Abstract
Background
ABO-incompatible (ABOi) kidney transplantation poses significant challenges in achieving successful outcomes. This study aimed to investigate the impact of various interventions and techniques on improving the success rates of ABOi kidney transplantation.
Methods
We conducted a retrospective observational analysis of patients who underwent ABOi kidney transplantation from November 2012 to March 2023. The study included a total of 105 patients. We collected and analyzed data on patient demographics, preoperative assessments, surgical details, and postoperative outcomes.
Results
The mean ages of the donors and recipients were 50.52±10.32 and 36.63±11.61 years, respectively. The majority of recipients were male (81.9%), while most donors were female (89.5%). The most common blood group among recipients was O (69.5%), and among donors, it was B (46.7%). The median durations of chronic kidney disease and dialysis were 12 months (interquartile range [IQR], 7–28 months) and 6 months (IQR, 2–12 months), respectively. Baseline antibody titers (anti-A and anti-B) ranged from 64.0 to 256.0, while on the day of surgery, they were ≤8. Perioperative complications included hypotension (10.5%), acute tubular necrosis (5.7%), delayed graft function (3.8%), and reexploration (3.8%) due to hematoma.
Conclusions
ABOi kidney transplantation is a viable option for recipients lacking available donors with an ABO-compatible match. Perioperative concerns, including hypoalbuminemia, heightened risk of infections, coagulopathies, aseptic precautions, and immunological surveillance, must be carefully addressed.
Historically, ABO incompatibility between a living kidney donor and the recipient was considered a contraindication for kidney transplantation [1]. However, advancements in immunosuppression and desensitization protocols over recent decades have made ABO-incompatible (ABOi) kidney transplantation a viable option for individuals with end-stage kidney disease [2].
ABOi kidney transplantation requires careful consideration and thorough planning to ensure the success of the procedure and to minimize complications during the perioperative period [3,4]. This approach demands close collaboration among the transplant team, blood bank, and immunology specialists, highlighting the need for a multidisciplinary strategy.
Different aspects of perioperative management, such as desensitization protocols, monitoring, optimization of immunosuppressive regimens, and management of antibody-mediated rejection, have become active areas of research. Specifically, preparing the recipient for ABOi transplantation through various desensitization protocols adds complexity to their perioperative care and increases the risk of complications, presenting daily challenges for anesthesiologists in transplant units. A thorough understanding of these key aspects of perioperative management can enhance surgical outcomes and improve the quality of life for these patients [5]. Previous studies on perioperative outcomes in ABOi kidney transplantation have yielded varied conclusions across different geographic regions. Our study focused on the ABOi kidney transplantations performed at our tertiary care center and aimed to provide insights into this under-researched area, drawing on our experience.
This retrospective observational study was conducted with approval from the Institutional Ethics Committee of Mahatma Gandhi Medical College and Hospital (No. MGMC&H/IEC/JPR/2023/1543, dated 19/05/2023). Informed consent was waived due to the retrospective nature of the study.
We included 105 ABOi recipients who underwent kidney transplantation at our institute from November 2012 to March 2023. The medical records of these patients, including preanesthesia checkups, intraoperative anesthesia and surgery notes, and postoperative charts, were thoroughly examined to collect perioperative data.
Preoperative demographic characteristics—including age, sex, duration of chronic kidney disease, duration of hemodialysis/peritoneal dialysis, presence of an arteriovenous fistula, and whether the donor was live or cadaveric—along with comorbidities such as hypertension, diabetes mellitus, pulmonary tuberculosis, hypothyroidism, and chronic obstructive pulmonary disease, were retrieved from patient records. Laboratory parameters, including blood tests, kidney and liver function tests, coagulation parameters, and serum biochemistry, were also collected. In the anesthesia approach, drugs were administered for both induction and maintenance. Intraoperative data, including ischemia times (warm, cold, and rewarming), requirements for intravenous fluids, albumin, blood, and blood loss, were reviewed. Perioperative complications, such as arrhythmias, psychosis, pulmonary edema, hypoglycemia, hypotension, acute rejection, acute tubular necrosis (ATN), delayed graft function (DGF), and reexploration due to hematoma, were recorded from operating room and post-kidney transplant unit notes.
Statistical analysis was conducted using Prism GraphPad for Mac, ver. 8 (GraphPad Software). Data were presented as mean±standard deviation. The unpaired t-test was used to compare continuous variables, while the chi-square test or Fisher exact test was used for categorical values. Survival analysis was performed using the Kaplan-Meier method, and group comparisons were made using the log-rank test. A P-value <0.05 was considered statistically significant.
From November 2012 to March 2023, our center performed a total of 1,700 kidney transplants, including ABO-compatible, ABOi, paired, human leucocyte antigen-incompatible, and deceased donor grafts (Fig. 1).
In our study population, the average age of recipients was 36.63±11.61 years, compared to 50.52±10.32 years for donors. The majority of recipients were male (81.9%), while most donors were female (89.5%). The most common blood group among recipients was O (69.5%), and among donors, it was B (46.7%). Among the patients, 11 (10.5%) received preemptive transplants, and four (3.8%) had undergone a previous kidney transplant. The median duration of chronic kidney disease and dialysis was 12 months (interquartile range [IQR], 7–28 months) and 6 months (IQR, 2–12 months), respectively. Among donors, 67 (63.8%) were first-degree relatives, and 38 (36.2%) were second-degree relatives. Among recipients, seven (6.7%) had a history of previous pregnancy, and 16 (15.2%) had a history of previous blood transfusion. The recorded comorbidities among recipients included hypertension (90.5%), diabetes mellitus (8.6%), hypothyroidism (3.8%), and pulmonary tuberculosis (11.4%) (Table 1).
On the day of the transplant, the mean blood urea level was 114.21 mg/dL, and the serum creatinine level was 10.27 mg/dL. Coagulation parameters, including thromboelastography, were within the normal range. The baseline antibody titer (anti-A and anti-B) ranged from 64 to 256, but on the day of surgery, it decreased to ≤8. The median number of plasmapheresis cycles required to achieve the target isoagglutinin titer was 4, ranging from 3 to 6. Additionally, the median number of units of fresh frozen plasma (FFP) transfused was 20, with a range of 16 to 26 (Table 2).
Recipients of ABOi transplants received a single 200 mg dose of rituximab approximately 21 days prior to the scheduled transplantation. Antibody removal was achieved through plasmapheresis conducted on alternate days, aiming for a 1:8 immunoglobulin G antibody titer before the kidney transplantation. One week before the transplant, patients began taking oral tacrolimus (0.05 mg/kg in two divided doses) and mycophenolate sodium (720 mg twice daily). The target tacrolimus trough level before the transplant was set at 8–12 ng/mL. During the intraoperative phase, a 500 mg dose of intravenous methylprednisolone was administered. Additionally, basiliximab (20 mg) was used as an induction agent on the day of surgery and again on postoperative day 4. Immediately following the transplant, oral prednisolone was initiated at a dosage of 40 mg/day, which was gradually tapered to 20 mg/day by the time of discharge.
The mean warm ischemia time, cold ischemia time, and rewarming ischemia time were 3.11, 49.83, and 43.15 minutes, respectively. The average intraoperative blood loss was approximately 300 mL. Only seven patients (6.7%) required an intraoperative blood transfusion, while albumin was administered to 44 patients (41.9%) due to low preoperative values. The intraoperative requirements for normal saline and balanced salt solution were 1,050 mL and 865 mL, respectively (Table 3). The perioperative complications observed included arrhythmias, psychosis, pulmonary edema, hypoglycemia, hypotension, acute rejection, ATN, DGF, and reexploration due to hematoma (Fig. 2).
Over the past few decades, the landscape of ABOi kidney transplantation has seen significant developments. Initially considered a contraindication, ABOi transplantation now accounts for up to one-third of kidney transplants in countries such as Japan [6]. This growing popularity is due to a better understanding of the immunological mechanisms behind hyperacute rejection, the phenomenon of accommodation, and the introduction of newer immunosuppressive medications. These advancements have not only led to successful outcomes but have also broadened the scope of kidney transplantation in ABOi cases.
In India, the limited scope of the deceased donor program—attributable to factors such as insufficient public awareness, social and cultural taboos, disinterest among local organizations, and inadequate recognition and management by caregivers—coupled with the lack of national-level registry data, has led to a heightened recognition of ABOi transplantation as a viable alternative in recent years.
One of the major factors affecting ABOi kidney transplants in developing countries, such as India, is the limited donor pool, which predominantly consists of female donors. ABOi kidney transplantation can expand the pool of potential donors by enabling organ transplants across blood group barriers [7]. The removal of antibodies against donor antigens is essential to prevent hyperacute rejection and ensure a successful outcome. Some well-established techniques used globally include plasma exchange, double filtration plasmapheresis, rituximab, splenectomy, and immunoadsorption. However, the overall expenses may increase significantly depending on the chosen therapy [8]. The duration and type of antibody reduction protocol may vary among transplant centers, which can influence postoperative graft survival. Nonetheless, the benefits of these procedures, in terms of improved patient outcomes and an expanded donor pool, outweigh the costs.
At our center, recipients undergo plasmapheresis to achieve the desired antibody titer of 1:8 (anti-A and/or anti-B). Plasmapheresis results in a significant loss of serum proteins, which necessitates replacement with substantial volumes of either FFP or albumin. Hypoproteinemia, the removal of protective antibodies during plasmapheresis, and the administration of FFP increase the risk of perioperative infections [9,10]. Despite the high susceptibility to bacterial, viral, and fungal infections during surgery, ABOi recipients may not show typical clinical signs and symptoms of infection, such as fever or an increased white blood cell count, due to intense immunosuppression. Therefore, stringent aseptic precautions are crucial during transplantation, and invasive procedures should be minimized [11].
Sometimes, there is inadequate time to optimize recipients in the preoperative period, and we must accept them in suboptimal conditions due to the high risk of titer rebound if the procedure is postponed. It is well-known that plasmapheresis leads to the depletion of fibrinogen and other clotting factors [12], which can increase the risk of perioperative bleeding and complications related to blood transfusions. To restore blood volume, leukodepleted packed red blood cells from the same blood group should be transfused, and coagulopathies can be addressed with FFP (either donor-specific or AB group), which lacks antibodies against the donor antigen [13].
Effective pain management during the perioperative period is crucial for kidney transplants to reduce complications and improve recovery outcomes. In ABOi kidney transplants, additional precautions are necessary when employing neuraxial pain control techniques such as epidural, intrathecal, or fascial plane blocks, due to the increased risk of hematoma associated with coagulation abnormalities [14,15]. Conducting additional tests, such as a thromboelastogram when available, can be beneficial to identify any preexisting coagulopathy on the day of surgery, thereby minimizing risks [16].
The limitations of our study include its retrospective nature, the short duration of follow-up, and the absence of a comparative group. ABOi kidney transplantation is a viable option for recipients who lack available donors with an ABO-compatible match. Perioperative concerns, including hypoalbuminemia, heightened infection risk, coagulopathies, aseptic precautions, and immunological surveillance, must be carefully addressed.
ARTICLE INFORMATION
Author Contributions
Conceptualization: PS, NK. Data curation: PS, VKG, SM (Saurabh Mittal). Formal analysis: all authors. Investigation: PS, SM (Sony Mandal). Methodology: PS, VKG, SM (Saurabh Mittal), NK, AM. Writing–original draft: PS. Writing–review & editing: all authors. All authors read and approved the final manuscript.
REFERENCES
1. Opelz G, Morath C, Süsal C, Tran TH, Zeier M, Döhler B. 2015; Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation. 99:400–4. DOI: 10.1097/TP.0000000000000312. PMID: 25050471.
2. Tydén G, Donauer J, Wadström J, Kumlien G, Wilpert J, Nilsson T, et al. 2007; Implementation of a protocol for ABO-incompatible kidney transplantation - a three-center experience with 60 consecutive transplantations. Transplantation. 83:1153–5. DOI: 10.1097/01.tp.0000262570.18117.55. PMID: 17496528.
3. Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. 2012; Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 93:603–9. DOI: 10.1097/TP.0b013e318245b2af. PMID: 22290268. PMCID: PMC3299822.
4. Naciri Bennani H, Abdulrahman Z, Allal A, Sallusto F, Delarche A, Game X, et al. 2016; Early post-transplant complications following ABO-incompatible kidney transplantation. J Nephropathol. 5:19–27. DOI: 10.15171/jnp.2016.04. PMID: 27047806. PMCID: PMC4790183.
5. Goyal VK, Solanki SL, Baj B. 2018; Pulmonary hypertension and post-operative outcome in renal transplant: a retrospective analysis of 170 patients. Indian J Anaesth. 62:131–5. DOI: 10.4103/ija.IJA_529_17. PMID: 29491519. PMCID: PMC5827480.
6. Okumi M, Toki D, Nozaki T, Shimizu T, Shirakawa H, Omoto K, et al. 2016; ABO-incompatible living kidney transplants: evolution of outcomes and immunosuppressive management. Am J Transplant. 16:886–96. DOI: 10.1111/ajt.13502. PMID: 26555133.
7. Shin M, Kim SJ. 2011; ABO incompatible kidney transplantation-current status and uncertainties. J Transplant. 2011:970421. DOI: 10.1155/2011/970421. PMID: 22174989. PMCID: PMC3235893.
8. Donauer J, Wilpert J, Geyer M, Schwertfeger E, Kirste G, Drognitz O, et al. 2006; ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a single center experience. Xenotransplantation. 13:108–10. DOI: 10.1111/j.1399-3089.2006.00293.x. PMID: 16623802.
9. de Weerd AE, Betjes MG. 2018; ABO-incompatible kidney transplant outcomes: a meta-analysis. Clin J Am Soc Nephrol. 13:1234–43. DOI: 10.2215/CJN.00540118. PMID: 30012630. PMCID: PMC6086717.
10. Scurt FG, Ewert L, Mertens PR, Haller H, Schmidt BM, Chatzikyrkou C. 2019; Clinical outcomes after ABO-incompatible renal transplantation: a systematic review and meta-analysis. Lancet. 393:2059–72. DOI: 10.1016/S0140-6736(18)32091-9. PMID: 31006573.
11. Habicht A, Bröker V, Blume C, Lorenzen J, Schiffer M, Richter N, et al. 2011; Increase of infectious complications in ABO-incompatible kidney transplant recipients--a single centre experience. Nephrol Dial Transplant. 26:4124–31. DOI: 10.1093/ndt/gfr215. PMID: 21622990.
12. Zöllner S, Pablik E, Druml W, Derfler K, Rees A, Biesenbach P. 2014; Fibrinogen reduction and bleeding complications in plasma exchange, immunoadsorption and a combination of the two. Blood Purif. 38:160–6. DOI: 10.1159/000367682. PMID: 25501972.
13. Chung Y, Ko DH, Lim J, Kim KH, Kim H. 2022; Choice of ABO group for blood component transfusion in ABO-incompatible solid organ transplantation: a questionnaire survey in Korea and guideline proposal. Ann Lab Med. 42:105–9. DOI: 10.3343/alm.2022.42.1.105. PMID: 34374356. PMCID: PMC8368232.
14. Goyal VK, Mandal S, Nimje GR, Shekhrajka P, Rana PS, Mittal S. 2023; Acute pain management after kidney transplantation: a current review of literature. Indian J Transpl. 17:402–9. DOI: 10.4103/ijot.ijot_49_23.
15. Mittal S, Goyal VK, Shekhrajka P, Bhardwaj M, Nimje GR, Singh P, et al. 2023; Role of intrathecal morphine for acute postoperative pain management in patients undergoing kidney transplant: a randomized controlled study. Exp Clin Transplant. 21:939–45. DOI: 10.6002/ect.2023.0063. PMID: 38263780.
16. Baj BB, Goyal VK, Shekhrajka P, Nimje GR, Mittal S. 2023; Perioperative management of a severely thrombocytopenic kidney transplant recipient using thromboelastography. Med J Armed Forces India. 79:718–21. DOI: 10.1016/j.mjafi.2023.01.011. PMID: 37981936. PMCID: PMC10654360.
Fig. 1
Study design. ABOc, ABO-compatible; ABOi, ABO-incompatible; HLAi, human leukocyte antigen-incompatible.
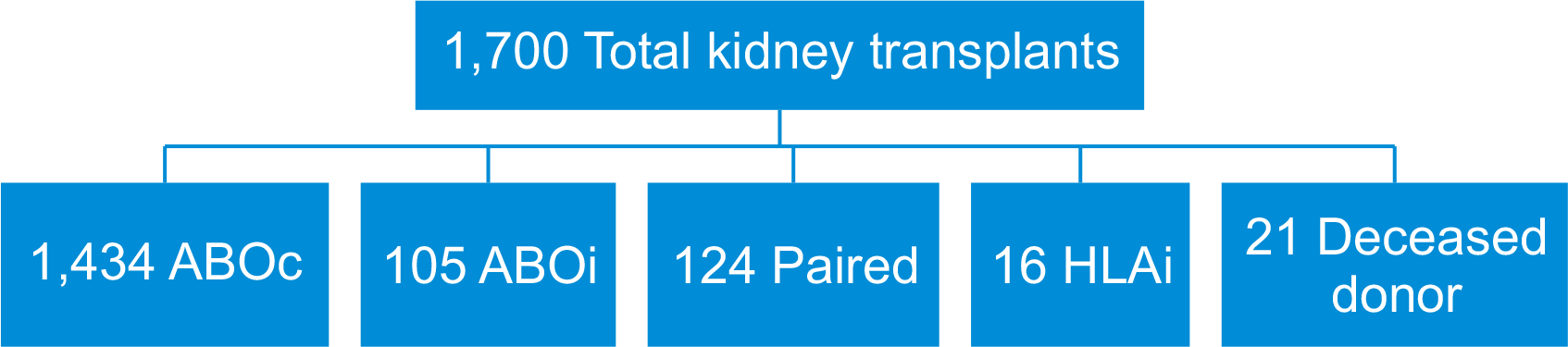
Fig. 2
Perioperative complications. AF, atrial fibrillation; ATN, acute tubular necrosis; DGF, delayed graft function.
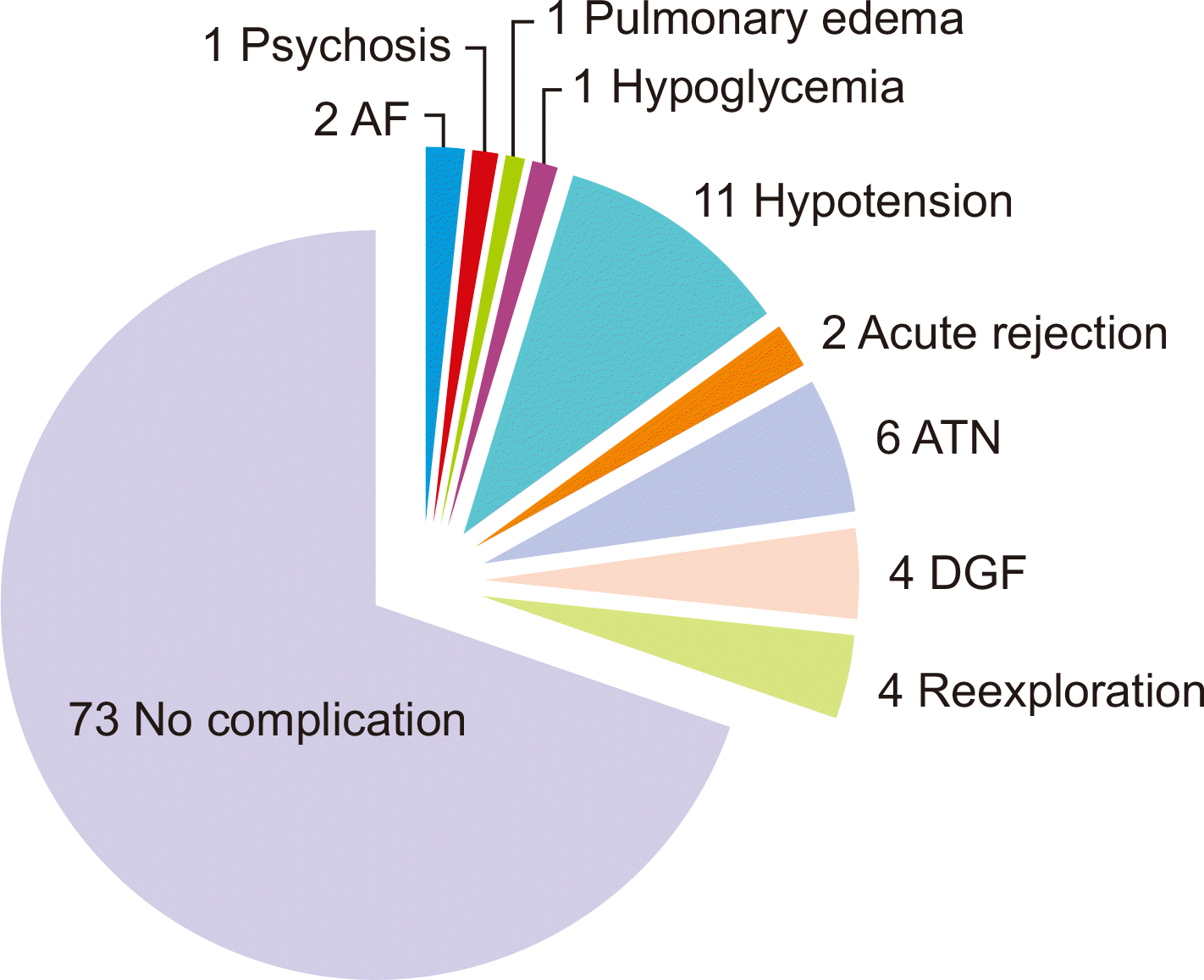
Table 1
Demographic variables of donors and recipients
Table 2
Recipients’ laboratory parameters on the day of surgery




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download