Dear Sir:
Currently, treatments for acute ischemic stroke (AIS) are based on reperfusion therapies; however, the time window for these interventions is limited to the first few hours after stroke onset. Therefore, alternative neuroprotective therapies are urgently required.
Acute blood flow disruption leads to early neuronal death in the core of the ischemic area, later, secondary processes, such as inflammation, excitotoxicity, and oxidative stress enlarge the infarct core into the surrounding tissue, the “penumbra,” [
1] which represents the perfect target for neuroprotective strategies. Adenosine receptors (ARs) have recently emerged as potential therapeutic targets in brain ischemia [
2], granting AR agonists a role to prevent “penumbra” evolution [
3-
5]. Grant et al. [
6] showed that pulsed electromagnetic fields (PEMFs) promote significant neuroprotection in a rabbit model of transient focal ischemia. This effect is explained by the selective agonist activity for A2A AR of PEMFs, first described by Varani et al. [
7].
Based on preclinical and early clinical experiences (phase 1 and 2 studies) [
8-
10], we designed a double-blind, placebo-controlled, randomized study to assess whether PEMF exposure was effective in reducing cerebral ischemic volume (primary endpoint) and promoting functional recovery (secondary endpoint) (I-NIC study, NCT02767778). I-NIC clinical trial protocol is provided as
Supplementary Materials.
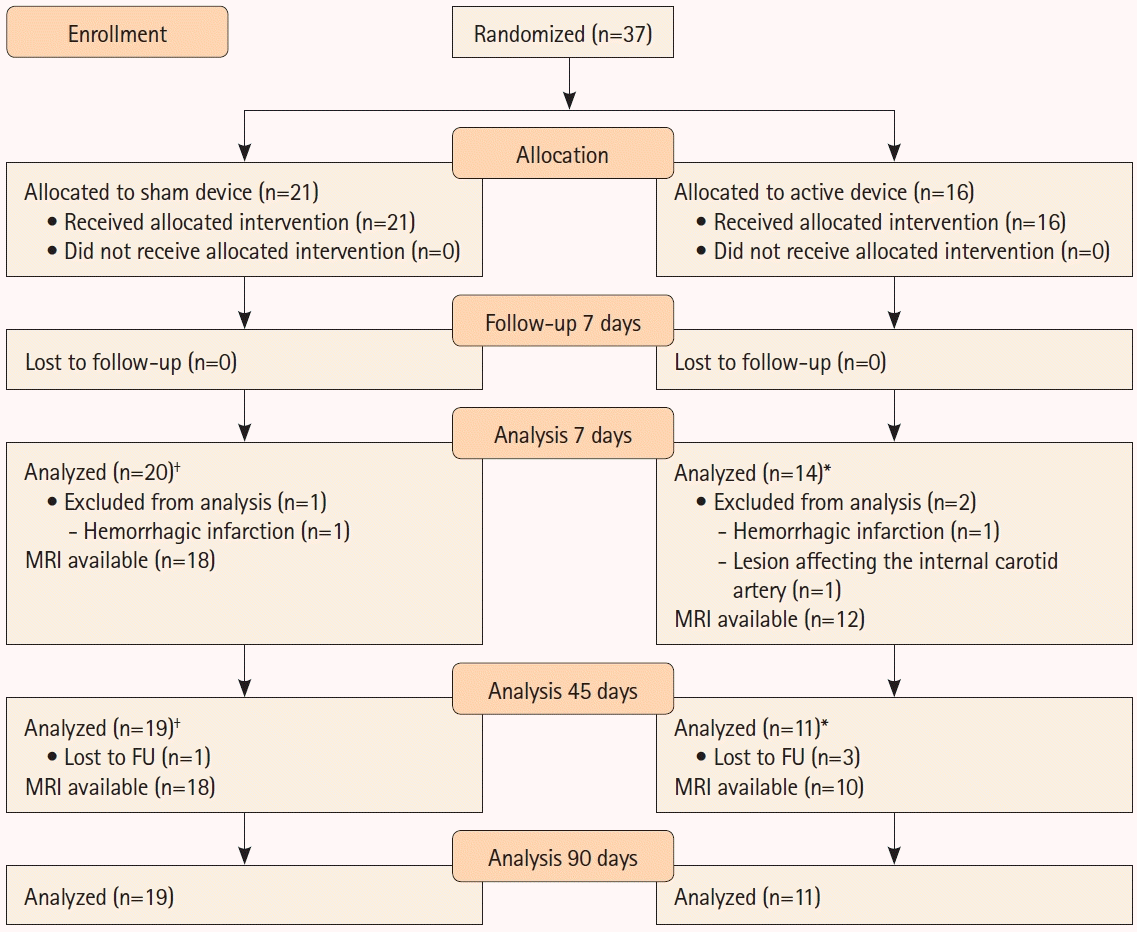
One hundred and sixty-eight patients were screened, and 37 patients with ischemic stroke in the middle cerebral artery (MCA) territory were enrolled: 16 patients were assigned to the active group and 21 to the placebo group (sham device) (
Figure 1). The detailed methodology is described in the
Supplementary Methods. There were no significant differences in the demographic and clinical characteristics at baseline between the groups (
Supplementary Table 1). Magnetic resonance imaging (MRI) was conducted at baseline and 7 and 45 days after stroke onset (
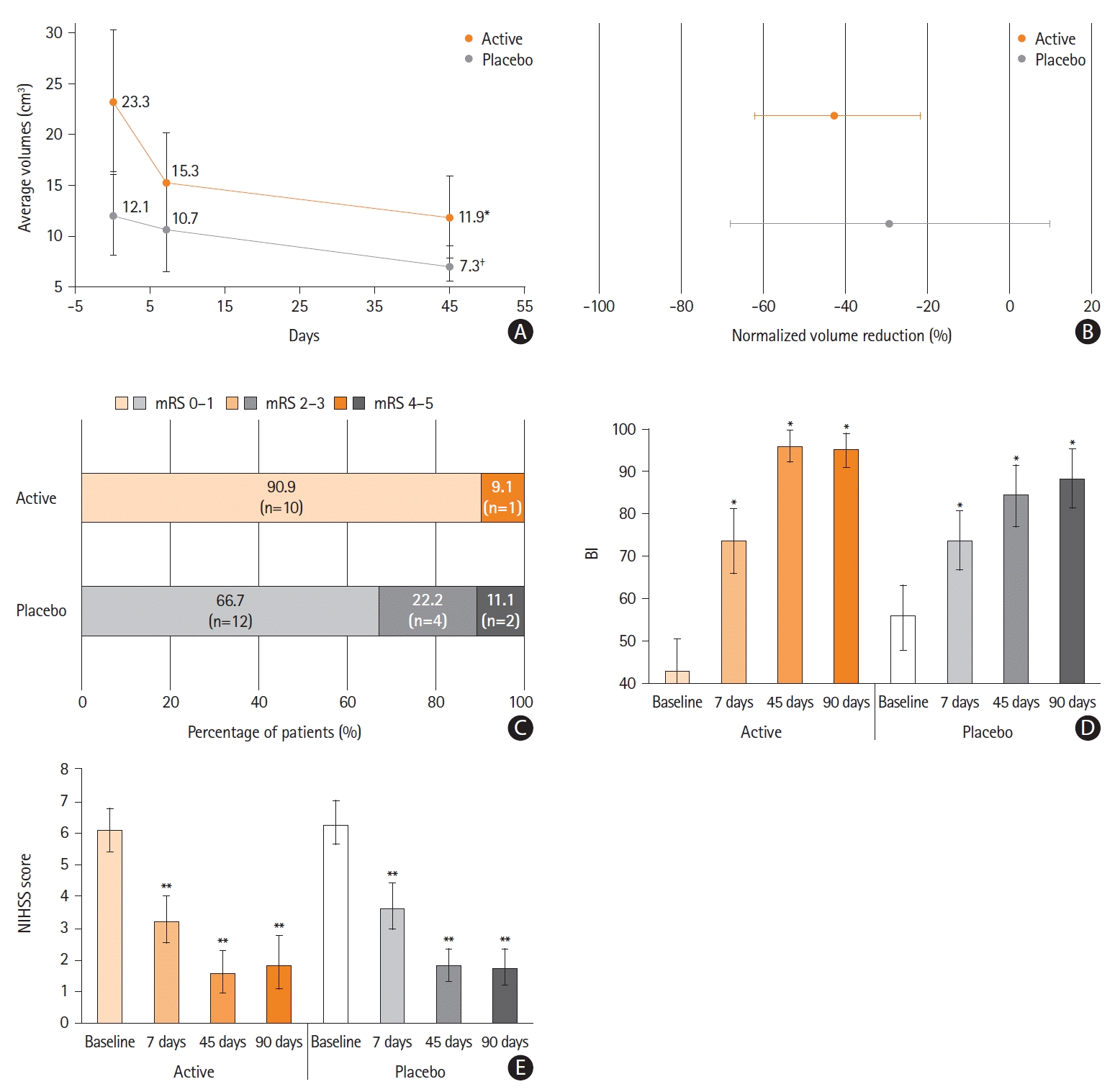
Supplementary Table 2). The average lesion volume decreased from 23.3±25.3 cm
3 to 11.9±12.8 cm
3 in the active group (
P=0.023), and from 12.1±17.7 cm
3 to 7.3±7.5 cm
3 in the placebo group (
P=0.065) (
Figure 2A and B,
Supplementary Figure 1).
Clinical scores, Barthel Index (BI), modified Rankin Scale (mRS) scores, and National Institutes of Health Stroke Scale (NIHSS) scores showed significant improvements at 7, 45, and 90 days compared with baseline in both groups (
Figure 2C-E and
Supplementary Table 3). Excellent outcome at 90 days (defined as a score on the mRS of 0 or 1) was achieved in 10 out of 11 patients (90.9%) in the active group and in 12 out of 18 patients (66.7%) in the placebo group (
Figure 2C).
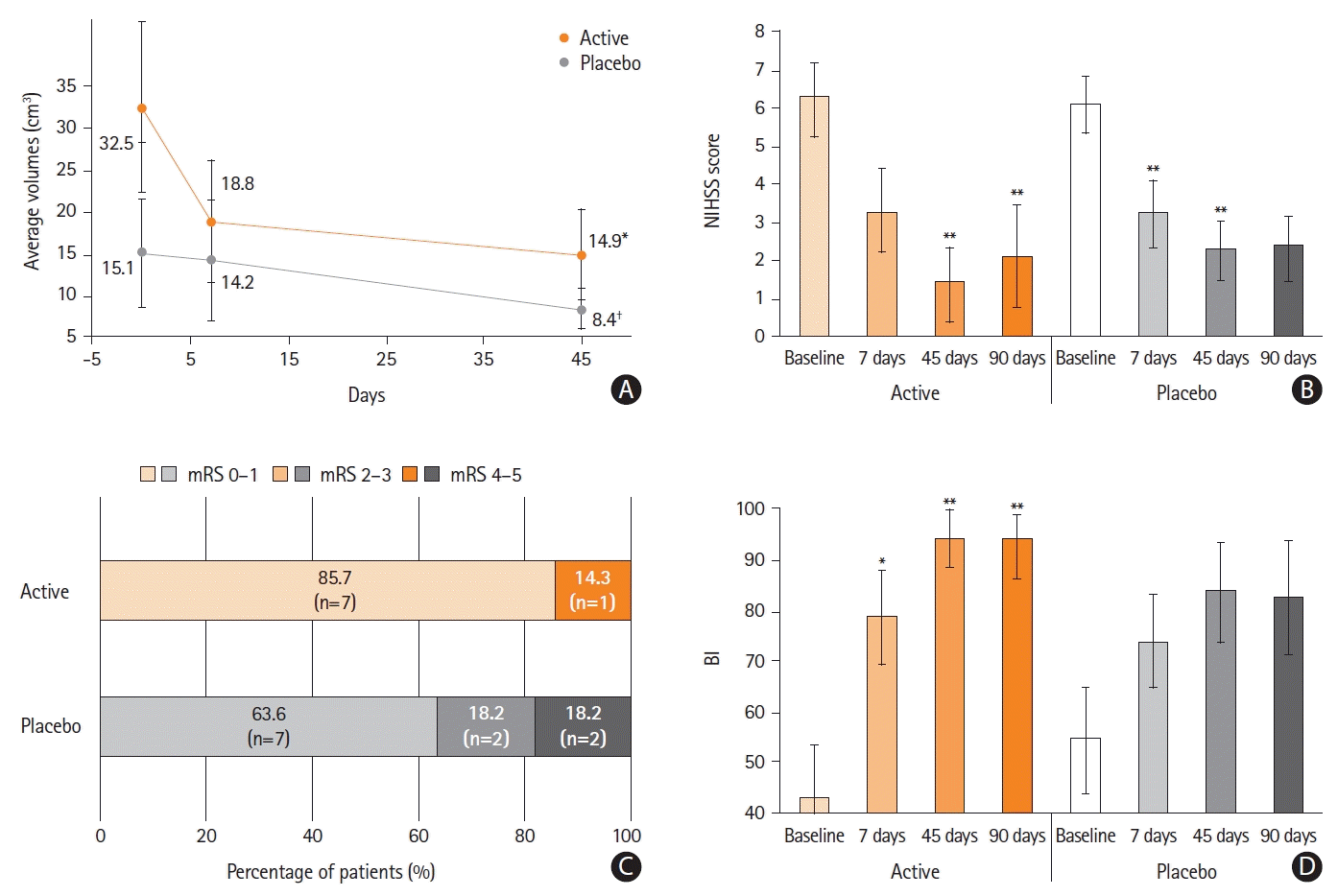
Twenty patients underwent reperfusion therapies: 12 in the placebo group and eight in the active group. PEMF treatment resulted in significant MRI volume reduction compared with baseline in the active group only (
P=0.04) (
Figure 3); and the average normalized MRI volume reduction at 45 days over baseline was 50.0% in the active group and 22.7% in the placebo group, with a significant difference between the groups (
P=0.04).
The clinical scores at 7, 45, and 90 days improved from baseline in both groups. Notably, the improvements in clinical scores were larger and earlier in the active group than in the placebo group, in line with the volumetric changes in the lesions. MRI volume reduction and BI clinical score improvement were the largest in the active group in the first 7 days after AIS, that is, when PEMF treatment was delivered. Moreover, the BI score reached the widest recovery as early as 45 days in the active group compared to 90 days in the placebo group.
Among patients who underwent reperfusion treatment, the BI score improved from 42.5±30.9 at baseline to 92.9±16.8 (P<0.01) at 90 days in the active group; whilst in the placebo group, BI increased from 54.2±37.2 at baseline to 82.7±37.2 (P=0.10) at 90 days.
The safety of PEMFs was carefully monitored during treatment; no severe adverse events that would require treatment interruption occurred (
Supplementary Table 4). At follow-up visits, the patients did not report any side effects that could be attributed to the PEMF treatment.
Our results show that PEMF treatment is safe, well tolerated, and efficiently deployed in stroke units. It is performed at the patient’s bed (
Supplementary Figures 2 and
3), does not require dedicated infrastructure or specialized personnel, does not extend the length of hospital stay, and costs are expected to be contained.
In summary, the instrumental and clinical results of the I-NIC study showed that PEMF treatment protects the central nervous system following ischemic stroke. At 45 days, the area of neuronal sufferance identified by MRI was significantly reduced over baseline in the actively treated patients only (
P=0.02), whereas no statistically significant reduction was observed in the placebo group (
Figure 2B and
Supplementary Figure 1).
Among patients receiving reperfusion therapy, PEMF treatment favored MRI volume reduction and clinical amelioration, showing that the treatments can be successfully combined to prevent the enlargement of structural damage.
The present study has limitations: (1) PEMF treatment was restricted to patients with lesions located in the MCA territory; (2) the difference in average lesion volume at baseline between the groups was wide; and (3) the low number of patients included, resulting from the stringent inclusion criteria and the clinical protocol request for three MRI exams over 45 days. Then, the COVID-19 pandemic further slowed enrolment and access to follow-up visits.
In the interim analysis, the primary endpoint of the study was reached and the safety and tolerability of PEMF treatment were proven; therefore, the study was interrupted.
This is the first human clinical trial to use PEMF treatment to limit neuronal damage in patients with ischemic stroke. Our results show that PEMF treatment should be considered to reduce the neuronal damage occurring in the “penumbra,” offering to clinicians the opportunity to extend the time for intervention to the first week after ischemic stroke.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download