INTRODUCTION
Colonoscopy is the gold standard for the early detection of colorectal cancer (CRC) and the removal of precancerous polyps.
1,2 Clear visualization of the entire colonic mucosa is essential for an effective colonoscopy, and this requires a large volume of fluid to pass into the colonic lumen for bowel preparation.
3 Traditionally, a 4-L isotonic solution containing polyethylene glycol (PEG) with an electrolyte has been used to induce osmotic activity, causing profuse diarrhea. However, to improve poor compliance caused by taking large volumes, ascorbate, a hypertonic osmotic substance, has been combined with 2-L PEG to reduce the volume of fluid intake. Recently, a 1-L PEG plus ascorbate (1-L PEG/Asc) preparation was developed to further reduce the solution volume by increasing the ascorbate content.
4 Additionally, a new formulation of oral sulfate solution (OSS), consisting of sodium sulfate, potassium sulfate, and magnesium sulfate, has been developed for low-volume bowel preparation; however, the high sulfate content of OSS results in a rotten egg smell and bitter taste.
5-7 To improve compliance by eliminating the unpleasant experience of using OSS, an oral sodium sulfate tablet (OST) has recently been developed as a bowel preparation agent in tablet format, with a tasteless and odorless nature.
8 In fact, in randomized clinical trials, OST had better bowel preparation efficacy (particularly in the right colon), higher compliance, and better safety than 1-L PEG/Asc and 2-L PEG/Asc , as well as no inferior adenoma detection rate (ADR).
8-12 Therefore, the use of 1-L PEG/Asc and OST as low-volume bowel preparation agents has gradually increased.
These low-volume bowel preparation agents have raised concerns regarding the risk of acute gastric mucosal injury due to incomplete dissolution of the tablet formula in water or hyperosmolarity originating from the concentrated solution.
13-16 In fact, a recent study has reported that OST may induce erosive gastritis.
17 Even if these lesions have not yet been determined to be clinically significant, a positive finding upon esophagogastroduodenoscopy (EGD) can influence endoscopic interpretation. Nevertheless, insufficient data on acute gastropathy due to the use of these bowel preparation agents limits the choice of low-volume agents for bowel preparation.
The elderly population is less likely to tolerate high-volume agents; therefore, the availability of low-volume agents is crucial for planning colonoscopies in elderly patients.
18,19 However, because old age is a known risk factor for inappropriate bowel preparation and poor safety, elderly individuals have often been excluded from clinical trials of low-volume agents subjects have often been excluded from clinical trials with low-volume agents because old age has been known as a risk factor for inappropriate bowel preparation and vulnerable safety and few randomized controlled trials reported the efficacy and safety of low-volume agents in elderly subjects.
18,20 Moreover, despite the increasing burden of elderly patients undergoing colonoscopy, no previous study has reported safety data for acute gastric mucosal lesions associated with low-volume agents such as 1-L PEG/Asc and OST in elderly subjects.
Therefore, we compared the incidence of acute gastropathy and the efficacy, and safety of 1-L PEG/Asc and OST according to age in healthy subjects who underwent both EGD and colonoscopy.
Go to :

SUBJECTS AND METHODS
1. Study design and population
This retrospective study included patients visiting the Health Promotion Center of a single academic hospital in South Korea between January 1, 2021, and December 31, 2022. Eligible participants included outpatients aged ≥18 years who underwent a scheduled EGD and colonoscopy on the same day for screening and were administered only OST (Orafang®; Pharmbio Korea Co. Ltd., Seoul, Korea) or 1-L PEG/Asc (CleanViewAL®; Taejoon Pharm Co., Ltd., Seoul, Korea) for bowel preparation before colonoscopy. Bowel preparation agents were selected by the subject’s preference as much as possible, and doctor’s recommendation was not intervened in the selection process. The study population was divided into three age groups as follows: those aged 18–49, 50–64, and ≥65 years were defined as the young, middle-aged, and old-aged groups, respectively. We collected patient demographic data, comorbidities, current medication use, EGD and colonoscopy findings, bowel preparation results, and several laboratory parameters from the day of the colonoscopy.
This study was approved by the Institutional Review Board of our study institution (KHNMC IRB 2023-10-037) and conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was waived by KHNMC IRB because of the retrospective study design.
2. Bowel preparation protocol
All participants were instructed to follow a low-residue diet for 3 days before the scheduled colonoscopy and a clear liquid diet for dinner the night before the colonoscopy. Both groups received preparation agents as a split-dosing regimen at home. The first dose was administered between 6:00 and 8:00 PM the day before the colonoscopy, while the second dose was administered between 6:00 and 8:00 AM on the day of the colonoscopy, at least 3 hours before the scheduled procedure. All patients underwent EGD and colonoscopy between 9:00 AM and noon. Patients in the OST group took the first sequence of 14 tablets with water and an additional 1 L of water over an hour, as well as a second sequence of the remaining 14 tablets, similar to the first. In contrast, patients in the 1-L PEG/Asc group were instructed to drink 500 mL of the study solution - a mixture of the study drug in powdered form and free water - followed by at least 500 mL of free water per dose.
3. Assessment of outcomes
The primary outcome of this study was the presence of acute gastropathy associated with bowel preparation agents in the EGD findings. In this study, acute gastric lesions caused by bowel preparation agents were divided into three categories: hyperemia, erosive lesions, and acute gastric mucosal lesion (AGML)-like blood clots or hematin (
Fig. 1). The location of the occurrence of acute gastric lesions was distinguished from other locations by the greater curvature (GC) of the body of the stomach, which frequently occurs due to its dependent position.
21 The severity of gastric mucosal injury was evaluated using the LANZA score based on five grades as follows: normal stomach or proximal duodenum=0, mucosal hemorrhage only=1, one or two erosions=2, numerous (3–10) erosions= 3, and a large number of erosions (>10) or ulcers=4.
22 In addition, retention of fluid ≥200 mL was recorded upon each EGD examination. The presence of gastropathy associated with bowel preparation agents was reviewed by four non-investigating gastroenterologists who were blinded to the administered preparation agents.
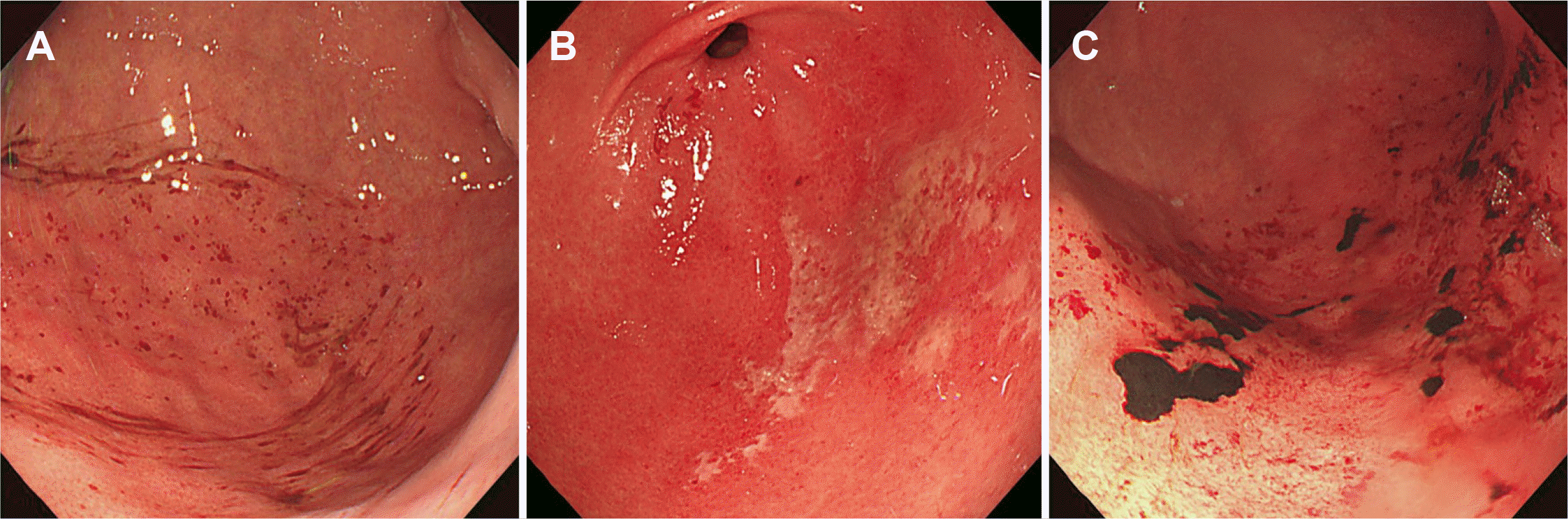 | Fig. 1Esophagogastroduodenoscopic findings of acute gastropathy: (A) hyperemia, (B) erosive lesions, and (C) acute gastric mucosal lesion-like blood clots or hematins. 
|
In this study, we used the Boston Bowel Preparation Scale (BBPS) for the bowel preparation score. The BBPS is a 4-point (0–3) scale used to assess three segments of the colon, namely, the right (cecum and ascending colon), transverse, and left (descending, sigmoid colon, and rectum) segments, giving a total score of 0 to 9.
23 Successful overall bowel preparation was defined as a BBPS score ≥2 for all segments, while high-quality bowel preparation was defined as a BBPS score of 9. Bowel cleansing scores were evaluated by highly experienced board-certified gastroenterologists who performed the endoscopic procedures. Other secondary outcomes included the polyp detection rate (PDR), ADR, and CRC rate. The PDR and ADR were calculated as the percentage of patients in the analyzed population with at least one polyp or adenoma, respectively. Furthermore, the study population underwent laboratory testing to determine serum levels of blood urea nitrogen (BUN), sodium, potassium, chloride, calcium, and phosphate on the day of the colonoscopy (post-bowel preparation).
4. Statistical analysis
Continuous variables are presented as the mean±standard deviation for clinical data and were compared using two-sample t-tests. In contrast, categorical variables are presented as numbers and percentages. These were analyzed using the chi-squared test or Fisher’s exact test for comparison between two groups and analysis of variance or the Kruskal–Wallis test for comparison between three groups. All statistical analyses were performed using SPSS software (version 20.0; IBM Corp., Armonk, NY, USA), and all statistical tests were two-sided with statistical significance set at p<0.05. The distribution of each laboratory parameter after the study medication was administered was determined as an overlay graph using the kernel density plot in Stata 16.1 software (StataCorp LP, College Station, TX, USA).
Go to :

DISCUSSION
To our knowledge, this large observational study was the first to compare acute gastropathy upon EGD examination according to age in groups taking OST or the 1-L PEG/Asc for bowel preparation. We found that acute gastropathy occurred more often in the OST group than in the 1-L PEG/Asc group across all age groups. Moreover, the difference in the incidence of acute gastropathy between the groups was largest in the young group, followed by the middle-aged and old-aged groups. Our results also showed that acute gastropathy associated with OST occurred regardless of age, while that associated with 1-L PEG/Asc occurred more frequently in older age groups, which is consistent with a previous study that reported an age ≥60 years was a risk factor for developing hemorrhagic gastropathy after taking bowel preparation agents.
13 Although OST-related gastropathy has been observed not infrequently, it does not have any particular clinical significance leading to additional medications or treatments.
Acute gastropathy caused by bowel preparation agents is characterized by erosive lesions of the antrum or longitudinal ulcers containing hematin in the stomach.
15,16 It is commonly found in portions dependent on gravity, such as the GC and posterior wall of the stomach.
13,14 Therefore, in this study, acute gastropathy was defined by dividing it into three stages: AGML-like lesions, which represent longitudinally directed ulcers with hematin; erosive lesions; and hyperemia, a milder form than the others. In addition, we classified acute gastropathy according to its location of occurrence – either on the GC side on the body of the stomach or in other location. We found that acute gastropathy occurred more frequently on the GC side than on the lesser curvature side, which was consistent with a previous study showing that most lesions occurred in the GC.
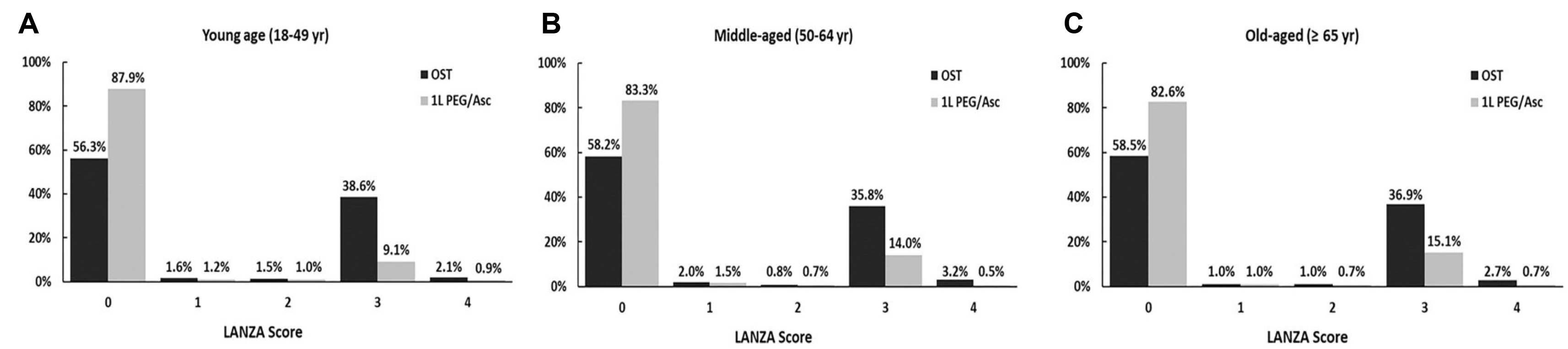
13 In this study, most patients with gastric mucosal injury had a severity score of 3, as measured using the LANZA score, which indicated the presence of 3–10 erosive lesions. In the 1-L PEG/Asc group, the proportion of patients with a severity score of 3 gradually increased with age; however, in the OST group, there was no significant difference in the proportion of patients with a severity score of 3 according to age. There was a significant difference between 1-L PEG/Asc and OST at the most severe point, with a score of 4 indicating deep mucosal injury (ulceration) or more than ten erosive lesions (0.7% vs. 2.5%). However, there were no significant differences between the groups in terms of age.
The mechanism of acute gastropathy caused by the ingestion of a preparation agent remains uncertain. The possible mechanism may be multifactorial and is likely related to the high osmolality of the concentrated solution and the direct thermal effect caused by insufficient dissolution from the ingestion of small amounts of water than the recommended amount of water in the instructed education.
13-15 Speculation regarding this cause might explain the results of our study showing that the OST group (tablet type), which is likely to be partially dissolved, suffered more gastric mucosal injury than the solution-type group. In a real-world experience, OST-related gastropathy is observed more frequently in patients who drink less water than the instructed education. Therefore, we could instruct subjects to drink about 250–500 mL of water before starting OST medications (pre-dosing) in order to prevent OST-related gastropathy
Aspirin or other NSAIDs are generally considered to be more likely to trigger acute gastric mucosal injury.
24 However, a previous study showed that the chronic use of aspirin had no effect on hemorrhagic gastropathy after bowel preparation, which is consistent with some of our findings.
13 In our study, the proportions of patients taking antiplatelet agents or NSAIDs in the young, middle-, and old-aged groups were 2.7%, 11.0%, and 28.6%, respectively. However, the proportions of the AGML-like lesions were 16.3%, 17.7%, and 17.9% in the OST group and 3.3%, 6.2%, and 5.2% in the 1-L PEG/Asc group, respectively. This indicates that the AGML-like lesions found in our study were more related to bowel preparation agents than to ulcerogenic drugs.
The incidence of nausea, vomiting, abdominal discomfort, and abdominal pain, which are gastrointestinal symptoms likely associated with acute gastropathy, appear to differ from the rate of acute gastropathy observed upon EGD. These results suggest that acute gastropathy upon EGD examination after the administration of bowel preparation agents may not cause clinically significant discomfort. In previous animal-model research using pigs, the macro- and microscopic acute gastric mucosal injury induced by bowel cleansing agents was characterized by the superficial nature, and spontaneous reversibility with healing occurring in ≤72 hours.
25,26 Regarding clinical safety, nausea and vomiting were significantly higher in the OST group than in the PEG/Asc group; however, in contrast, a previous study reported higher nausea and vomiting in the PEG/Asc group than in the OST group.
8,9 In our study, the OST group had higher rates of acute gastropathy observed upon EGD than the 1-L PEG/Asc group (42.3% vs. 14.7%, p<0.001). Therefore, the more frequent gastrointestinal symptoms in the PEG/Asc group may be due to the disgusting odor or taste of the concentrated agents and the high dose of ascorbate rather than due to acute gastropathy.
24 It is supported by previous findings that 1-L PEG/Asc contains 40.6 g of ascorbate causing more abdominal discomfort, bloating, nausea, and vomiting than 2-L PEG/Asc containing 9.4 g ascorbate (34.6% vs. 18.4%, p=0.003).
27 Conversely, the gastrointestinal symptoms in the OST group may have occurred because the subjects consumed more accessible tasteless tablets and less free water than necessary.
Notably, the middle- and old-aged groups often chose OST due to the tasteless nature of the tablet formation in contrast to the unpleasant taste and smell they experienced while consuming bowel cleansing agents in solution form during a previous colonoscopy. However, younger age groups might have chosen 1-L PEG/Asc because they have no previous experience with liquid preparation agents and prefer the less expensive solution form, which is one-fifth the price of the OST in our country. In previous studies comparing the effectiveness and safety of OST and low-volume PEG/Asc, satisfaction with taste or smell was higher for OST, and the proportion of subjects willing to use the same agent in the next colonoscopy was also significantly higher for OST.
8,9 These results suggest that the proportion of patients who choose tablet forms of bowel preparation agents will increase. Furthermore, as this form may be preferred by the elderly, research on the safety of its use in the elderly may become significant.
10
In this study, we found that the OST group had a significantly better bowel preparation effect than the 1-L PEG/Asc group in the young age group; however, there was no difference between these two groups in the middle- and old-aged groups, which is consistent with previous studies reporting no difference in BBPS between OST and 1-L PEG/Asc in 28 patients aged ≥65 years.
9 One previous study comparing 2-L PEG/Asc and OST also reported no difference in the bowel cleansing effect in people aged >65, whereas OST had a significantly better bowel preparation effect than 2-L PEG/Asc in patients aged ≤65 years, similar to our study.
10 Another study comparing OST and 1-L PEG/Asc showed that the bowel preparation effect of OST was superior, especially in the right colon, at all ages.
8
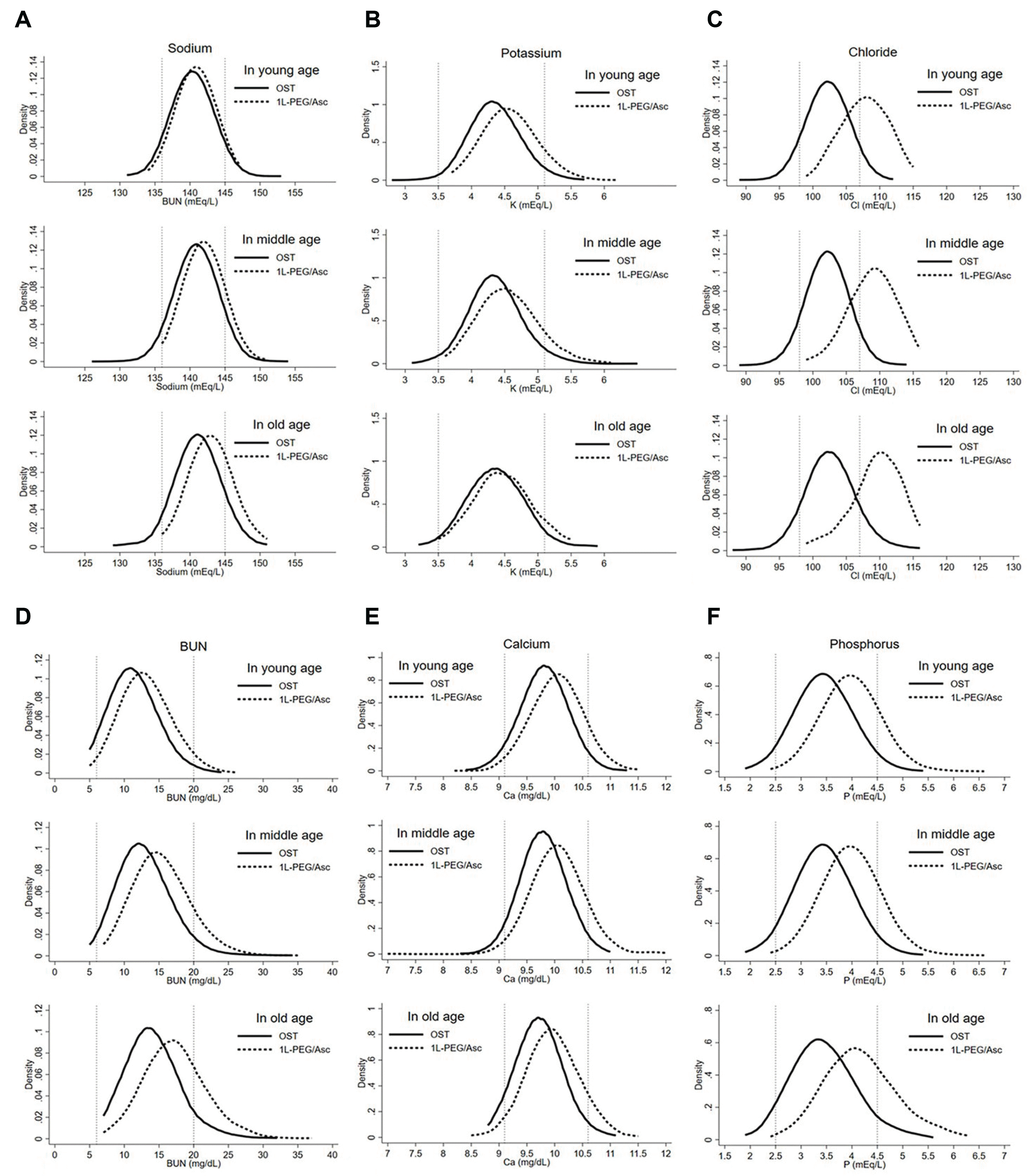
Our results regarding the distribution of each laboratory parameter are consistent with those of previous studies. Patients who took OST or 1-L PEG/Asc commonly had decreased BUN, calcium, phosphate, and potassium and increased sodium; however, changes in chloride and phosphate levels had different patterns between the OST and 1-L PEG/Asc groups.
8,28 Therefore, the distribution of chloride and phosphate values between the two groups shows a more apparent difference than other parameters like the results in this study. We also found that the differences in sodium, chloride, BUN, and phosphate between the OST and PEG groups increased with age, while that for potassium increased with a younger age. Moreover, there was no difference in the distribution of calcium between the groups according to age. In contrast, Di Palma et al.
8 reported that all parameters showed no significant difference between elderly and non-elderly groups. This might be due to their smaller number of study participants than that in our study, and their numbers may therefore not reach statistical significance for subgroup analysis by age. Other previous studies have stated that most changes in laboratory values after taking bowel preparation agents tended to be tolerable and rarely clinically meaningful.
8,9,28,29 In this study, we found that the shift of the laboratory parameters’ distribution after taking 1-L PEG/Asc was more outside the normal range than that with OST, and this was more pronounced in older groups. Therefore, the use of 1-L PEG may raise concerns regarding the risk of more hypernatremia and electrolyte shifting.
30 Additional large-scale prospective studies are necessary to check electrolyte changes observed in this study, as our finding is limited by an observational study design.
This study had some limitations. First, this retrospective study did not collect dietary habits, all medication history, and lifestyle patterns, which may be associated with the occurrence of gastropathy. Considering that AGML-like erosion or ulcer is extremely rare in EGD performed on healthy, asymptomatic subjects, these findings were classified as OST-related gastropathy in our study. Even though, we could not collect all medication history, we collected ulcerogenic medication history, such as antiplatelet agent, anticoagulant, and non-steroidal anti-inflammatory drug. However, a prospective study is mandatory to control all these risk factors. Second, OST-related gastropathy may be influenced by drug compliance, water intake, and timing of administration, which could not be controlled in a real-world practice. Therefore, a prospective study controlling these variables are necessary. Third, our results might not be generalizable because a healthy population undergoing colonoscopy and EGD for screening may indicate a selective bias. Fourth, due to the retrospective nature of health check-up in our study, we could not survey satisfaction after taking bowel preparation agents, or symptoms correlated with acute gastropathy due to a lack of follow-up at the clinic. Finally, we were only able to conduct laboratory tests after bowel preparation; therefore, we were unable to compare the changes in laboratory parameters before and after bowel preparation.
In conclusion, acute gastropathy was more strongly associated with OST than with 1-L PEG/Asc during bowel preparation in all age groups. Therefore, physicians should take acute gastropathy associated with low-volume agents into consideration during bowel preparation for all age groups.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download