INTRODUCTION
Chronic gastritis (CG) is the chronic inflammation of the gastric mucosa associated with varying degrees of superficial and glandular epithelial damage.
1 Helicobacter pylori (
H. pylori) is a gram-negative bacterium and the most common cause of CG. Approximately 50% of the world’s population is infected with
H. pylori.
2 The percentage is higher in developing countries and more in men than women.
3 Long-term infection increases the risk of progression to gastric cancer. Gastric carcinoma is the second most common malignancy worldwide.
4,5 An accurate diagnosis of
H. pylori-associated gastritis is crucial in clinical practice because
H. pylori-associated gastritis indicates the early phase of gastric carcinogenesis.
6-8 H. pylori infection is a treatable condition. Therefore, early detection and eradication improve the quality of life with a decreased severity of dyspeptic symptoms and a decrease in the risk of gastric malignancy. The Updated Sydney System (USS) for grading and classifying CG was conceived to provide a unified and standardized application to interpret antral biopsies.
9 In the USS, the following five histological parameters are graded as mild, moderate, or severe: chronic inflammation, activity (neutrophilic infiltration), atrophy, intestinal metaplasia, and
H. pylori density.
9 Few studies have examined
H. pylori gastritis and its association with histomorphological features by the USS in central India. Therefore, this study evaluated the histopathological changes in chronic gastritis using the USS to determine the prevalence and correlation of
H. pylori gastritis with other histological variables. These results offer new insights and details regarding
H. pylori gastritis-associated histopathological features and its prevalence in central India.
Go to :

DISCUSSION
Chronic gastritis was the major cause of dyspepsia in the present study, comprising 83.84% (109/130), similar to Sharma et al.
10, who reported an 89% prevalence in Jammu and Kashmir. A lower prevalence of histological gastritis was reported in a study in Thailand (58.7%) and Malaysia (78.2%) among total dyspeptic patients.
11,12 The common age group of CG in this study was 31–40 and 41–50 years, similar to Sharma et al.
10 and Mujawar et al.
13, whereas Pruthi et al.
14 reported 61–70 and 51–60 years to the most common age group. In the present study, CG showed a male predominance, similar to other studies.
13,15-17 The prevalence of
H. pylori in the present study (46.78%) was comparable to Garg et al.
18 (43.67%) from Ludhiana, whereas studies from other parts of India reported a higher prevalence of
H. pylori (46– 67%).
15,16,19,20 The prevalences of
H. pylori from Pakistan,
21 Brazil,
22 Thailand,
11 Malaysia,
12 and Kosovo
23 were reported to be 62.5%, 58,5%, 48.2%, 6.8%, and 23.38%, respectively. This variation was attributed mainly to a varied geographical area, socioeconomic status, and sanitation standards.
RUT in biopsy specimens is also a good choice for rapid detection of the organism. In the present study, the sensitivity and specificity of RUT were similar to Dandin et al.
19 Pruthi et al.
14, reported very low specificity (37.5%) but relatively comparable sensitivity (62.5%) with the present findings (79.59%). The reason for 10 false negative tests in the present study could be attributed to low bacterial density. In contrast, six false positive results could be better explained by the presence of other urease-producing bacteria like
Proteus mirabilis and
Klebsiella pneumonie.
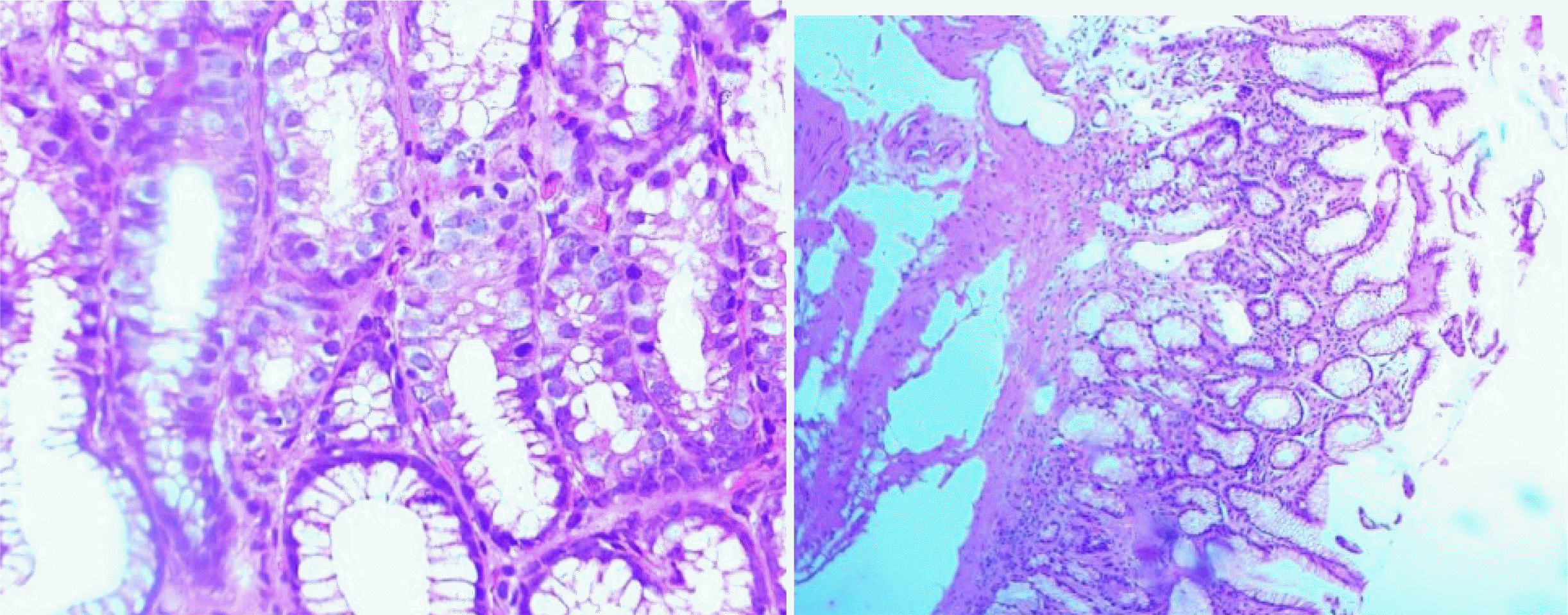
Using the USS of CG, chronic inflammation was present in 100% of cases, with 53.2%, 40.3%, and 6.42% mild, moderate, and severe inflammation, respectively. Sharma et al.
10, Garg et al.
18, and Manxhuka-Kerliu et al.
24, also found that the majority of cases had mild inflammation, similar to the present study. In contrast, Pruthi et al.
14 and Türkay et al.
25 reported the moderate grade of chronic inflammation to be the prevailing grade in CG. Sharma et al.
10, Pruthi et al.
14, and Türkay et al.
25 reported that
H. pylori colonization increased as the degree of chronic inflammation agreement with the present study. A significant association was observed between
H. pylori and a moderate grade of inflammation.
Neutrophilic infiltration (activity) was present in 50% of cases, similar to Shafii et al.
26 (49.26%), whereas Sharma et al.
10 and Garg et al.
18 observed 39.33% and 33.3%, respectively. The association between
H. pylori and neutrophilic activity was significant in various studies similar to the present study.
13,15,17,18,21 Pruthi et al.
14 and Türkay et al.
25 reported an increase in
H. pylori positivity as the degree of activity increased, but the present study observed no statistically significant association between neutrophilic activity and
H. pylori. This might be because of variation in the prevalence of
H. pylori in antral biopsies despite multiple site biopsies.
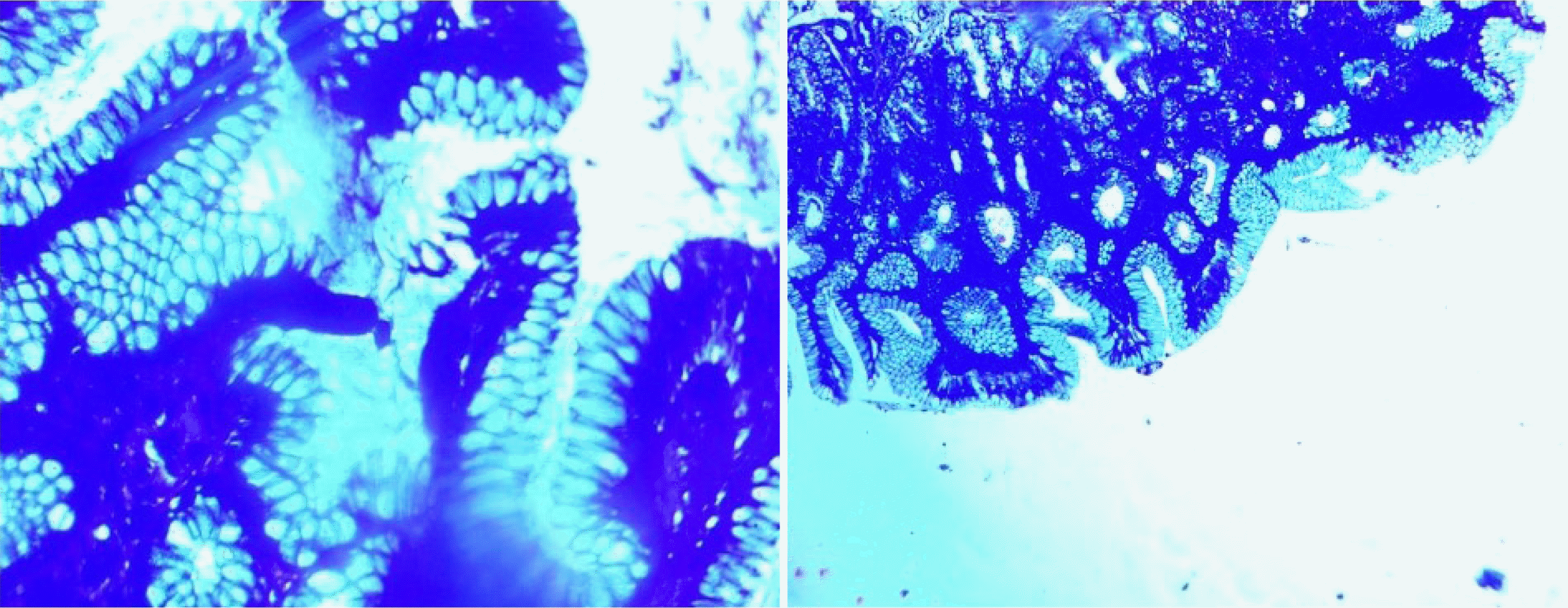
A higher prevalence (9.2%) of intestinal metaplasia was observed in the present study, compared to Sharma et al.
10 (7.87%), Atisook et al.
22 (8.2%), and Garg et al.
18 (7%). A mild degree of intestinal metaplasia was significantly related to
H. pylori in the present study, similar to Türkay et al.
25 and Shafii et al.
26 On the other hand, Nai et al.
8, Sharma et al.
10, and Garg et al.
18 observed no significant correlation.
Atrophy was observed in 23 (21.10%) of total CG cases. The results were comparable to Shafii et al.
26 (17.65%), while lower values were reported by Sharma et al.
10 (12.36%), Atisook et al.
22 (11.6%), Garg et al.
18 (12.3%), and Manxhuka-Kerliu et al.
24 (14.94%). Similar to the present study, a non-significant association of
H. pylori with atrophy was observed in many studies.
13-15,18 This could be explained by the bacteria remaining and multiplying on epithelial cells. Thus, atrophy is an unfavorable factor for their growth.
Only 11 (10.09%) cases of lymphoid aggregates and follicles were found. Out of these 11 cases, 63.6% showed
H. pylori positivity. On the other hand, Sharma et al.
10 and Shafii et al.
26 reported lymphoid follicles and aggregates in 29.2% and 45.58% of cases, respectively, with
H. pylori positivity observed in 80.64% (50 out of 62) and 76.92% (20 out of 26 cases) of those cases, respectively. The association of
H. pylori colonization with lymphoid aggregates was not significant in the present study compared to these studies. Nevertheless, there was a very high association between lymphoid follicle formation and CG in the present study.
Among the 51 cases of
H. pylori, the most frequent histological finding was chronic inflammation (100%), followed by lymphoid aggregates (63.6%), and the least was neutrophilic activity (56%). Sharma et al.
10 and Hemalata et al.
27 also reported chronic inflammation in 100% of
H. pylori-positive cases and neutrophilic activity in 66.67% and 80% of cases, respectively. In contrast, Nai et al.
8, reported more neutrophilic activity in
H. pylori-negative cases (72.3%) than in
H. pylori-positive cases (53%). Sharma et al.
10 and Hemalata et al.
27 reported a lower rate of intestinal metaplasia: 7.87% and 8%, respectively. Lymphoid aggregates were present in only 63.6% of
H. pylori- positive cases in the present study, whereas Sharma et al.
10 and Shafii et al.
26 observed them in 80.64% and 76.92% of cases, respectively.
The correlation among various histological parameters of the USS of CG was calculated and significant correlations were observed between chronic inflammation and neutrophilic activity (r=0.458, p-value<0.001), chronic inflammation and lymphoid hyperplasia (r=0.251, p-value=0.008), and atrophy and intestinal metaplasia (r=0.245, p-value=0.01), similar to Garg et al.
18 On the other hand, the present study observed a statistically significant correlation between neutrophilic activity and atrophy (r=0.312, p-value=0.001) in contrast to Garg et al.
18
CG patients with different ABO blood groups showed a maximum association of
H. pylori in A
+ patients, followed by AB
+. By contrast, Mattos et al.
28 and Baqir et al.
29 found
H. pylori to be more prevalent in the “O” blood group than other blood groups. This discrepancy between the present study and others might be due to the low prevalence of
H. pylori and regional variability.
The major limitations of this study were the small sample size, no topographical study, using only an antral biopsy, and no special stains (e.g., alcian blue-periodic acid Schiff) to detect the incomplete intestinal metaplasia. Therefore, an assessment of multiple gastric biopsy specimens is necessary to provide a global picture of H. pylori infection in the stomach. On the other hand, maximum prevalence has been demonstrated through the body greater curvature. In the present study, the author has done two antral biopsies to observe the histopathological changes because of a H.pylori infection and found a smaller prevalence of H.pylori infection than biopsies on the sites of body greater curvature. The histological findings other than the USS associated with H. pylori infection were also not evaluated. The strength of this study was interobserver reproducibility because at least two pathologists studied each biopsy. Nevertheless, studies with a larger sample size and biopsies from different sites of the stomach should be conducted to validate the importance of the USS in an Indian scenario.
H. pylori was significantly associated with the degree of chronic inflammation and intestinal metaplasia. The presence of intestinal metaplasia is a significant risk factor for progression to gastric cancer. This paper suggests a vigorous search for H. pylori if chronic inflammation and intestinal metaplasia are observed in antral gastric biopsies. The USS is extremely useful for analyzing gastric biopsies, and it offers a standardized and unified approach for the diagnosis of H. pylori-associated gastritis.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download