Abstract
Background/Aims
Methods
Results
Conclusions
Notes
Funding Source
This work was supported by the National Key R&D Program of China (2023YFC2507300), National High-Level Hospital Clinical Research Funding (2022-PUMCH-B-022, 2022-PUMCHC- 018, 2022-PUMCH-A-074, 2022-PUMCH-A-179), the Capital Health Research and Development of Special Foundation (2022-2-4014), CAMS Innovation Fund for Medical Sciences (2022-I2M-C&T-B-011), National Natural Science Foundation of China (81970495), National Key Clinical Specialty Construction Project (ZK108000), and Peking Union Medical College Teaching Reform in Undergraduate Education (2023zlgl008).
Conflict of Interest
Qian J is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
Conceptualization: Zhan W, Bai X, Yang H, Qian J. Data curation: Zhan W, Bai X, Yang H. Formal analysis: Zhan W. Funding acquisition: Yang H, Qian J. Investigation: Zhan W. Methodology; Project administration; Resources: Zhan W, Bai X, Yang H, Qian J. Software: Zhan W. Supervision: Bai X, Yang H, Qian J. Validation: Zhan W, Bai X, Yang H, Qian J. Visualization: Zhan W, Bai X. Writing - original draft: Zhan W. Writing - review & editing: Zhan W, Bai X, Yang H, Qian J. Approval of final manuscript: all authors.
Supplementary Material
Supplementary Table 1.
Supplementary Fig. 1.
Supplementary Fig. 2.
REFERENCES
Fig. 1.
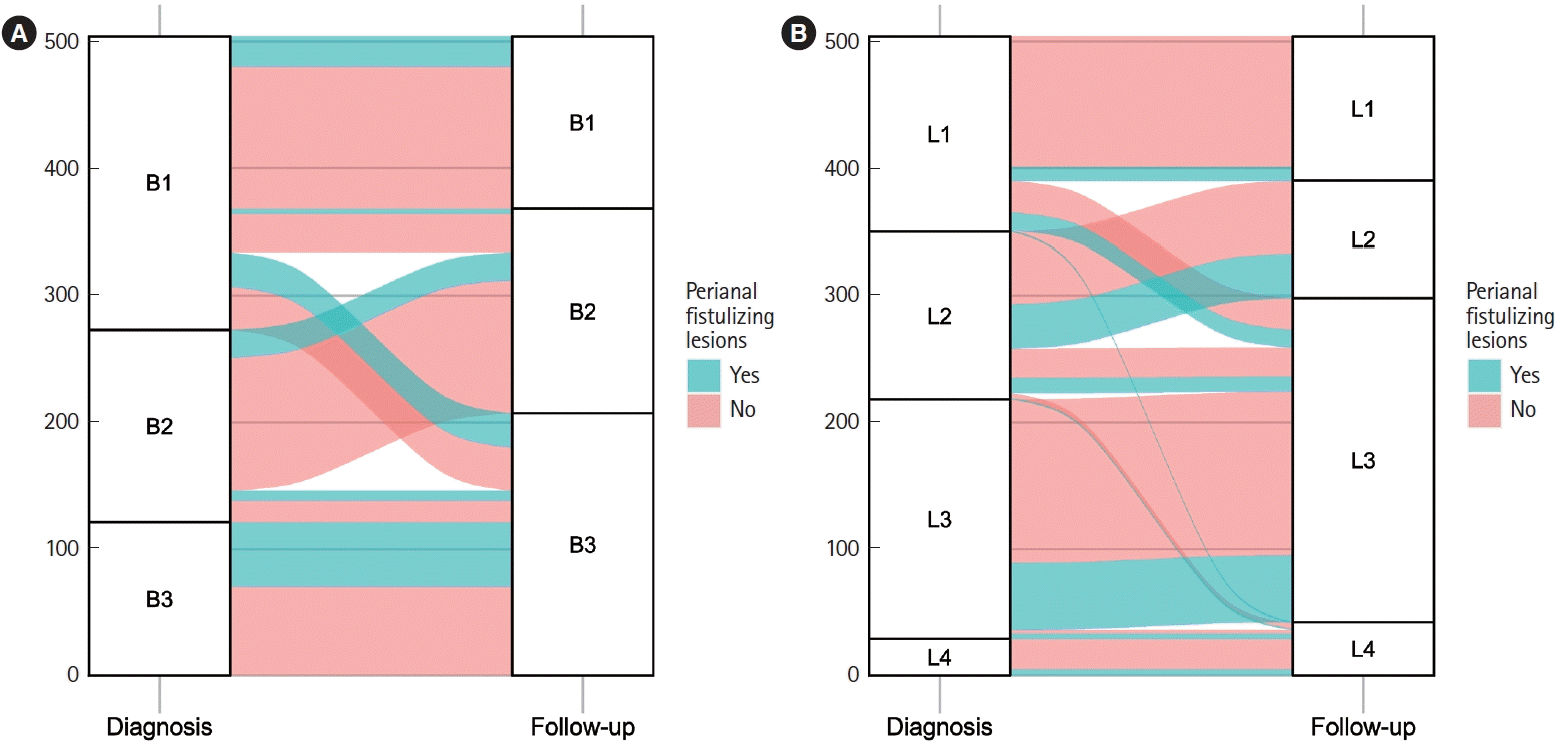
Fig. 2.
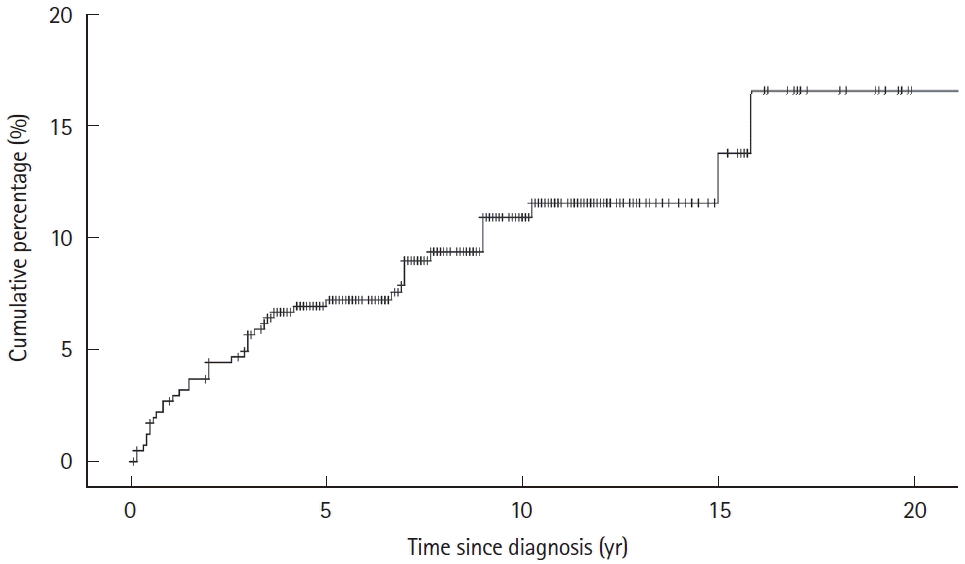
Fig. 3.
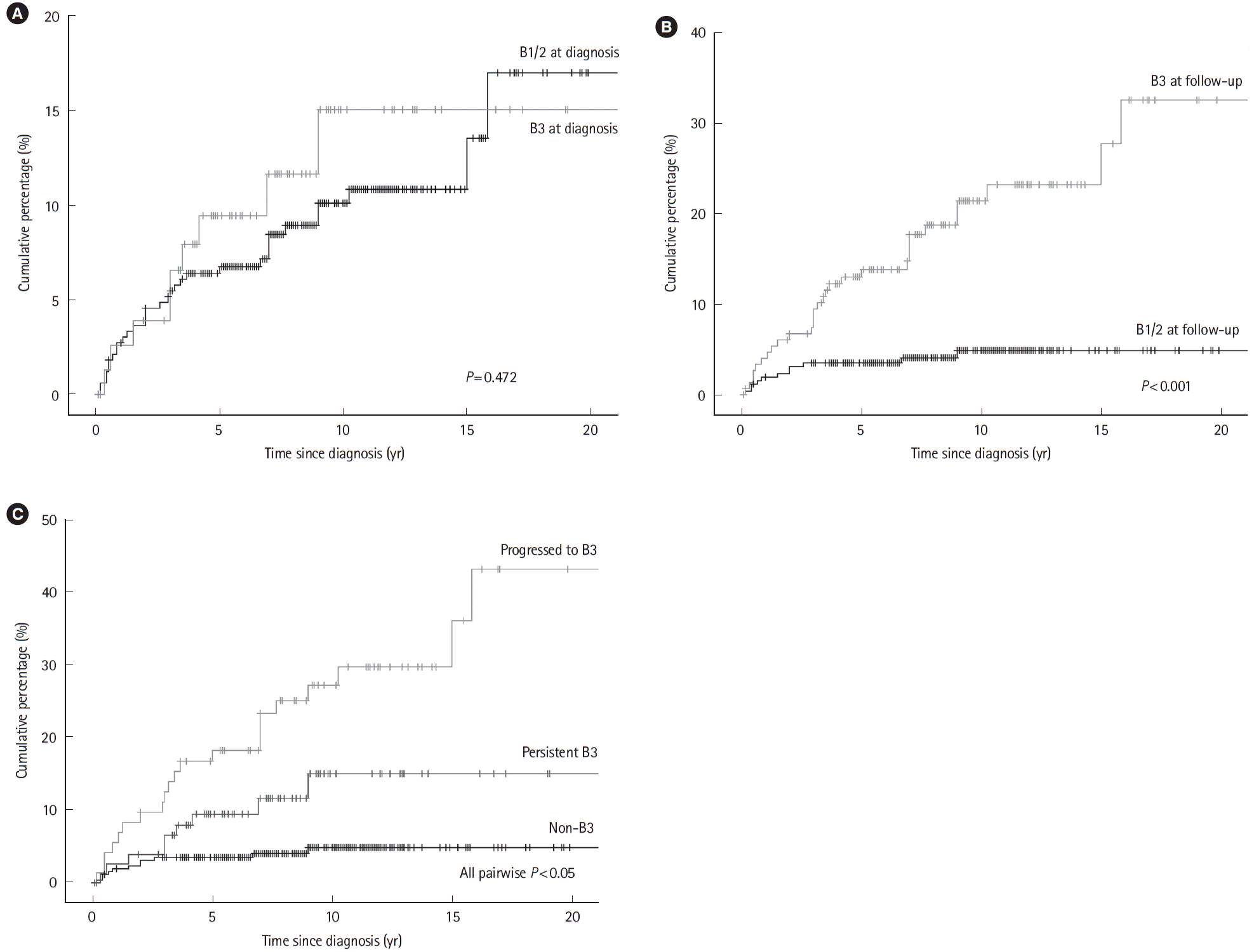
Table 1.
| Characteristic | Perianal lesions, No. (%) | No perianal lesion, No. (%) | P-value |
|---|---|---|---|
| Sex | < 0.001 | ||
| Male | 110 (80.9) | 239 (64.9) | |
| Female | 26 (19.1) | 129 (35.1) | |
| Smoking history | 0.740 | ||
| Never | 107 (78.7) | 287 (78.0) | |
| Former | 16 (11.8) | 38 (10.3) | |
| Ongoing | 13 (9.6) | 43 (11.7) | |
| A classificationa | < 0.001 | ||
| A1 | 19 (14.0) | 28 (7.6) | |
| A2 | 98 (72.1) | 215 (58.4) | |
| A3 | 19 (14.0) | 125 (34.0) | |
| L classification at diagnosisa | 0.001 | ||
| L1 | 26 (19.1) | 128 (34.8) | |
| L2 | 48 (35.3) | 84 (22.8) | |
| L3 | 57 (41.9) | 132 (35.9) | |
| L4 | 5 (3.7) | 24 (6.5) | |
| L classification at follow-upa | < 0.001 | ||
| L1 | 11 (8.1) | 103 (28.0) | |
| L2 | 35 (25.7) | 57 (15.5) | |
| L3 | 79 (58.1) | 177 (48.1) | |
| L4 | 11 (8.1) | 31 (8.4) | |
| B classification at diagnosisa | < 0.001 | ||
| B1 | 55 (40.4) | 176 (47.8) | |
| B2 | 30 (22.1) | 122 (33.2) | |
| B3 | 51 (37.5) | 70 (19.0) | |
| B classification at follow-upa | < 0.001 | ||
| B1 | 24 (17.6) | 112 (30.4) | |
| B2 | 26 (19.1) | 135 (36.7) | |
| B3 | 86 (63.2) | 121 (32.9) | |
| Disease activity at diagnosisb | 0.010 | ||
| Remission | 17 (12.5) | 59 (16.0) | |
| Mild | 30 (22.1) | 80 (21.7) | |
| Moderate | 53 (39.0) | 177 (48.1) | |
| Severe | 36 (26.5) | 52 (14.1) | |
| Overall extraintestinal manifestation | 77 (56.6) | 172 (46.7) | 0.049 |
| Overall medical therapy | |||
| Corticosteroid | 110 (80.9) | 270 (73.4) | 0.082 |
| Immunomodulator | 77 (56.6) | 203 (55.2) | 0.771 |
| Biologic | 51 (37.5) | 54 (14.7) | < 0.001 |
| Overall severe complications | |||
| Obstruction | 30 (22.1) | 124 (33.7) | 0.012 |
| Perforation | 46 (33.8) | 105 (28.5) | 0.250 |
| Massive gastrointestinal bleeding | 15 (11.0) | 47 (12.8) | 0.597 |
| Overall Crohn’s disease-related surgery | 36 (26.5) | 175 (47.6) | < 0.001 |
a Subgroups were categorized according to the Montreal classification: age classification (A1, ≤16 yr; A2, 17-40 yr; A3, >40 yr); location classification (L1, ileal; L2, colonic; L3, ileocolonic; L4, isolated upper disease), and behavior classification (B1, non-structuring, non-penetrating; B2, structuring; B3, penetrating). [20]
b Subgroups were categorized according to the Harvey-Bradshaw Index. [19]
Table 2.
Table 3.
| Variable | Hazard ratio (95% CI) | P-value |
|---|---|---|
| B3 trendsa | < 0.001 | |
| Non-B3 | (Ref.) | |
| Progressed to B3 | 9.90 (4.60–21.33) | < 0.001 |
| Initial B3 | 4.72 (1.91–11.66) | 0.001 |
| CD-related surgery before perianal fistulizing lesions | 0.08 (0.03–0.20) | < 0.001 |
| Immunomodulator therapy before perianal fistulizing lesions | 0.42 (0.21–0.84) | 0.014 |
| Disease activity at diagnosis | 0.024 | |
| Remission | (Ref.) | |
| Mild | 2.11 (0.23–19.19) | 0.506 |
| Moderate | 6.29 (0.84–47.14) | 0.073 |
| Severe | 5.59 (0.70–44.47) | 0.104 |
| Extraintestinal manifestation | 2.03 (1.04–3.93) | 0.037 |
a B3 refers to penetrating behavior according to the Montreal classification. [20]
Table 4.
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| B3 trendsa | 0.002 | |
| Non-B3 | (Ref.) | |
| Progressed to B3 | 0.16 (0.05–0.54) | 0.003 |
| Persistent B3 | 1.13 (0.41–3.13) | 0.817 |
| Disease activity at diagnosis | 0.005 | |
| Remission | (Ref.) | |
| Mild | 8.96 (1.20–66.72) | 0.032 |
| Moderate | 0.47 (0.12–1.78) | 0.266 |
| Severe | 1.17 (0.26–5.18) | 0.840 |
| L classification at follow-upb | 0.935 | |
| L1 | (Ref.) | |
| L2 | 0.84 (0.16–4.58) | 0.842 |
| L3 | 1.16 (0.24–5.65) | 0.853 |
| L4c | - | - |
a B3 refers to penetrating behavior according to the Montreal classification [20]
b Subgroups were categorized according to the Montreal classification: location classification (L1, ileal; L2, colonic; L3, ileocolonic; L4, isolated upper disease). [20]
Table 5.
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| B3 trendsa | 0.008 | |
| Non-B3 | (Ref.) | |
| Progressed to B3 | 0.91 (0.25–3.26) | 0.882 |
| Persistent B3 | 3.91 (1.36–11.23) | 0.011 |
| L classification at follow-upb | 0.061 | |
| L1 | (Ref.) | |
| L2 | 0.29 (0.05–1.67) | 0.166 |
| L3 | 0.69 (0.15–3.26) | 0.641 |
| L4 | 2.67 (0.37–19.47) | 0.333 |
| Crohn’s disease-related surgery | 0.38 (0.13–1.09) | 0.072 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download