Dear Sir:
The coronavirus disease 2019 (COVID-19) pandemic prompted the development and emergency-use authorization of various vaccines, which played a major role in reducing severe illness, hospitalization, and mortality rates [
1]. However, ongoing reports have raised concerns about vaccine side effects, such as alopecia, and multiple sclerosis [
2-
4]. Although some studies have investigated the association between vaccines and stroke, there has been no research specifically focusing on the association between different vaccine types and ischemic stroke. Therefore, we aimed to analyze the association between all vaccine types and ischemic stroke risk by using Vigibase, the global individual case safety report database of the World Health Organization (WHO).
We utilized VigiBase, the WHO’s international collection of case reports managed by the Uppsala Monitoring Centre (UMC; Uppsala, Sweden;
https://who-umc.org/vigibase/) in this study (
Supplementary Method and
Supplementary Figure 1). It includes 89,065,592 adverse drug reactions (ADR) reports from 1969 to 2023 covering nearly 170 countries and territories. The Institutional Review Boards of Kyung Hee University (KHUH 2022-06-042) and the UMC (license 7014049) approved the use of confidential and electronic processes for patient data.
Of the ischemic stroke reports in VigiBase between 1969 and 2023 (n=21,011), vaccine-associated reports (n=5,843) were categorized into five groups according to vaccine types: influenza vaccines; COVID-19 mRNA vaccines; Ad5-vectored COVID-19; inactivated whole-virus COVID-19 vaccines; and others (anthrax, brucellosis, cholera, dengue vaccines, diphtheria, Ebola, encephalitis,
Haemophilus influenzae type b, hepatitis A, hepatitis B, leptospirosis, measles, meningococcal, mumps, papillomavirus, pertussis, plague, pneumococcal, polio, rabies, rotavirus diarrhea, rubella, smallpox, tetanus toxoids, tuberculosis, typhoid, typhus, varicella zoster, and yellow fever vaccines) (
Supplementary Table 1).
VigiBase is based on the Medical Dictionary for Regulatory Activities (MedDRA) version 26.0 (
https://www.meddra.org/) framework for the organization of ADRs and uses the following five classes: system organ class, high-level group term, high-level term, preferred term, and lowest-level term [
3]. The preferred search terms “embolic stroke,” “ischaemic stroke,” “thrombotic stroke,” and “vertebrobasilar stroke” were used to collect every ADR globally reported by vaccinators (
Supplementary Table 2). Relying on the WHO causality assessment recommendations, all ADRs were “suspected” when estimating disproportionality signals of vaccines for ischemic stroke.
To identify whether the vaccines were associated more with ischemic stroke reports relative to all other drugs in VigiBase, we performed a disproportionality analysis for all vaccine types and each vaccine. If the proportion of ischemic stroke reports is significantly higher for a vaccine than for any other medicinal product, the disproportionality signal indicates a significant association between the vaccine and ischemic stroke reports. We estimated two indicators, information component (IC) and reporting odds ratio (ROR), which are commonly used measures in pharmacovigilance for signaling the disproportionate association between a medicinal product and ADRs. IC
025 (the lower interval of the 95% confidence interval [CI]) for IC and 95% CI for ROR are calculated, assuming Jeffrey’s prior in the Bayesian analyses [
3]; an IC
025 of >0.00 and ROR of >1.00 are indicative of statistical significance. For a comparison between vaccines, the Kruskal-Wallis test was used for continuous variables and Fischer’s exact test for categorical variables, determining statistical significance with a two-tailed test (
P<0.05) [
5]. All analyses were performed using SAS software (version 9.4; SAS Inc., Cary, NC, USA).
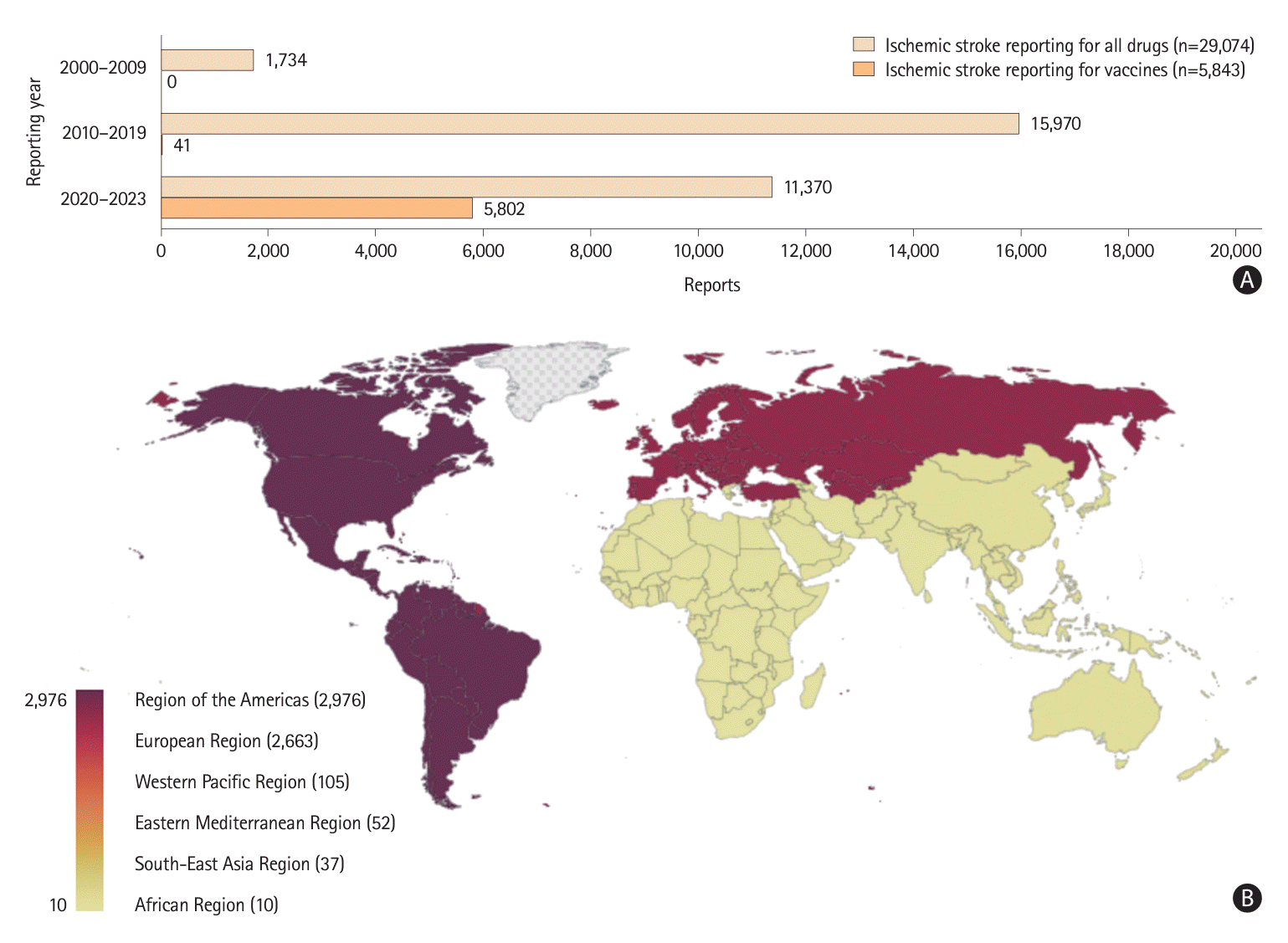
In our analysis, we identified 5,843 reports of vaccine-induced ischemic stroke from among 89,065,592 reports (
Supplementary Figure 2). The number of reported cases of vaccine-induced ischemic stroke has increased significantly since 2020, especially in the United States and Europe (
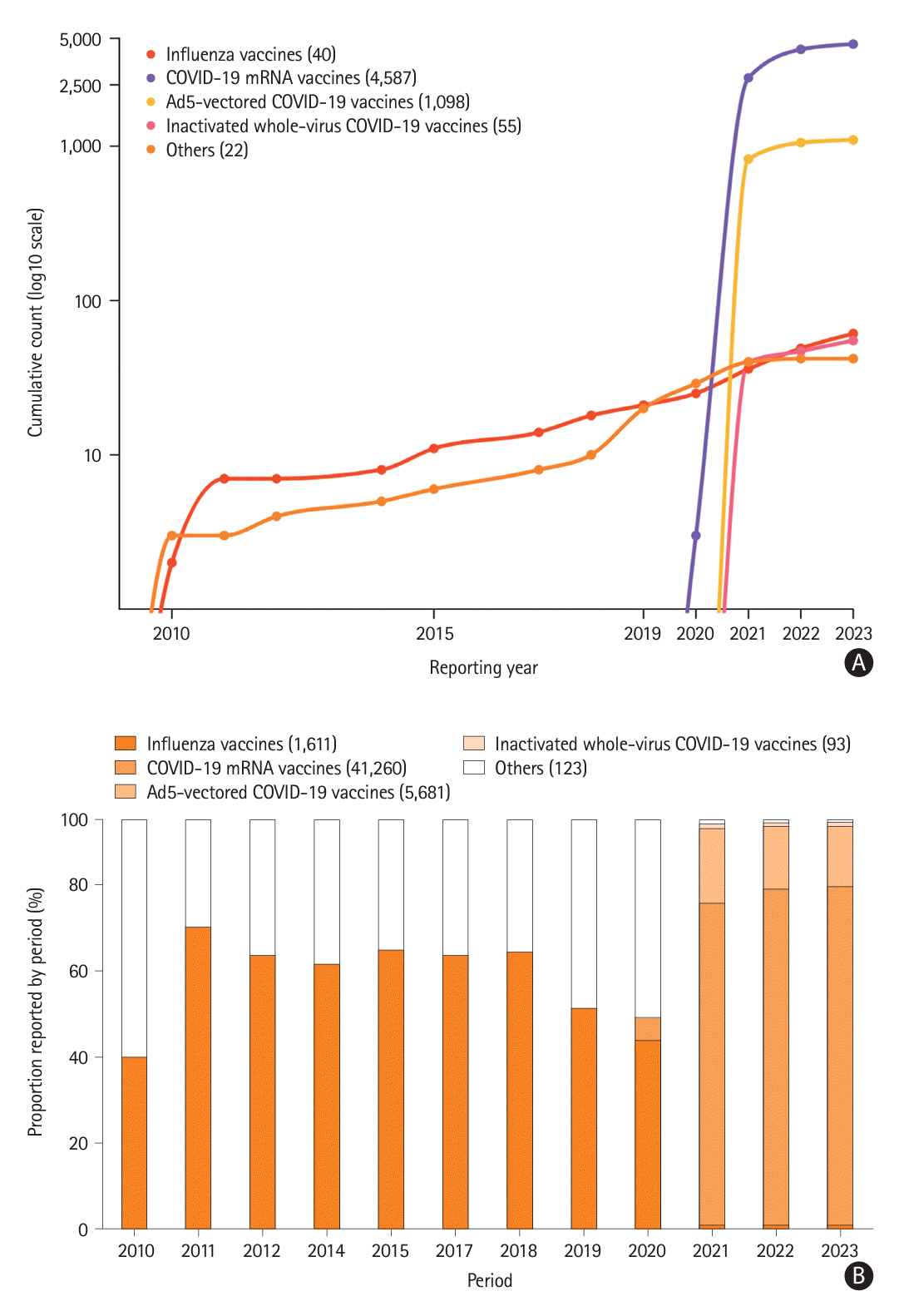
Figure 1). Before the pandemic, most cases of vaccine-induced ischemic stroke were attributed to influenza vaccines. However, as COVID-19 vaccination began globally, the incidence of COVID-19 vaccine-associated ischemic stroke has been reported to be several times higher than that associated with other vaccines (
Figure 2). Especially, two types of COVID-19 vaccines are particularly associated with ischemic stroke: the COVID-19 mRNA vaccine (ROR, 8.33 [95% CI, 8.03–8.65]; IC, 2.42 [IC
025, 2.38]), and Ad5-vectored COVID-19 (ROR, 4.23 [95% CI, 3.98–4.50]; IC, 1.97 [IC
025, 1.87]) (
Table 1). This trend was more pronounced in individuals aged ≥45 years; no significant difference was observed between the sexes (
Supplementary Table 2). Furthermore, by employing MedDRA-preferred terms, we found that coronary symptoms were the most common concomitant adverse events (
Supplementary Table 3 and
Supplementary Figure 3).
Unlike previous research that primarily focused on the association between COVID-19 vaccines and stroke, our study advances the topic by identifying specific vaccine types, notably mRNA and Ad5-vectored, that are more closely associated with ischemic stroke. We conducted a comprehensive investigation of vaccine-induced ischemic stroke cases using VigiBase, which revealed a significant increase in ischemic stroke incidence since the initiation of mass vaccination in 2020. A substantial number of cases have been directly linked to COVID-19 vaccination, suggesting that vaccination may increase stroke risk. Additionally, approximately 4% of patients experienced fatal outcomes, a figure that is relatively lower than the global mortality rate of stroke.
These findings can be explained by the unique mechanism of the vaccines, which is distinct from that of traditional vaccines. During the immunization process, the mRNA and vector of the vaccine introduced into the body generate various viral particles, such as the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) spike protein, leading to interaction with platelets [
6]. This sequence may ultimately trigger cerebral venous sinus thrombosis and vaccine-induced thrombotic thrombocytopenia, potentially leading to ischemic stroke [
7,
8]. However, several limitations must be considered when interpreting the study results. First, the participants’ lifestyles and health conditions should be considered more thoroughly, because they can influence study results. Second, the reports are predominantly from Western countries, where post-vaccination monitoring is more accessible; there are limitations in identifying regional and racial differences. Furthermore, because the report only provides information at the time of reporting and does not incorporate the results of subsequent monitoring, it remains unclear whether the mortality outcome can be trusted.
Our study highlights the significant association between several COVID-19 vaccine types and ischemic stroke while overcoming the limitations of previous research, which failed to establish a specific association. The findings highlight the need for thorough monitoring and research on the potential adverse reactions following vaccination.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download