue of mice in the control group was light pink in color, smooth, soft and elastic to the touch, with no nodules or hemorrhage points, and that of mice in the bleomycin group was grayish white, rough, hard and inelastic to the touch. The nodules were distinct and abundant with multiple hemorrhages points. The lung tissue of mice in the bleomycin + hemin group was slightly darker in color, slightly rougher, with reduced elasticity, and with a small number of nodules and hemorrhage points in some areas.
After HE and Masson staining (Fig. 2B–D), we found that the alveolar structure of the lung tissue of the mice in the control group was complete and clear, with thin alveolar walls, normal spacing, no edema or inflammatory cell exudation, and only a small amount of scattered blue collagen fibers. The lung tissue structure of the mice in the bleomycin group was severely damaged, with thickening of the alveolar wall, an increase in the width of the septa, and the appearance of large, dark blue collagen fibers between the lung interstitium. The bleomycin + hemin group showed varying degrees of reduction in all of the above pathological impairments.
Immunohistochemistry revealed only a small number of scattered brownish-yellow α-SMA- and collagen I-positive cells in the control group (Fig. 3). In the bleomycin group, the expression of SMA and collagen I was obviously increased (p < 0.01). Compared with those in the bleomycin group, the expression of α-SMA and collagen I was significantly lower in the hemin pretreatment group (p < 0.01).
MDA (p < 0.01) content was significantly increased, and SOD and CAT (p < 0.01) activity were markedly decreased in the lung tissue of the bleomycin group compared to those of the control group (Fig. 4). Hemin pretreatment significantly inhibited the bleomycin-induced increase in MDA (p < 0.05) production and the decrease in SOD and CAT (p < 0.01) activity.
As shown in Fig. 5, bleomycin stimulation significantly elevated the serum levels of IL-6 and TNF-α (p < 0.01) compared to those in the control group. The serum concentrations of IL-6 and TNF-α (p < 0.01) were markedly lower in the bleomycin + hemin group than in the bleomycin group.
Western blotting revealed that bleomycin stimulation significantly increased the expression of TGF-β1 (p < 0.01) and the phosphorylation of p38, ERK1/2 and JNK (p < 0.01) in mouse lung tissues compared with those in the control group. Hemin pretreatment markedly inhibited the bleomycin-induced increase in TGF-β1 expression (p < 0.05) and p38 (p < 0.05), ERK1/2 (p < 0.01) and JNK phosphorylation (p < 0.05) (Fig. 6 and Supplementary Fig. 1).
As shown in Fig. 7 and Supplementary Fig. 2, the expression of SIRT1, PGC-1α and HO-1 (p < 0.01) and the phosphorylation level of AMPK (p < 0.01) in the lung tissues of the mice in the bleomycin group were significantly lower than those in the control group, while the phosphorylation level of NF-κB p65 (p < 0.01) was significantly greater than that in the control group. Hemin pretreatment partially increased SIRT1 (p < 0.05), PGC-1α (p < 0.01) and HO-1 (p < 0.01) expression and AMPK (p < 0.01) phosphorylation and decreased NF-κB p65 (p < 0.01) phosphorylation.
PF is a devastating chronic pulmonary disease [30]. Epidemiological studies have shown that the incidence of PF is approximately 0.9 to 13/100,000 [31]. Dove's study showed a 9.8% increase in PF mortality in the United States over the past 10 years [32]. The pathogenesis of PF is complex and diverse, existing therapeutic drugs have the disadvantages of poor efficacy and many side effects [33], and the search for safe and effective anti-PF drugs has become an important research topic for a wide range of scholars. Therefore, the aim of the present study was to investigate the protective role of hemin in bleomycin-induced PF and its possible mechanisms to provide a new option for the treatment of PF.
The bleomycin-induced PF model is widely used in experimental studies, and its pathological process can be divided into three phases: early (inflammatory), intermediate (pro-fibrotic) and late (fibrotic). Bleomycin may cause patchy parenchymal inflammation of variable intensity, epithelial cell damage with reactive hyperplasia, basement membrane damage, interstitial damage and intra-alveolar fibrosis by provoking DNA strand breaks and oxidative damage [34-36]. The present study showed that bleomycin stimulation resulted in a marked decrease in survival and terminal body weight; a significant increase in the lung index; substantial inflammatory cell infiltration in the alveolar lumen and interstitium; and increased collagen deposition in mice, in agreement with the findings of Yang et al. [37]. After hemin treatment, these changes were significantly improved, and the degree of PF was mitigated.
α-SMA is a marker of myofibroblast activation and accelerates the development of PF by participating in the synthesis of extracellular matrix components such as collagen I, collagen III and fibronectin. TGF-β1 is one of the most important pro-fibrotic proteins, and its downstream MAPK pathway, as a key signaling pathway in vivo, mediates cellular processes such as inflammation, fibrosis and cancer progression and regulates the expression of multiple inflammatory mediators and cytokines [15,38]. The MAPK pathway has been confirmed to be closely associated with lung fibroblast activation and extracellular matrix deposition [39,40]. Studies have shown that increased ROS levels lead to activation of the MAPK pathway, which is associated with PF [41,42]. Dong et al. [43] reported that in H2O2-induced human alveolar epithelial A549 cells, MDA production was excessive, and SOD and GSH-Px activities were reduced, accompanied by activation of the MAPK pathway, whereas Sarcodon aspratus reduced apoptosis and alleviated oxidative damage to cells by decreasing the phosphorylation levels of ERK, JNK and p38. Yang et al. [44] reported that berberine has a protective effect on bleomycin-induced PF, and the underlying mechanism may be related to inhibition of the p38 MAPKa (pT180/Y182) sites, reduction of inflammatory cell aggregation and cytokine secretion, attenuation of the inflammatory response, downregulation of TGF-β1 and α-SMA expression, reduction of collagen production and inhibition of the conversion of lung fibroblasts into myofibroblasts. The present study showed that there was a significant increase in MDA production and a marked decrease in SOD and CAT activity; moreover, the expression of TGF-β1 and the phosphorylation of p38, ERK1/2 and JNK were markedly elevated, and the expression of α-SMA and collagen I increased in the lung tissue of the bleomycin group mice. However, hemin pretreatment notably inhibited MDA overexpression, increased SOD and CAT activities, decreased the expression and phosphorylation levels of proteins related to the TGF-β1/MAPK pathway, and decreased the positive cellular expression of α-SMA and collagen I, suggesting that hemin probably ameliorated bleomycin-induced PF by inhibiting TGF-β1/MAPK pathway activation, attenuating oxidative damage and reducing extracellular matrix deposition.
AMPK is a recognized cellular bioenergetic sensor and metabolic regulator. AMPK protects the liver, heart, lung and kidney from fibrosis [45]. SIRT1 is a histone deacetylase that exerts anti-inflammatory effects by inhibiting the production of several proinflammatory cytokines by inducing the activation of PGC-1α and the downregulation of NF-κB [46,47]. PGC-1α is a primary regulator of mitochondrial biogenesis, oxidative phosphorylation and mitochondrial antioxidant defense and is responsible for maintaining a metabolic steady state. PGC-1α and NF-κB regulate each other during inflammation and play an important role in the vicious cycle of OS [48]. HO-1 is a membrane-bound enzyme encoded by the HMOX-1 gene and can be strongly induced by a variety of factors, including hemin, UV light and heat shock [49-53]. HO-1 acts both as a key cytoprotective molecule against oxidative damage and as a key anti-inflammatory protein that can attenuate NF-κB-mediated inflammatory responses [54,55]. The literature has shown that Sanghuangporus sanghuang acts by regulating the TLR4/NF-κB/MAPK, Keap1/Nrf2/HO-1, CaMKK/AMPK/Sirt1 and TGF-β/SMAD3 pathways and exerts anti-inflammatory, antioxidant and antiapoptotic effects, thereby relieving bleomycin-induced PF in mice [15]. Park et al. [56] reported that hemin prevents UV-induced skin cancer, and the underlying mechanism may be related to activation of the AMPK/HO-1 pathway. Ali et al. [57] reported that hemin ameliorates ovarian ischemia‒reperfusion injury by regulating the HO-1 and p-JNK/p-NF-κB p65/iNOS pathways, increasing antioxidant capacity and inhibiting the inflammatory response. In the present study, after bleomycin stimulation, the expression of SIRT1, PGC-1α and HO-1 and the phosphorylation of AMPK were significantly reduced, and the phosphorylation of NF-κB p65 and the serum IL-6 and TNF-α levels were significantly increased in mouse lung tissues; however, hemin pretreatment significantly inhibited the bleomycin stimulation-induced changes in these indices. These results suggest that hemin possibly inhibits OS and the inflammatory response by modulating the AMPK/SIRT1/PGC-1α/HO-1/NF-κB pathway, thereby ameliorating bleomycin-induced PF.
In conclusion, hemin likely alleviated bleomycin-induced PF by regulating expression and phosphorylation of proteins related to the TGF-β1/MAPK and AMPK/SIRT1/PGC-1α/HO-1/NF-κB pathways, inhibiting oxidative stress and the inflammatory response, and reducing extracellular matrix deposition; however, the exact mechanism involved requires further investigation.
Supplementary data including two figures can be found with this article online at https://doi.org/10.4196/kjpp.2024.28.6.559
Notes
REFERENCES
1. Santos G, Fabiano A, Mota PC, Rodrigues I, Carvalho D, Melo N, Novais-Bastos H, Alexandre AT, Moura CS, Guimarães S, Pereira JM, Carvalho A, Morais A. 2023; The impact of nintedanib and pirfenidone on lung function and survival in patients with idiopathic pulmonary fibrosis in real-life setting. Pulm Pharmacol Ther. 83:102261–102267. DOI: 10.1016/j.pupt.2023.102261. PMID: 37758002.
2. Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. 2019; Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med. 65:56–69. DOI: 10.1016/j.mam.2018.08.004. PMID: 30130563. PMCID: PMC6374163.
3. Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. 2016; Drug treatment of idiopathic pulmonary fibrosis: systematic review and network meta-analysis. Chest. 149:756–766. DOI: 10.1016/j.chest.2015.11.013. PMID: 26836914.
4. Justet A, Klay D, Porcher R, Cottin V, Ahmad K, Molina Molina M, Nunes H, Reynaud-Gaubert M, Naccache JM, Manali E, Froidure A, Jouneau S, Wemeau L, Andrejak C, Gondouin A, Hirschi S, Blanchard E, Bondue B, Bonniaud P, Tromeur C, et al. ; OrphaLung Network. 2021; Safety and efficacy of pirfenidone and nintedanib in patients with idiopathic pulmonary fibrosis and carrying a telomere-related gene mutation. Eur Respir J. 57:2003198–2003201. DOI: 10.1183/13993003.03198-2020. PMID: 33214205.
5. Collins BF, Raghu G. 2019; Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. Eur Respir Rev. 28:190022–190036. DOI: 10.1183/16000617.0022-2019. PMID: 31578210. PMCID: PMC9489066.
6. Lancaster LH, de Andrade JA, Zibrak JD, Padilla ML, Albera C, Nathan SD, Wijsenbeek MS, Stauffer JL, Kirchgaessler KU, Costabel U. 2017; Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 26:170057–170066. DOI: 10.1183/16000617.0057-2017. PMID: 29212837. PMCID: PMC9488585.
7. Spagnolo P, Kropski JA, Jones MG, Lee JS, Rossi G, Karampitsakos T, Maher TM, Tzouvelekis A, Ryerson CJ. 2021; Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol Ther. 222:107798–107808. DOI: 10.1016/j.pharmthera.2020.107798. PMID: 33359599. PMCID: PMC8142468.
8. Mastruzzo C, Crimi N, Vancheri C. 2002; Role of oxidative stress in pulmonary fibrosis. Monaldi Arch Chest Dis. 57:173–176. PMID: 12619377.
9. Todd NW, Luzina IG, Atamas SP. 2012; Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 5:11–34. DOI: 10.1186/1755-1536-5-11. PMID: 22824096. PMCID: PMC3443459.
10. Blanc PD, Annesi-Maesano I, Balmes JR, Cummings KJ, Fishwick D, Miedinger D, Murgia N, Naidoo RN, Reynolds CJ, Sigsgaard T, Torén K, Vinnikov D, Redlich CA. 2019; The occupational burden of nonmalignant respiratory diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am J Respir Crit Care Med. 199:1312–1334. DOI: 10.1164/rccm.201904-0717ST. PMID: 31149852. PMCID: PMC6543721.
11. Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. 2006; Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 103:13180–13185. DOI: 10.1073/pnas.0605669103. PMID: 16924102. PMCID: PMC1551904.
12. Saito A, Horie M, Micke P, Nagase T. 2018; The role of TGF-β signaling in lung cancer associated with idiopathic pulmonary fibrosis. Int J Mol Sci. 19:E3611–E3624. DOI: 10.3390/ijms19113611. PMID: 30445777. PMCID: PMC6275044.
13. Yang W, Li X, Qi S, Li X, Zhou K, Qing S, Zhang Y, Gao MQ. 2017; lncRNA H19 is involved in TGF-β1-induced epithelial to mesenchymal transition in bovine epithelial cells through PI3K/AKT signaling pathway. PeerJ. 5:e3950–e3964. DOI: 10.7717/peerj.3950. PMID: 29062612. PMCID: PMC5649593.
14. Arthur JS, Ley SC. 2013; Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 13:679–692. DOI: 10.1038/nri3495. PMID: 23954936.
15. Chien LH, Deng JS, Jiang WP, Chou YN, Lin JG, Huang GJ. 2023; Evaluation of lung protection of Sanghuangporus sanghuang through TLR4/NF-κB/MAPK, keap1/Nrf2/HO-1, CaMKK/AMPK/Sirt1, and TGF-β/SMAD3 signaling pathways mediating apoptosis and autophagy. Biomed Pharmacother. 165:115080–115092. DOI: 10.1016/j.biopha.2023.115080. PMID: 37392658.
16. Wang D, Yan Z, Bu L, An C, Deng B, Zhang J, Rao J, Cheng L, Zhang J, Zhang B, Xie J. 2019; Protective effect of peptide DR8 on bleomycin-induced pulmonary fibrosis by regulating the TGF-β/MAPK signaling pathway and oxidative stress. Toxicol Appl Pharmacol. 382:114703–114734. DOI: 10.1016/j.taap.2019.114703. PMID: 31398421.
17. Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, Abraham E, Darley-Usmar V, Thannickal VJ, Zmijewski JW. 2018; Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 24:1121–1127. Erratum in: Nat Med. 2018;24:1627-1644. DOI: 10.1038/s41591-018-0087-6. PMID: 29967351. PMCID: PMC6081262.
18. Xuan LL, Hou Q.
19. Cheng X, Yin M, Sun X, Zhang Z, Yao X, Liu H, Xia H. 2023; Hemin attenuated LPS-induced acute lung injury in mice via protecting pulmonary epithelial barrier and regulating HO-1/NLRP3-mediated pyroptosis. Shock. 59:744–753. DOI: 10.1097/SHK.0000000000002101. PMID: 36821407.
20. Shimzu K, Takahashi T, Iwasaki T, Shimizu H, Inoue K, Morimatsu H, Omori E, Matsumi M, Akagi R, Morita K. 2008; Hemin treatment abrogates monocrotaline-induced pulmonary hypertension. Med Chem. 4:572–576. DOI: 10.2174/157340608786241972. PMID: 18991742.
21. Even B, Fayad-Kobeissi S, Gagliolo JM, Motterlini R, Boczkowski J, Foresti R, Dagouassat M. 2018; Heme oxygenase-1 induction attenuates senescence in chronic obstructive pulmonary disease lung fibroblasts by protecting against mitochondria dysfunction. Aging Cell. 17:e12837–e12848. DOI: 10.1111/acel.12837. PMID: 30341816. PMCID: PMC6260925.
22. Tian S, Ge X, Wu K, Yang H, Liu Y. 2014; Ramipril protects the endothelium from high glucose-induced dysfunction through CaMKKβ/AMPK and heme oxygenase-1 activation. J Pharmacol Exp Ther. 350:5–13. DOI: 10.1124/jpet.114.212928. PMID: 24741076.
23. Kosuru R, Kandula V, Rai U, Prakash S, Xia Z, Singh S. 2018; Pterostilbene decreases cardiac oxidative stress and inflammation via activation of AMPK/Nrf2/HO-1 pathway in fructose-fed diabetic rats. Cardiovasc Drugs Ther. 32:147–163. DOI: 10.1007/s10557-018-6780-3. PMID: 29556862.
24. Yang H, Zhao LF, Zhao ZF, Wang Y, Zhao JJ, Zhang L. 2012; Heme oxygenase-1 prevents liver fibrosis in rats by regulating the expression of PPARγ and NF-κB. World J Gastroenterol. 18:1680–1688. DOI: 10.3748/wjg.v18.i14.1680. PMID: 22529699. PMCID: PMC3325536.
25. Cho RL, Lin WN, Wang CY, Yang CC, Hsiao LD, Lin CC, Yang CM. 2018; Heme oxygenase-1 induction by rosiglitazone via PKCα/AMPKα/p38 MAPKα/SIRT1/PPARγ pathway suppresses lipopolysaccharide-mediated pulmonary inflammation. Biochem Pharmacol. 148:222–237. DOI: 10.1016/j.bcp.2017.12.024. PMID: 29309760.
26. Ma WH, Li M, Ma HF, Li W, Liu L, Yin Y, Zhou XM, Hou G. 2020; Protective effects of GHK-Cu in bleomycin-induced pulmonary fibrosis via anti-oxidative stress and anti-inflammation pathways. Life Sci. 241:117139–117151. DOI: 10.1016/j.lfs.2019.117139. PMID: 31809714.
27. Canesin G, Di Ruscio A, Li M, Ummarino S, Hedblom A, Choudhury R, Krzyzanowska A, Csizmadia E, Palominos M, Stiehm A, Ebralidze A, Chen SY, Bassal MA, Zhao P, Tolosano E, Hurley L, Bjartell A, Tenen DG, Wegiel B. 2020; Scavenging of labile heme by hemopexin is a key checkpoint in cancer growth and metastases. Cell Rep. 32:108181–108123. DOI: 10.1016/j.celrep.2020.108181. PMID: 32966797. PMCID: PMC7551404.
28. Collino M, Pini A, Mugelli N, Mastroianni R, Bani D, Fantozzi R, Papucci L, Fazi M, Masini E. 2013; Beneficial effect of prolonged heme oxygenase 1 activation in a rat model of chronic heart failure. Dis Model Mech. 6:1012–1020. DOI: 10.1242/dmm.011528. PMID: 23592614. PMCID: PMC3701220.
29. Huang C, Gong H, Mu B, Lan X, Yang C, Tan J, Liu W, Zou Y, Li L, Feng B, He X, Luo Q, Chen Z. 2022; BAF-L modulates histone-to-protamine transition during spermiogenesis. Int J Mol Sci. 23:1985–2001. DOI: 10.3390/ijms23041985. PMID: 35216101. PMCID: PMC8877947.
30. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. 2011; An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 183:788–824. DOI: 10.1164/rccm.2009-040GL. PMID: 21471066. PMCID: PMC5450933.
31. Yue YL, Zhang MY, Liu JY, Fang LJ, Qu YQ. 2022; The role of autophagy in idiopathic pulmonary fibrosis: from mechanisms to therapies. Ther Adv Respir Dis. 16:1–21. DOI: 10.1177/17534666221140972. PMID: 36468453. PMCID: PMC9726854.
32. Dove EP, Olson AL, Glassberg MK. 2019; Trends in idiopathic pulmonary fibrosis-related mortality in the United States: 2000-2017. Am J Respir Crit Care Med. 200:929–931. DOI: 10.1164/rccm.201905-0958LE. PMID: 31225965.
33. Richeldi L, Collard HR, Jones MG. 2017; Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952. DOI: 10.1016/S0140-6736(17)30866-8. PMID: 28365056.
34. Moore BB, Hogaboam CM. 2008; Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 294:L152–L160. DOI: 10.1152/ajplung.00313.2007. PMID: 17993587.
35. Lown JW, Sim SK. 1977; The mechanism of the bleomycin-induced cleavage of DNA. Biochem Biophys Res Commun. 77:1150–1157. DOI: 10.1016/S0006-291X(77)80099-5. PMID: 71149.
36. Sausville EA, Peisach J, Horwitz SB. 1976; A role for ferrous ion and oxygen in the degradation of DNA by bleomycin. Biochem Biophys Res Commun. 73:814–822. DOI: 10.1016/0006-291X(76)90882-2. PMID: 64249.
37. Yang JY, Tao LJ, Liu B, You XY, Zhang CF, Xie HF, Li RS. 2019; Wedelolactone attenuates pulmonary fibrosis partly through activating AMPK and regulating Raf-MAPKs signaling pathway. Front Pharmacol. 10:151–162. DOI: 10.3389/fphar.2019.00151. PMID: 30890932. PMCID: PMC6411994.
38. Johnson GL, Lapadat R. 2002; Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 298:1911–1912. DOI: 10.1126/science.1072682. PMID: 12471242.
39. Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Maruoka S, Horie T. 2001; Transforming growth factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-Jun-NH2-terminal kinase-dependent pathway. Am J Respir Crit Care Med. 163:152–157. DOI: 10.1164/ajrccm.163.1.2005069. PMID: 11208641.
40. Guo H, Jian Z, Liu H, Cui H, Deng H, Fang J, Zuo Z, Wang X, Zhao L, Geng Y, Ouyang P, Tang H. 2021; TGF-β1-induced EMT activation via both Smad-dependent and MAPK signaling pathways in Cu-induced pulmonary fibrosis. Toxicol Appl Pharmacol. 418:115500–115510. DOI: 10.1016/j.taap.2021.115500. PMID: 33744278.
41. Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. 2003; NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 22:3898–3909. DOI: 10.1093/emboj/cdg379. PMID: 12881424. PMCID: PMC169052.
42. Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. 2005; Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 120:649–661. DOI: 10.1016/j.cell.2004.12.041. PMID: 15766528.
43. Dong H, Yang J, Wang Y, Jiang Y, Chen J, Zhang W, Lu Y, Chen L, Chen Y. 2020; Polysaccharide SAFP from Sarcodon aspratus attenuates oxidative stress-induced cell damage and bleomycin-induced pulmonary fibrosis. Int J Biol Macromol. 164:1215–1236. DOI: 10.1016/j.ijbiomac.2020.07.120. PMID: 32693133.
44. Yang Z, Bian M, Ma J, Dong Y, Yang D, Qiu M, Gao Z. 2023; Berberine regulates pulmonary inflammatory microenvironment and decreases collagen deposition in response to bleomycin-induced pulmonary fibrosis in mice. Basic Clin Pharmacol Toxicol. 132:154–170. DOI: 10.1111/bcpt.13818. PMID: 36433932.
45. Jiang S, Li T, Yang Z, Yi W, Di S, Sun Y, Wang D, Yang Y. 2017; AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res Rev. 38:18–27. DOI: 10.1016/j.arr.2017.07.001. PMID: 28709692.
46. Gao R, Ma Z, Hu Y, Chen J, Shetty S, Fu J. 2015; Sirt1 restrains lung inflammasome activation in a murine model of sepsis. Am J Physiol Lung Cell Mol Physiol. 308:L847–L853. DOI: 10.1152/ajplung.00274.2014. PMID: 25659903. PMCID: PMC4398874.
47. Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. 2010; AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 298:E751–E760. DOI: 10.1152/ajpendo.00745.2009. PMID: 20103737. PMCID: PMC2853213.
48. Rius-Pérez S, Torres-Cuevas I, Millán I, Ortega ÁL, Pérez S. 2020; PGC-1α, inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev. 2020:1452696–1452715. DOI: 10.1155/2020/1452696. PMID: 32215168. PMCID: PMC7085407.
49. Yoshida T, Biro P, Cohen T, Müller RM, Shibahara S. 1988; Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 171:457–461. DOI: 10.1111/j.1432-1033.1988.tb13811.x. PMID: 3345742.
50. Keyse SM, Tyrrell RM. 1989; Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 86:99–103. DOI: 10.1073/pnas.86.1.99. PMID: 2911585. PMCID: PMC286411.
51. Tyrrell RM, Applegate LA, Tromvoukis Y. 1993; The proximal promoter region of the human heme oxygenase gene contains elements involved in stimulation of transcriptional activity by a variety of agents including oxidants. Carcinogenesis. 14:761–765. DOI: 10.1093/carcin/14.4.761. PMID: 8472344.
52. Janssen YM, Marsh JP, Absher MP, Gabrielson E, Borm PJ, Driscoll K, Mossman BT. 1994; Oxidant stress responses in human pleural mesothelial cells exposed to asbestos. Am J Respir Crit Care Med. 149:795–802. DOI: 10.1164/ajrccm.149.3.8118652. PMID: 8118652.
53. Motterlini R, Foresti R, Bassi R, Calabrese V, Clark JE, Green CJ. 2000; Endothelial heme oxygenase-1 induction by hypoxia. Modulation by inducible nitric-oxide synthase and S-nitrosothiols. J Biol Chem. 275:13613–13620. DOI: 10.1074/jbc.275.18.13613. PMID: 10788478.
54. Fredenburgh LE, Perrella MA, Mitsialis SA. 2007; The role of heme oxygenase-1 in pulmonary disease. Am J Respir Cell Mol Biol. 36:158–165. DOI: 10.1165/rcmb.2006-0331TR. PMID: 16980551. PMCID: PMC2176110.
55. Ameeramja J, Perumal E. 2017; Protocatechuic acid methyl ester ameliorates fluoride toxicity in A549 cells. Food Chem Toxicol. 109:941–950. DOI: 10.1016/j.fct.2016.12.024. PMID: 28012895.
56. Park EJ, Kim YM, Chang KC. 2017; Hemin reduces HMGB1 release by UVB in an AMPK/HO-1-dependent pathway in human keratinocytes HaCaT cells. Arch Med Res. 48:423–431. DOI: 10.1016/j.arcmed.2017.10.007. PMID: 29089150.
57. Ali FF, Mokhemer SA, Elroby Ali DM. 2022; Administration of hemin ameliorates ovarian ischemia reperfusion injury via modulation of heme oxygenase-1 and p-JNK/p-NF-κBp65/iNOS signaling pathway. Life Sci. 296:120431–120440. DOI: 10.1016/j.lfs.2022.120431. PMID: 35218766.
Fig. 1
Effect of hemin on the survival rate, terminal body weight and lung indices of mice with pulmonary fibrosis.
(A) Survival rate. The survival rates were recorded, estimated by the Kaplan–Meier method and were compared by a log-rank test. There were fifteen mice in each group. (B) Terminal body weight. (C) Lung indices. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. The data are presented as the means ± standard deviations. There were six mice in each group. **p < 0.01 compared with the control group. ##p < 0.01 compared with the bleomycin group.
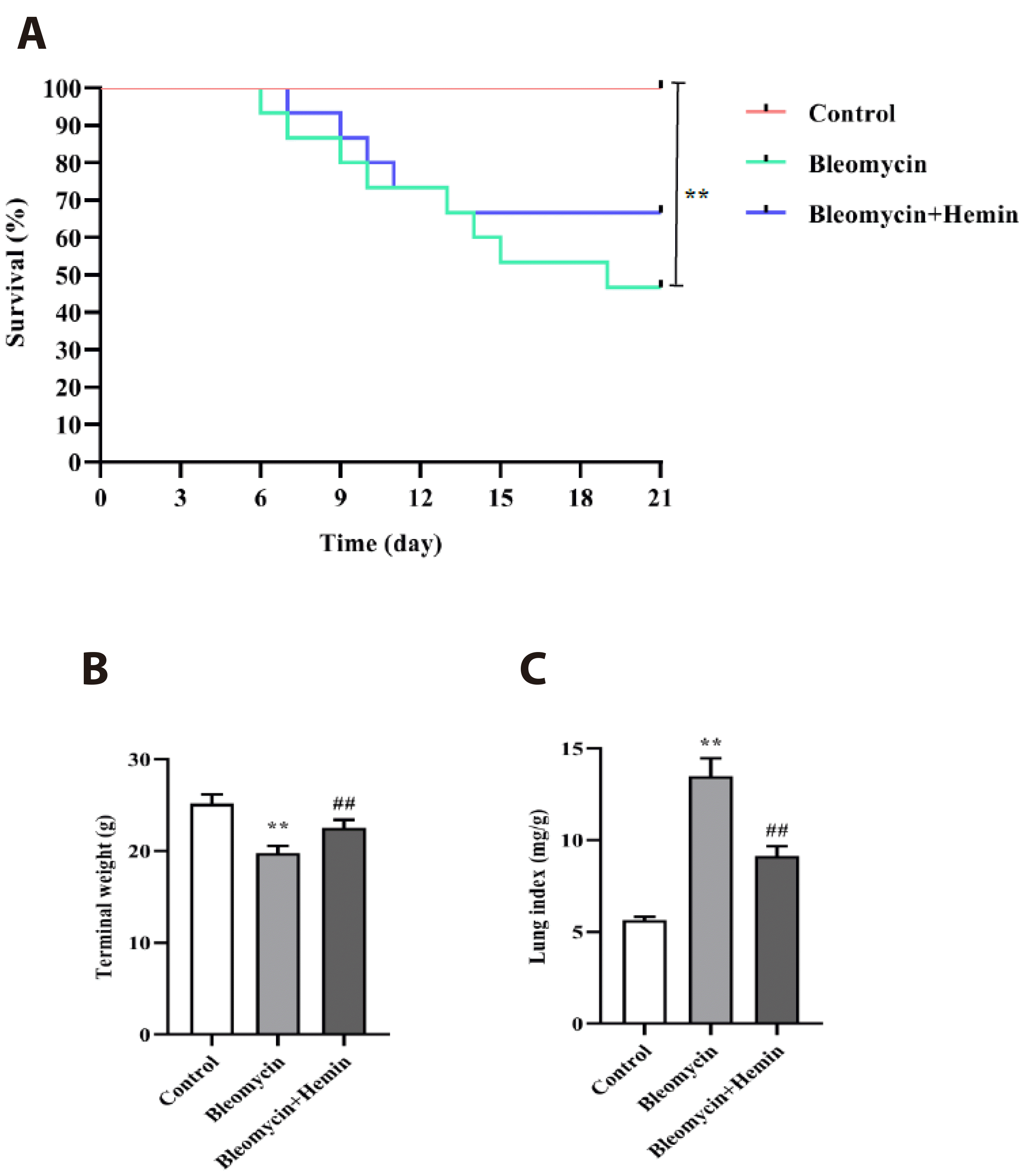
Fig. 2
Effect of hemin on morphological and pathological changes in the lung tissue of mice with pulmonary fibrosis.
(A) Gross morphology of lung tissue. (B) HE staining (400×). The black arrow in the figure indicates the location of pulmonary inflammation. (C) Masson staining (200×). The black arrow in the figure indicates collagen fibers. (D) Quantification of collagen expression levels by area percentage of positively stained collagen. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. The data are presented as the means ± standard deviations. There were six mice in each group. **p < 0.01 compared with the control group. ##p < 0.01 compared with the bleomycin group.

Fig. 3
Immunohistochemical staining of
α-SMA and collagen I in the lung tissue of mice with pulmonary fibrosis (400×). (A) Immunohistochemical staining of α-SMA. (B) Immunohistochemical staining of collagen I. The black arrow in the figure indicates positive cells. (C) and (D) present the areas of positively stained α-SMA and collagen I to represent their expression levels, respectively. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. The data are presented as the means ± standard deviations. There were six mice in each group. **p < 0.01 compared with the control group. ##p < 0.01 compared with the bleomycin group.
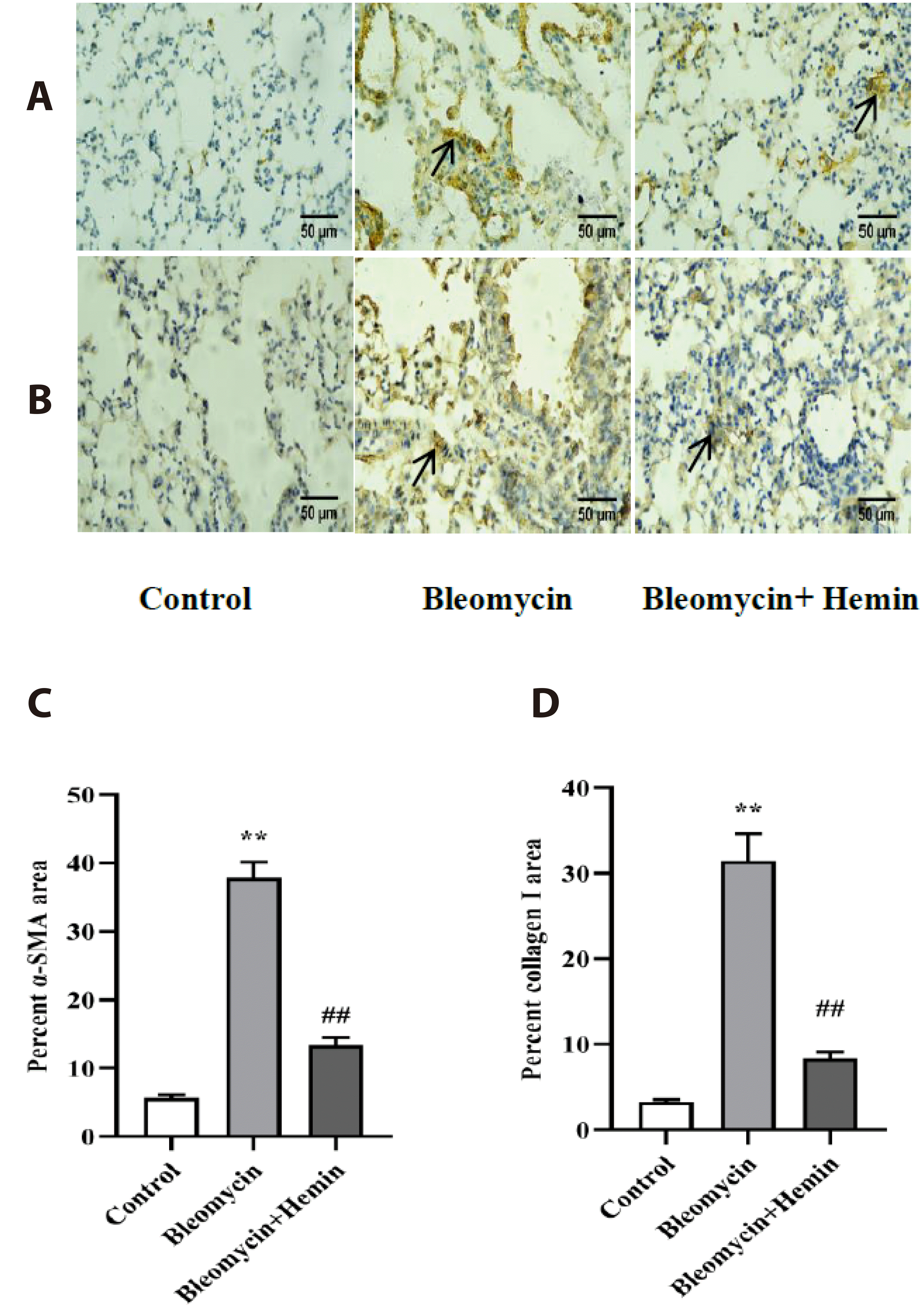
Fig. 4
Effect of hemin on the levels of MDA, SOD and CAT in the lung tissue of mice with pulmonary fibrosis.
(A) MDA levels in the lung tissue of mice in each group. (B) The activity of SOD in the lung tissue of the mice in each group. (C) The activity of CAT in the lung tissue of the mice in each group. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. The data are presented as the means ± standard deviations. There were six mice in each group. MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase. **p < 0.01 compared with the control group. #p < 0.05 and ##p < 0.01 compared with the bleomycin group.
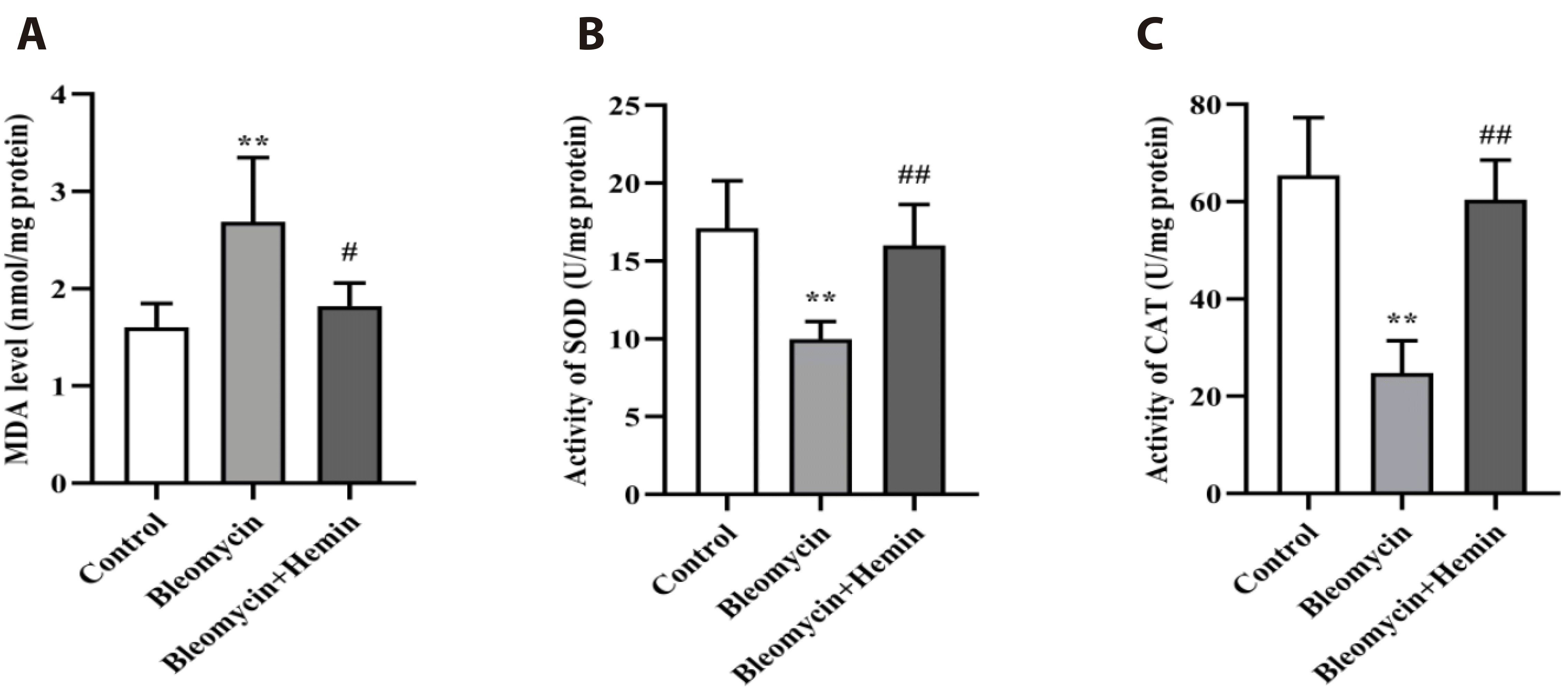
Fig. 5
Effect of hemin on the serum levels of interleukin (IL)-6 and tumor necrosis factor-
α (TNF-α) in mice with pulmonary fibrosis. (A) Serum IL-6 levels of the mice in each group. (B) Serum TNF-α levels of the mice in each group. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. The data are presented as the means ± standard deviations. There were six mice in each group. **p < 0.01 compared with the control group. ##p < 0.01 compared with the bleomycin group.
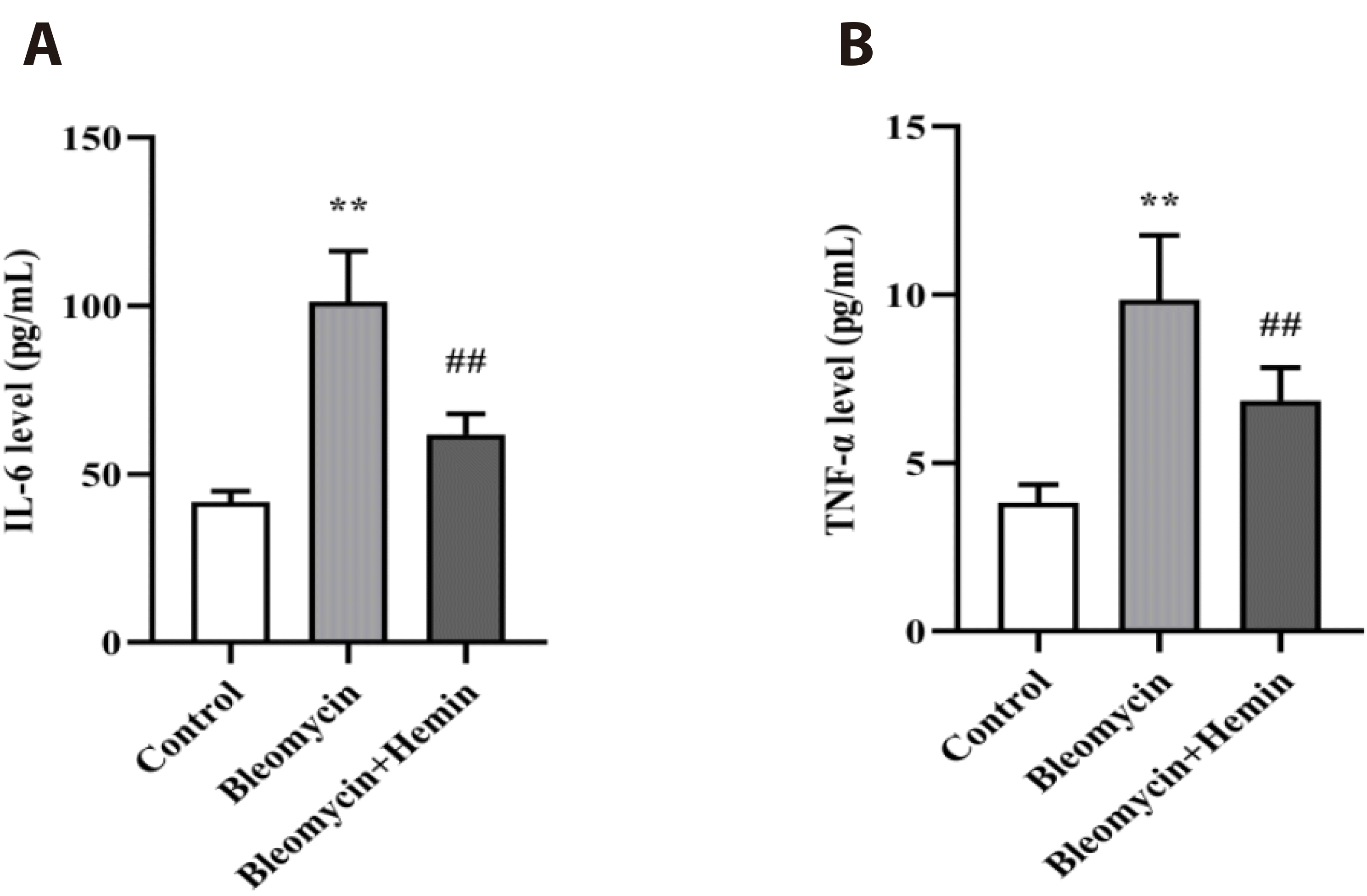
Fig. 6
Effect of hemin on the TGF-
β1/MAPK signaling pathway in the lung tissue of mice with pulmonary fibrosis (PF). (A) Western blotting detection of the expression of proteins related to the TGF-β1/MAPK pathway in the lung tissue of mice with PF. (B) TGF-β1/GAPDH. (C) p-p38/p38. (D) p-ERK1/2/ERK1/2. (E) p-JNK/JNK. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. The data are presented as the means ± standard deviations. Three mice were in each group. TGF-β1, transforming growth factor-β1; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun-n-terminal kinase. **p < 0.01 compared with the control group. #p < 0.05 and ##p < 0.01 compared with the bleomycin group.
Fig. 7
Effect of hemin on the AMPK/SIRT1/PGC-1
α/HO-1/NF-κB signaling pathway in the lung tissue of mice with pulmonary fibrosis (PF). (A) Western blotting detection of the expression of proteins related to the AMPK/SIRT1/PGC-1α/HO-1/NF-κB pathway in the lung tissue of mice with PF. (B) p-AMPK/AMPK ratio. (C) SIRT1/GAPDH ratio. (D) PGC-1α/GAPDH ratio. (E) HO-1/GAPDH ratio. (F) p-NF-κB p65/NF-κB p65 ratio. The significance of difference was determined by one-way ANOVA followed by Tukey’s test. The data are presented as the means ± standard deviations. Three mice were in each group. AMPK, adenosine monophosphate-activated protein kinase; SIRT1, silent information regulator 1; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator-1α; HO-1, heme oxygenase-1; NF-κB, nuclear transcription factor-κB. **p < 0.01 compared with the control group. #p < 0.05 and ##p < 0.01 compared with the bleomycin group.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download