Abstract
Background and Objectives
Metformin, a commonly used antidiabetic drug, has been reported to exhibit promising anticancer effects across various tumor types. This study investigated the effectiveness of combining metformin with radiotherapy (RT) to treat head and neck squamous cell carcinoma (HNSCC).
Materials and Method
In vitro experiments were conducted in which FaDu and SCC-25 cells were treated with metformin, followed by irradiation. Cell proliferation was evaluated by MTT assay, and apoptosis was assessed by staining with annexin V-fluorescein isothiocyanate/propidium iodide, followed by flow cytometry. Western blotting was performed to evaluate changes in apoptotic markers. In vivo experiments were performed using a murine AT-84 allograft model, where tumor volume was measured and serum samples were collected to assess the level of vascular endothelial growth factor (VEGF).
Results
The combination of metformin and RT significantly reduced cell proliferation in a dose- and time-dependent manner, and led to a significant increase in the apoptotic rate, accompanied by the upregulation of cleaved caspase-8 and FoxO3, and the downregulation of Bcl-2. The combination treatment also exhibited antiangiogenic effects, as shown by the reduced hypoxia-inducible factor-1 alpha level and inhibited tube formation in the endothelial cells. The combined therapy in the mouse model led to marked decrease in tumor volume and the serum VEGF level in comparison to both the control group and the RT alone.
Head and neck cancer is the 6th to 8th most common cancer worldwide with an annual incidence of approximately 660000 cases, and is therefore a major global health challenge [1,2]. Despite significant reductions in smoking rates over the past decade, there has been a concerning increase in incidence of human papillomavius-related head and neck cancer, affecting smokers as well as younger individuals and socioeconomically privileged populations [3,4].
Head and neck cancer is commonly treated with radiotherapy (RT), a widely used and efficacious therapeutic approach. However, it can activate molecular signaling pathways associated with tumor radioresistance, involving proteins such as protein kinase B (Akt) and mammalian target of rapamycin (mTOR) [5,6]. These pathways are critical effectors of tyrosine kinase receptors, including epidermal growth factor receptor, which activates them through phosphatidylinositol 3-kinase (PI3K) and Akt-T308 kinase [7]. In turn, Akt activates mTOR, leading to increased cellular growth, proliferation, and resistance to cytotoxic agents [8]. Furthermore, mTOR regulates gene expression and translation by phosphorylating p70S6-kinase and inhibiting the translation initiation inhibitor eIF4E-binding protein 1 [9]. Moreover, as recent studies have highlighted the role of PI3K/Akt/mTOR pathway activation in conferring radioresistance, there is a great deal of research interest in inhibitors of this pathway. These findings offer valuable insights into the mechanisms of tumor radioresistance, with potential implications for developing targeted therapies to overcome treatment resistance.
Metformin, a widely used biguanide drug for type 2 diabetes, effectively reduces glucose levels by decreasing hepatic glucose production and increasing fatty acid oxidation and glucose utilization [10,11]. Recent studies have provided compelling evidence for its protective effects against cancer and potential to improve cancer outcomes [12]. A case–control study demonstrated a dose–response relationship in that higher metformin dose, longer duration of use, and increased prescription frequency were associated with reduced cancer incidence [13]. Compared to sulfonylurea, metformin was found to decrease the risk of cancer, with type 2 diabetes patients treated with sulfonylureas showing significantly higher cancer-related mortality rates. In addition, a 10-year study in individuals newly prescribed metformin for type 2 diabetes demonstrated a 37% decrease in the occurrence of cancer. The anticancer activity of metformin has also been shown to be effective in experimental models of pancreatic and prostate cancer [14]. Metformin has been reported as a remedy for chemoresistance, effectively reducing the population of breast cancer stem cells in breast cancer [15]. In endometrial cancer cell lines, metformin inhibits cell proliferation through activation of AMPK, leading to suppression of the mTOR pathway [16]. Recently, it was reported that metformin can bidirectionally regulate autophagy by modulating AMPK-related signaling pathways, such as the AMPK/mTOR pathway. Although the role of metformin in glucose and fatty acid metabolism is well established [17], extensive research has focused on evaluating its therapeutic utility in both in vitro and in vivo cancer models. Nevertheless, the precise mechanisms underlying the general anticancer effects of metformin, including apoptosis, cell cycle, and inflammatory effects, remain unknown.
The primary objective of this study was to investigate the immediate effects of metformin on head and neck cancer and to further explore how metformin, as an adjuvant to RT, influences in vitro and in vivo models of head and neck squamous cell carcinoma (HNSCC).
Primary antibodies against the following molecules were used in immunoblotting analysis: AMPK and phospho-AMPK, Bcl-2, Cdk2, and FoxO3 (Cell Signaling Technology, Danvers, MA, USA); p53 and phospho-p53 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); cleaved caspase-8 (Novus Biologicals, Littleton, CO, USA); and β-actin (SigmaAldrich, St. Louis, MO, USA). All secondary antibodies, conjugated with horseradish peroxidase (HRP), were obtained from Santa Cruz Biotechnology. Metformin and vascular endothelial growth factor (VEGF) enzyme-linked immunosorbent assay kits were purchased from Sigma-Aldrich.
For the in vitro experiments, FaDu (HTB-43; ATCC, Manassas, VA, USA) cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin, and SCC-25 cells (CRL-1628; ATCC) cells were cultured in DMEM/F12 medium containing 10% FBS, 1% penicillin–streptomycin, and 40 ng/mL hydrocortisone. Human umbilical vein endothelial cells (HUVECs) (Lonza, Basel, Switzerland) were cultured in complete EGM-2 medium (Clonetics EGM-2 SingleQuots; Lonza). Cells were used after 4-10 passages in this study. The cells were subjected to gamma irradiation with a total dose of 8 Gy (Gammacell 3000 Elan; MDS Nordion, Ottawa, ON, Canada) covering the entire plate. Then the cells were incubated for 48 h before harvesting.
For the in vivo experiments, AT-84 murine oral squamous carcinoma cells (a kind gift from the Sung Lab) were cultured in complete RPMI-1640 medium containing 10% FBS and 1% penicillin–streptomycin. Metformin was administered in doses of 1.2-9.6 mM after irradiation. Media with and without VEGF were used as positive and negative controls, respectively.
Protein lysates from tumor samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked using Tris-buffered saline–Tween 20 and 5% low-fat milk, followed by incubation with primary antibodies. After washing, HRP-conjugated secondary antibodies were used, and protein bands were visualized using enhanced chemiluminescence reagents. Protein expression levels were quantified by densitometry.
HUVECs were seeded in triplicate at a density of 3×104 cells/well in 100 μL VEGF-EGM-2 medium with metformin with or without radiation. Cells were cultured concurrently in media with and without VEGF as positive and negative controls, respectively. The treated cells were plated on 96-well plates coated with Basement Membrane Matrix (BD Matrigel; BD Biosciences, Franklin Lakes, NJ, USA) and incubated at 37°C for 18 h. Tube formation was quantified as the total number of tube branches (in pixels) counted using a microscope at ×40 magnification.
Apoptosis was evaluated using an ApoScan annexin V fluorescein isothiocyanate (FITC) apoptosis detection kit (BioBud, Seongnam, South Korea) according to the manufacturer’s instructions. Following 48 h of treatment, FaDu cells were collected, rinsed with cold phosphate-buffered saline, and suspended in 500 μL binding buffer. To the binding buffer was added 1.25 μL annexin V solution, and the cells were incubated for 15 min at room temperature in the dark. Subsequently, staining was performed with propidium iodide (PI). Flow cytometric analysis was performed to assess apoptosis with annexin V labeling for early-stage apoptosis and PI staining for medium and late stages of apoptosis. The apoptotic rate was calculated by dividing the percentage of annexin V-positive and PI-negative cells by the total number of cells in the gated region.
Twenty-eight 5-week-old female C3H/HeN mice with an average body weight of 21±2.0 g, were purchased from Central Lab Animal Inc. (Seoul, South Korea) and acclimated for 1 week before commencement of the experiment. Mice were housed in sterile filter-top cages with hygienic bedding and provided a standard irradiated diet and autoclaved acidified water (pH 2.8) ad libitum. All experimental procedures were approved and overseen by the Institutional Animal Care and Use Committee of the Catholic University of Korea (IACUC, Seoul, South Korea; approval number CUMC-2011-0084-01).
For the allograft procedure, AT-84 cells were collected and injected subcutaneously into the flank of each mouse at a concentration of 1×107 cells in 50 μL phosphate-buffered saline. After approximately 2 weeks, when the tumor size exceeded 100 mm3, the mice were randomly divided into three groups of eight mice each: non-treated (NT) group, RT group, and combination of RT and metformin (RT+Met) group.
Animals in the RT group were irradiated every 3 days over a period of 2 weeks, amounting to a total of four sessions, with a dose of 4.5 Gy/min in each session. In the RT+Met group, mice were provided with metformin (at a concentration of 0.06% in AIN-93M-based diet) throughout the period of the experiment. Monitoring of all mice continued for 18 days, corresponding to the duration of the treatment regimen, after which they were euthanized on day 19. Tumor dimensions were assessed biweekly using calipers, and tumor volume was determined using the formula (length×width2)/2.
Whole blood samples were obtained from the retroorbital veins (retroorbital plexus) of mice using capillary tubes immediately before sacrifice. The samples were left at room temperature for 2 h, and serum was separated by centrifugation at 3000×g and 4°C for 15 min. Tumor tissue samples were harvested immediately upon sacrifice, and sections of each tissue were fixed in 4% paraformaldehyde solution to prepare paraffin blocks for immunostaining. In addition, protein lysates were obtained from 0.5 g of each tumor tissue and quantitatively assayed using Bradford reagent (Bio-Rad, Hercules, CA, USA).
Data are presented as the mean±standard deviation. Statistical analyses were performed using SPSS ver. 24.0 software (IBM Corp., Armonk, NY, USA). Student’s t test was used to compare data between treatment groups for all experiments. In all analyses, p<0.05 was taken to indicate statistical significance.
The RT+Met group showed substantial inhibition of FaDu cell growth, which was both dose- and time-dependent, compared to the NT and RT groups (Fig. 1). However, no inhibitory effect on SCC-25 cell growth was observed in the combined treatment group. This effect was observed in both in vitro cell culture experiments and in the in vivo tumor model using C3H/HeN mice. The tumors in the RT+Met group were significantly smaller and had significantly delayed growth compared to the other treatment groups. These observations indicated that the combination of metformin and RT effectively suppressed cell proliferation and inhibited tumor growth of head and neck cancer. The concentration of metformin and irradiation dosage were examined in preliminary experiments accounting for variables, such as cell type, incubation period, and dose considerations (data not shown).
Flow cytometric analysis using Annexin V-FITC and PI demonstrated a dose-dependent increase in the number of apoptotic cells in the RT+Met group, particularly for FaDu cells (Fig. 2). The most significant apoptotic effect was observed at a concentration of 2.4 mM metformin in combination with RT. Furthermore, the expression levels of cleaved caspase-8, Bcl-2, and FoxO3 supported these findings. Cleaved caspase-8 and FoxO3 were significantly elevated in the RT+Met group, particularly in cells treated with 2.4 mM metformin (Fig. 3A and C). In contrast, Bcl-2 expression was significantly decreased in the RT+Met group, particularly in cells treated with 2.4 mM metformin (Fig. 3B). These findings indicate that the combination treatment enhanced apoptotic signaling pathways, leading to increased cell death and the suppression of head and neck cancer cell proliferation (Figs. 3D, 4A).
In the RT+Met group, FaDu cells showed significantly increased phosphorylation of p53 and AMPK in a dose-dependent manner at 48 h after irradiation (Fig. 5). The enhanced activation of p53 and AMPK indicated the effectiveness of the combination treatment in promoting antitumor responses in head and neck cancer.
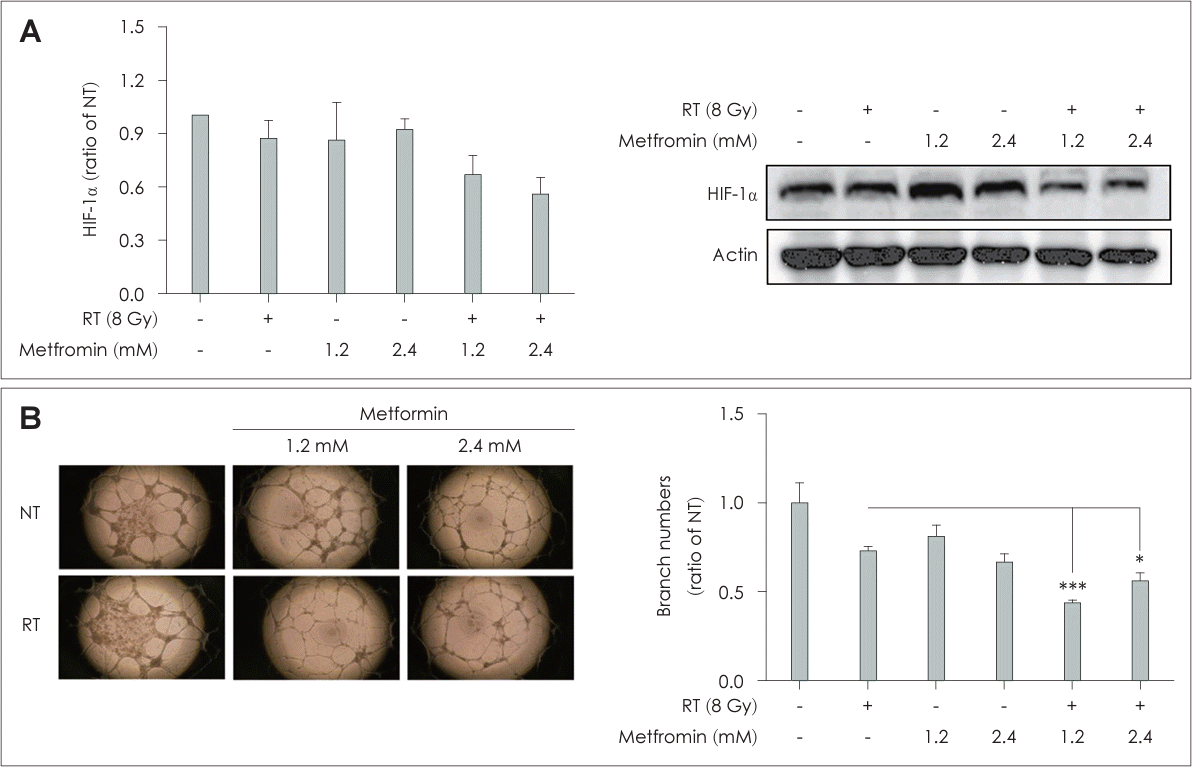
The levels of hypoxia-inducible factor-1 alpha (HIF-1α) and VEGF expression were significantly decreased in the RT+Met group compared to the other groups (Figs. 4B and 6A). In addition, tube formation assay using HUVECs showed a significant reduction in the number of branches in the RT+Met group compared to the other groups (Fig. 6B). These results provided evidence for the antiangiogenic effect of the combined metformin and RT treatment.
Metformin, a widely used drug for type 2 diabetes, has been reported to inhibit the proliferation of various cancer cell types, including breast, gastric, pancreatic, thyroid, and liver cancers [15,18-21]. Our study revealed significant antiproliferative effects even at lower doses of metformin when combined with RT compared to radiation treatment alone. Even a low dose of metformin (<5 mM) improved the growth inhibitory effect of RT on head and neck cancer cells.
Apoptosis plays an essential role in impeding tumor progression, and our findings emphasize that the concurrent application of irradiation and metformin increases apoptotic cell death in head and neck cancer cells. The results of Annexin V/PI staining confirmed a dose-dependent increase in number of apoptotic cells in the RT+Met group, providing additional evidence for the increased proapoptotic effects. The altered expression levels of cleaved caspase-8, Bcl-2, and FoxO3 in the RT+Met group indicated the activation of both intrinsic and extrinsic apoptotic pathways. The downregulation of Bcl-2, an antiapoptotic protein, and the upregulation of cleaved caspase-8 and FoxO3 provide mechanistic insights into the enhanced apoptotic response induced by the combined treatment. These findings suggest that the combination of metformin and RT effectively triggers apoptosis in head and neck cancer cells, potentially contributing to the inhibition of tumor growth. A recent study showed similar results, with decreased Bcl-2 mRNA expression and altered Bax/Bcl-2 ratio in response to combined treatment with metformin and low-dose radiation in cyclophosphamide-induced apoptosis in Wistar rats, even in the absence of cancer [22].
In the present study, p53 expression and AMPK phosphorylation were significantly increased in the RT+Met group. The activation of p53 and AMPK is closely associated with tumor suppression and cellular stress response as part of the tumor suppressor signaling pathway. Other researchers have reported the involvement of p53 and AMPK in regulating cell cycle arrest and apoptosis [23-25]. For example, metformin was suggested to increase cell intrinsic radiosensitivity and, when combined with radiation, activate AMPK and p53 to alleviate metabolic and genotoxic stress [23]. Our results also indicate that the combination of metformin and RT promotes the activation of p53 and AMPK, which may contribute to the enhanced antitumor response observed in head and neck cancers.
Angiogenesis is a critical factor in tumor growth and metastasis, and its inhibition is a mechanism for suppressing tumor growth [26,27]. Our data demonstrate that combined treatment with metformin and RT exerts an antiangiogenic effect. The downregulation of HIF-1α and VEGF expression, along with the reduction of tube formation by HUVECs, indicated the inhibition of angiogenesis in both in vitro and in vivo models in the RT+Met group. These findings suggest that the combination treatment disrupts proangiogenic signaling pathways, potentially limiting blood supply to the tumor and impeding its growth and metastasis. Consistent with our findings, previous studies reported the downregulation of proangiogenic factors with radiation and metformin combined therapy in different experimental setups [28,29].
In summary, our study provides compelling evidence supporting the potential for cotreatment with metformin and RT to control head and neck cancer. The synergistic effects observed in cell proliferation inhibition, apoptosis promotion, p53 and AMPK signaling pathway activation, and angiogenesis suppression highlight the promising therapeutic implications of this approach. However, further investigations are needed to elucidate the underlying mechanisms and optimize the treatment protocol for clinical applications in the field of head and neck cancer.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea (NRF), funded by the government of Korea (grant no. NRF-2021R1C1C1004073, No. 2011-0012555).
Notes
Author Contribution
Conceptualization: Jiyoung Yeo, Jun-Ook Park. Data curation: Jiyoung Yeo, Dong-Hyun Lee. Formal analysis: Jiyoung Yeo, Ah Young Bae, Da Hye Moon. Funding acquisition: Jiyoung Yeo, Jun-Ook Park. Investigation: Dong-Hyun Lee, Ah Young Bae, Da Hye Moon, Jooin Bang, Ji-Sun Kim. Methodology: Dong-Hyun Lee, Ji-Sun Kim. Supervision: Jun-Ook Park. Validation: Jun-Ook Park. Visualization: Ji-young Yeo. Writing—original draft: Ji-young Yeo. Writing—review & editing: Jiyoung Yeo, Jun-Ook Park.
REFERENCES
1. Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dent J. 2022; 233(9):780–6.
2. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020; 6(1):92.

3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3):209–49.

4. Michaud JM, Zhang T, Shireman TI, Lee Y, Wilson IB. Hazard of cervical, oropharyngeal, and anal cancers in HIV-infected and HIV-uninfected Medicaid beneficiaries. Cancer Epidemiol Biomarkers Prev. 2020; 29(7):1447–57.

5. Horn D, Hess J, Freier K, Hoffmann J, Freudlsperger C. Targeting EGFR-PI3K-AKT-mTOR signaling enhances radiosensitivity in head and neck squamous cell carcinoma. Expert Opin Ther Targets. 2015; 19(6):795–805.

6. Su YC, Lee WC, Wang CC, Yeh SA, Chen WH, Chen PJ. Targeting PI3K/AKT/mTOR signaling pathway as a radiosensitization in head and neck squamous cell carcinomas. Int J Mol Sci. 2022; 23(24):15749.
7. Marquard FE, Jücker M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem Pharmacol. 2020; 172:113729.

8. Perri F, Pacelli R, Della Vittoria Scarpati G, Cella L, Giuliano M, Caponigro F, et al. Radioresistance in head and neck squamous cell carcinoma: biological bases and therapeutic implications. Head Neck. 2015; 37(5):763–70.

9. Liu Y, Hidayat S, Su WH, Deng X, Yu DH, Yu BZ. Expression and activity of mTOR and its substrates in different cell cycle phases and in oral squamous cell carcinomas of different malignant grade. Cell Biochem Funct. 2007; 25(1):45–53.

11. Szymczak-Pajor I, Wenclewska S, Śliwińska A. Metabolic action of metformin. Pharmaceuticals (Basel). 2022; 15(7):810.
12. Podhorecka M, Ibanez B, Dmoszyńska A. Metformin–its potential anti-cancer and anti-aging effects. Postepy Hig Med Dosw (Online). 2017; 71:170–5.
13. Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res. 2010; 16(6):1695–700.

14. Duncan BB, Schmidt MI. Metformin, cancer, alphabet soup, and the role of epidemiology in etiologic research. Diabetes Care. 2009; 32(9):1748–50.

15. Samuel SM, Varghese E, Koklesová L, Líšková A, Kubatka P, Büsselberg D. Counteracting chemoresistance with metformin in breast cancers: targeting cancer stem cells. Cancers (Basel). 2020; 12(9):2482.

16. Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation—implications for a novel treatment strategy. Gynecol Oncol. 2010; 116(1):92–8.
17. Feingold KR. Role of glucose and lipids in the atherosclerotic cardiovascular disease in patients with diabetes. In : Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, editors. Endotext [Internet]. South Dartmouth, MA: MDText.com;2000. [cited 2023 October 29]. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK278947.
18. Tseng HH, Chen YZ, Chou NH, Chen YC, Wu CC, Liu LF, et al. Metformin inhibits gastric cancer cell proliferation by regulation of a novel Loc100506691-CHAC1 axis. Mol Ther Oncolytics. 2021; 22:180–94.

19. Chen K, Qian W, Jiang Z, Cheng L, Li J, Sun L, et al. Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol Cancer. 2017; 16(1):131.

20. Kim TS, Lee M, Park M, Kim SY, Shim MS, Lee CY, et al. Metformin and dichloroacetate suppress proliferation of liver cancer cells by inhibiting mTOR complex 1. Int J Mol Sci. 2021; 22(18):10027.

21. Han B, Cui H, Kang L, Zhang X, Jin Z, Lu L, et al. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumour Biol. 2015; 36(8):6295–304.
22. El Kiki SM, Omran MM, Mansour HH, Hasan HF. Metformin and/or low dose radiation reduces cardiotoxicity and apoptosis induced by cyclophosphamide through SIRT-1/SOD and BAX/Bcl-2 pathways in rats. Mol Biol Rep. 2020; 47(7):5115–26.

23. Muaddi H, Chowdhury S, Vellanki R, Zamiara P, Koritzinsky M. Contributions of AMPK and p53 dependent signaling to radiation response in the presence of metformin. Radiother Oncol. 2013; 108(3):446–50.

24. Zhou X, Kuang Y, Liang S, Wang L. Metformin inhibits cell proliferation in SKM-1 cells via AMPK-mediated cell cycle arrest. J Pharmacol Sci. 2019; 141(4):146–52.

25. Roque DR, Zhang L, Wysham WZ, Han J, Sun W, Yin Y, et al. The effects of NT-1044, a novel AMPK activator, on endometrial cancer cell proliferation, apoptosis, cell stress and in vivo tumor growth. Front Oncol. 2021; 11:690435.

26. Wang M, Lin Y, Shi W, Chen X, Mi Z, Jia Z, et al. Topical metformin suppresses angiogenesis pathways induced by pulsed dye laser irradiation in animal models. Exp Dermatol. 2022; 31(3):393–7.
27. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005; 23(5):1011–27.

Fig. 1.
Combination treatment with metformin and radiotherapy (RT) inhibited cell proliferation and tumor growth. FaDu (A) and SCC-25 (B) cells were irradiated at a dose of 8 Gy and treated with various concentrations of metformin (0.0–9.6 mM) for 48 h. The RT and metformin group showed significant inhibition of cell proliferation compared to the non-treated (NT) and RT groups. Data are means±standard deviations of 3–4 independent experiments (*p<0.05, ****p<0.0001; Student’s t test).
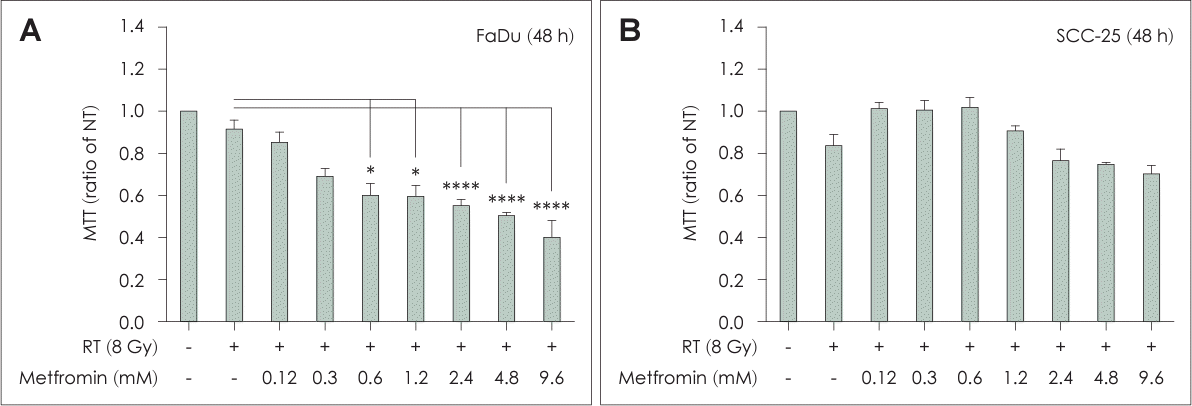
Fig. 2.
Combination treatment with metformin and radiotherapy (RT) induced apoptosis in head and neck squamous cell carcinoma. FaDu cells were treated with different concentrations of metformin (1.2 and 2.4 mM) with or without irradiation at a dose of 8 Gy for 48 h. Apoptosis was analyzed by flow cytometry using annexin V–fluorescein isothiocyanate and propidium iodide staining. The RT and metformin group exhibited a dose-dependent increase in apoptotic cells compared to the other groups. Data are means±standard deviations (n=3) (*p<0.05; Student’s t test). NT, non-treated.
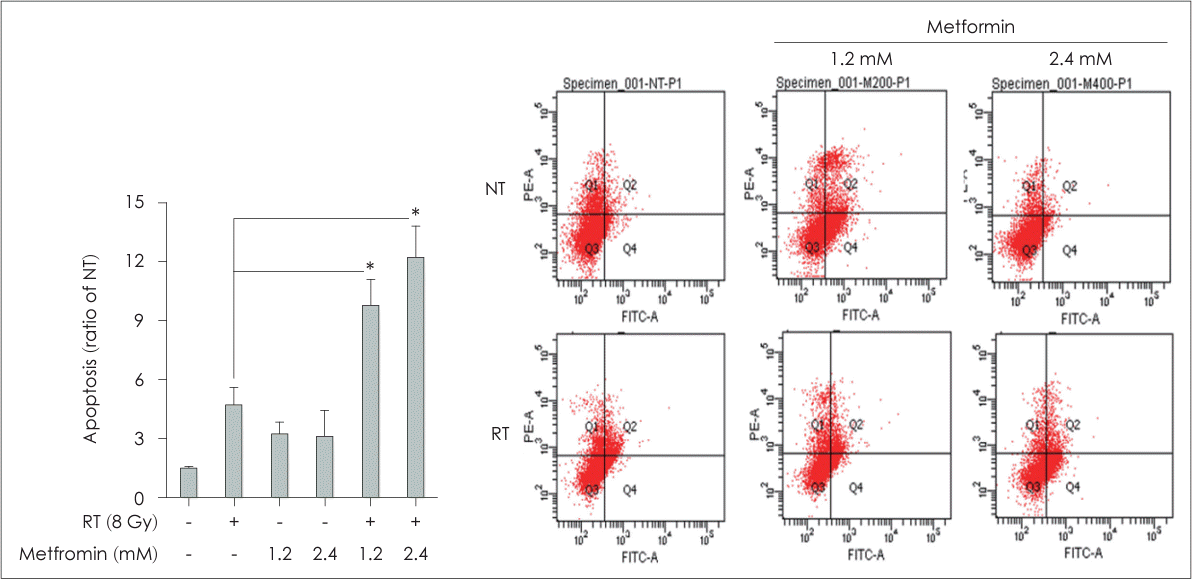
Fig. 3.
Combination treatment with metformin and radiotherapy (RT) affected the expression of cleaved caspase-8 (A), Bcl-2 (B), FoxO3 (C), and Cdk2 (D) in head and neck squamous cell cancers. FaDu cells were treated with metformin (0, 1.2, or 2.4 mM) with or without irradiation at a dose of 8 Gy for 48 h. Cell lysates were analyzed by immunoblotting for cleaved caspase-8, Bcl-2, FoxO3, and Cdk2. The RT and metformin group showed significant changes in the expression levels of these proteins compared to the other groups. Representative immunoblots are shown. Data are means±standard deviations (n=3) (*p<0.05; Student’s t test). NT, non-treated.
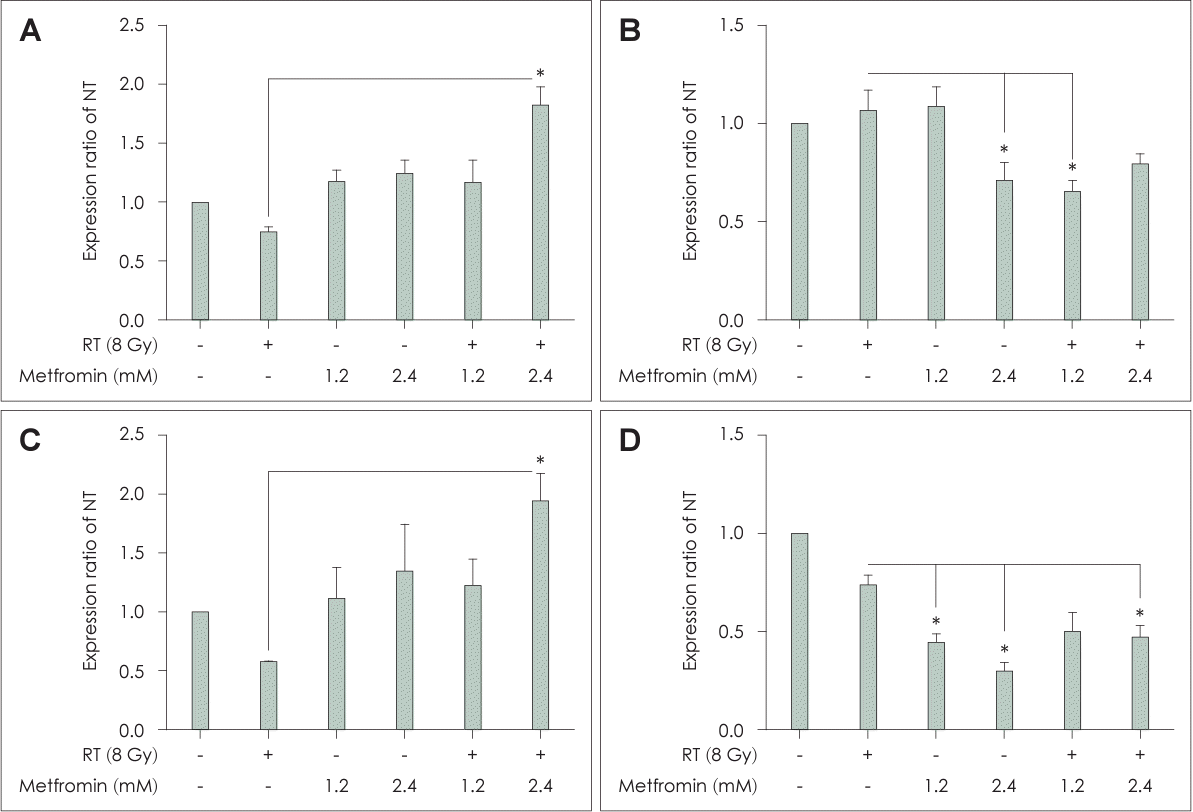
Fig. 4.
Combination treatment with metformin and radiotherapy (RT) suppressed tumor growth in C3H/HeN mice with AT-84 cell allografts. A: C3H/HeN mice were implanted subcutaneously with AT-84 cells. Treatment was initiated when tumor volumes exceeded 100 mm3. Mice were treated with a combination of metformin and irradiation every 3 days for 18 days. Tumor volumes were measured every 3 days prior to irradiation. B: Serum samples were collected after sacrificing the mice and the levels of vascular endothelial growth factor (VEGF) were measured through an enzyme-linked immunosorbent assay. Data are means±standard deviations (n=3) (*p<0.05, ***p<0.001; Student’s t test).
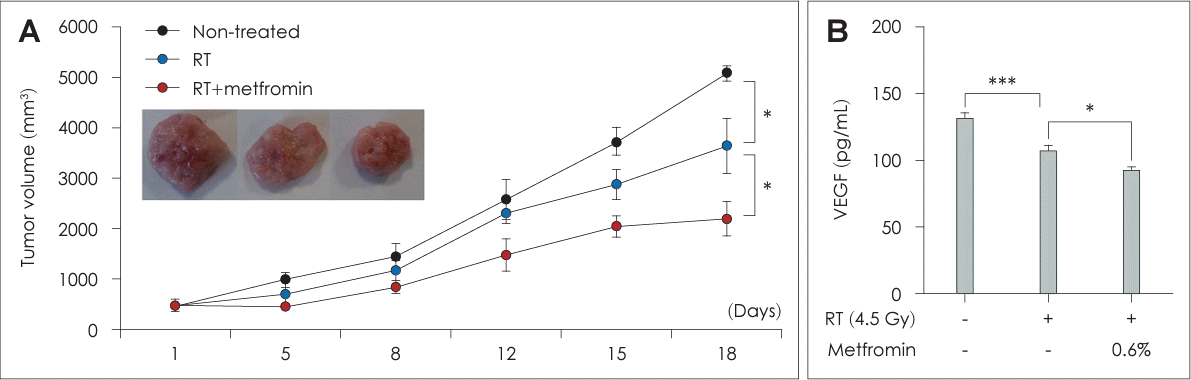
Fig. 5.
Combination treatment with metformin and radiotherapy (RT) increased the phosphorylation of p53 (A) and AMPK (B) in head and neck squamous cell cancers. FaDu cells were treated with metformin (0, 1.2, or 2.4 mM) with or without irradiation at a dose of 8 Gy for 48 h. Cell lysates were analyzed by immunoblotting for p53, phospho-p53, AMPK, and phospho-AMPK. The RT and metformin group showed dose-dependent increases in the phosphorylation of p53 and AMPK compared to the other groups. Representative immunoblots are shown. Data are means±standard deviations (n=3) (*p<0.05; Student’s t test). NT, non-treated.
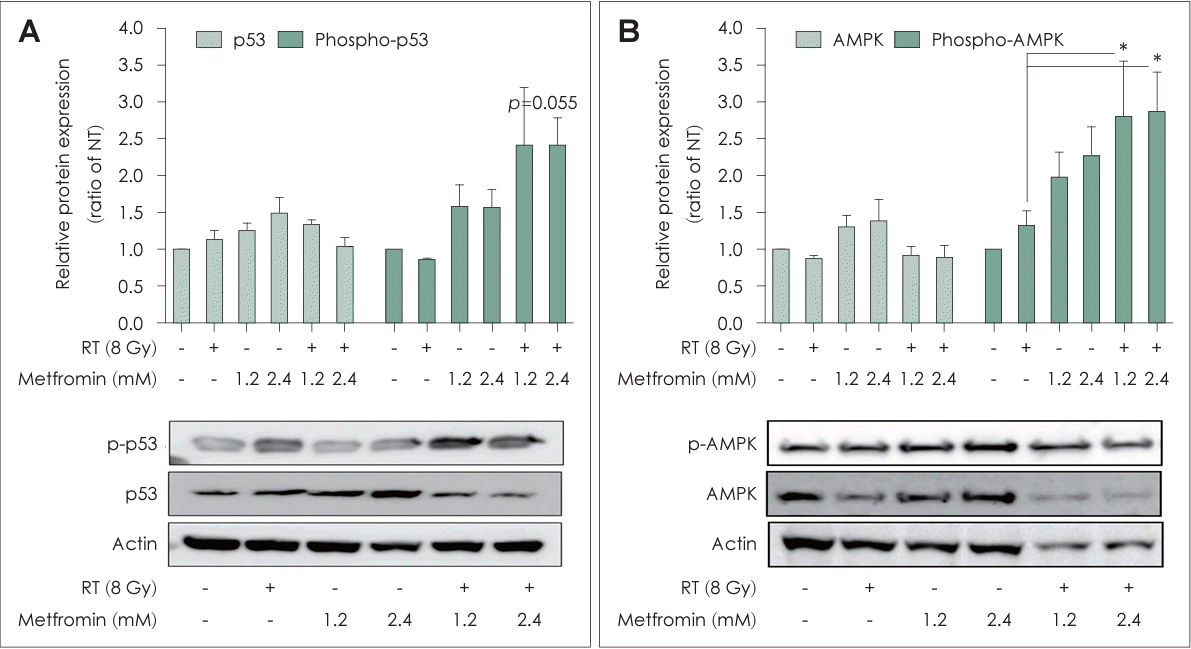
Fig. 6.
Combination treatment with metformin and radiotherapy (RT) inhibited angiogenesis in head and neck squamous cell cancers. A: FaDu cells were treated with metformin (0, 1.2, or 2.4 mM) with or without irradiation at a dose of 8 Gy for 48 h. Cell lysates were analyzed for hypoxia-inducible factor-1 alpha (HIF-1α) expression by immunoblotting. The RT and metformin group showed a significant decrease in HIF-1α expression. B: Human umbilical vein endothelial cells were treated with metformin with or without 8 Gy irradiation. Tube formation was quantified after 18 h of incubation. Data are means±standard deviations (n=3) (*p<0.05, ***p<0.001; Student’s t test). NT, nontreated.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download