Abstract
Background/Aims
Methods
Results
Conclusions
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Con D declares no conflicts of interest. De Cruz P has received research funding from Janssen, AbbVie, Takeda, Shire and Ferring. De Cruz P has been on advisory boards and received speaker fees from Janssen, AbbVie, Takeda, Celltrion, Shire, Ferring and Pfizer. De Cruz P is supported by a National Health and Medical Research Council (NHMRC) Emerging Leader Fellowship.
REFERENCES
Fig. 1.
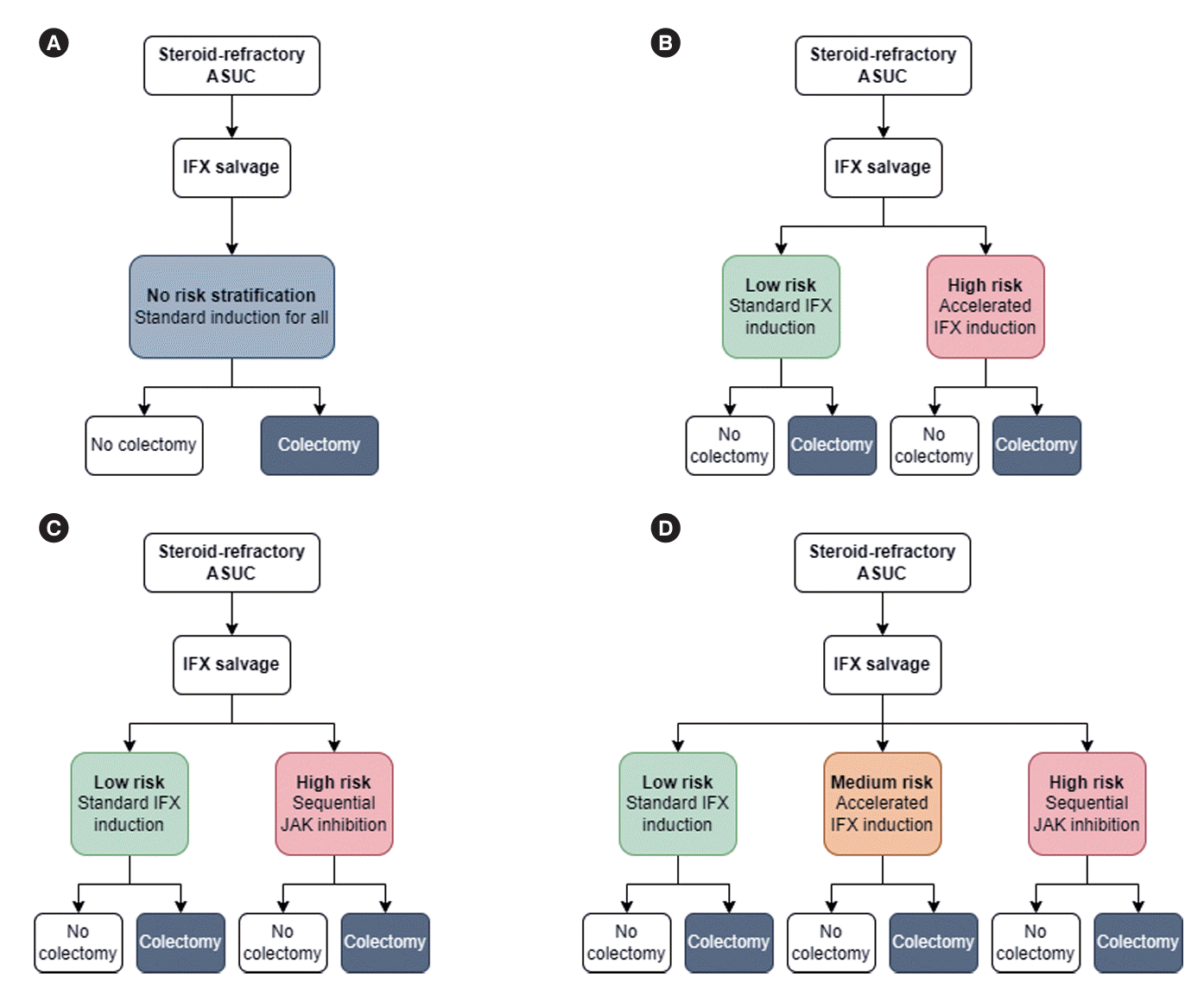
Fig. 2.
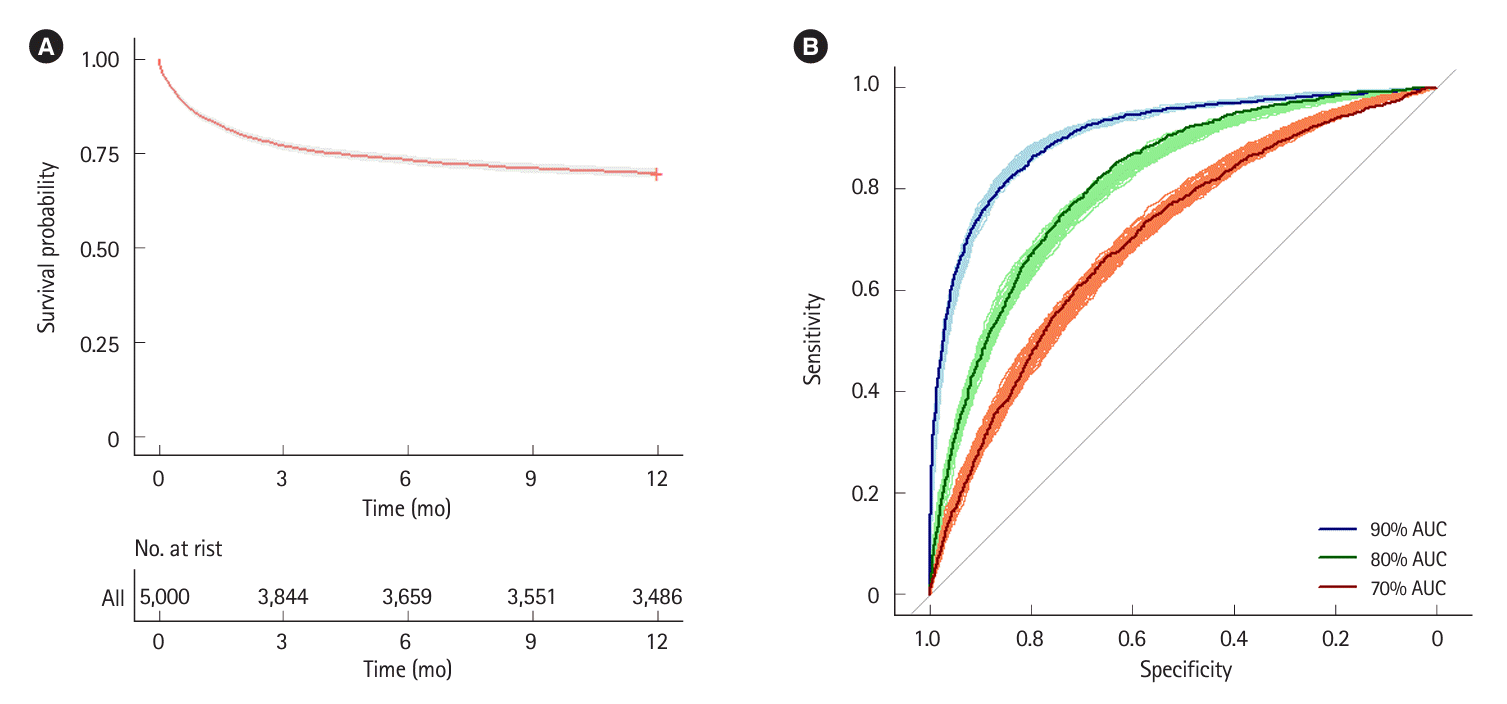
Fig. 3.
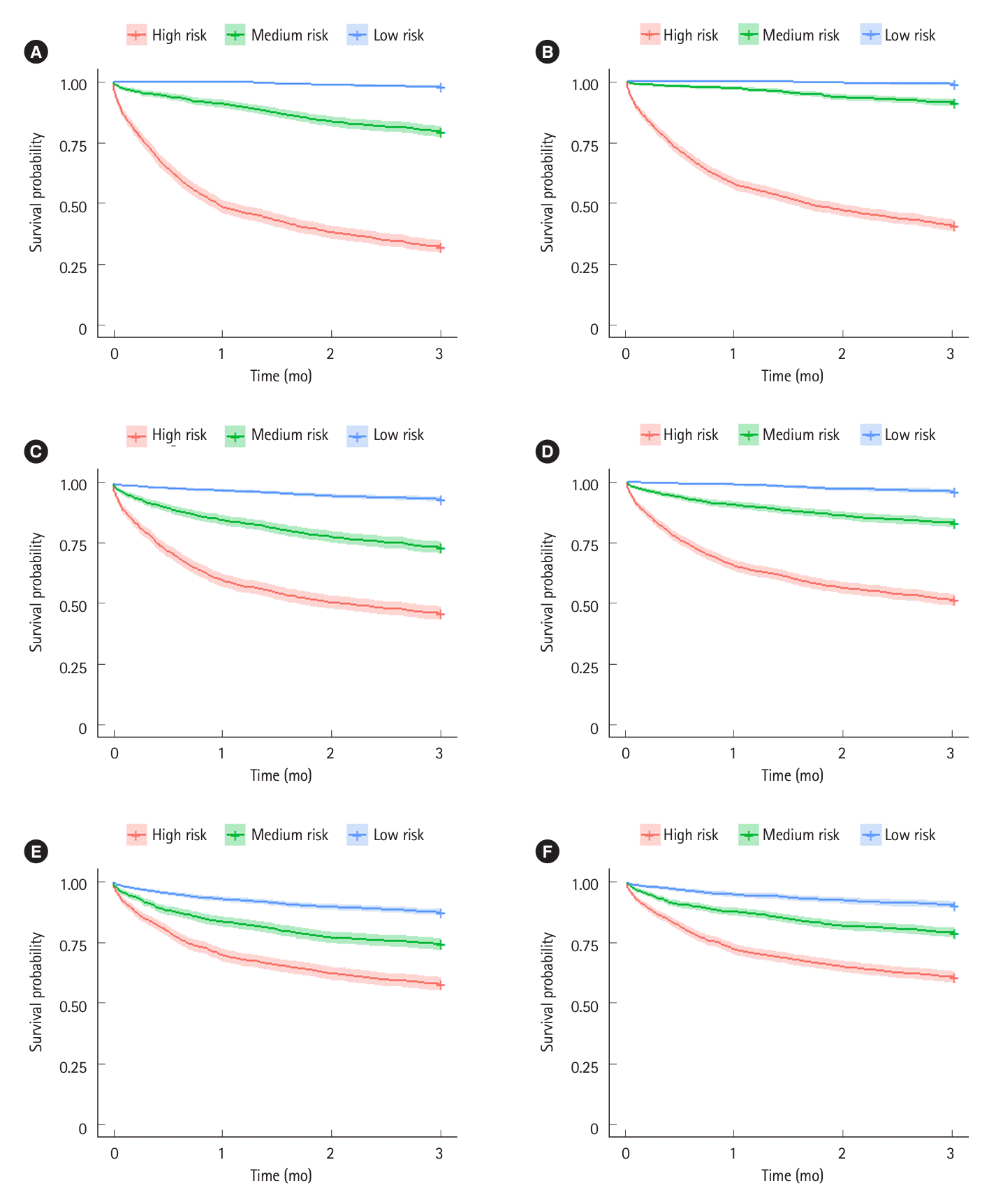
Table 1.
| Study (year) |
SD infliximab |
AD infliximab |
Propensity score matching | ||
|---|---|---|---|---|---|
| No. | Colectomy-free rate | No. | Colectomy-free rate | ||
| Syal et al. (2021) [14] | 29 | 86% (3 mo); 79% (12 mo) | 34 | 85% (3 mo); 80% (12 mo) | No |
| Gibson et al. (2019) [15] | 87 | 62% (6 mo) | 58 | 67% (6 mo) | No |
| Sebastian et al. (2019) [4] | 102 | 80% (3 mo); 72% (12 mo) | 29 | 76% (3 mo); 69% (12 mo) | Yes: 12 mo colectomy-free rate 42% in SD vs. 69% in AD (53% residual risk reduction) |
| Chao et al. (2019) [16] | 37 | 95% (3 mo) | 35 | 86% (3 mo) | Partial, specific results not described |
| Nalagatla et al. (2019) [17] | 132 | 86% (3 mo); 73% (12 mo) | 81 | 80% (3 mo); 72% (12 mo) | No |
| Shah et al. (2018) [18] | 120 | 72% (12 mo) | 26 | 73% (12 mo) | Yes: 12 mo colectomy-free rate 71% in SD vs. 81% in AD (34% residual risk reduction) |
| Gibson et al. (2015) [19] | 35 | 63% (1 mo) | 15 | 93% (1 mo) | No |
Table 2.
| Study (year) | Tofacitinib dose | No. | Colectomy-free rate | Setting |
|---|---|---|---|---|
| Uzzan et al. (2021) [20] | TOF 10 mg BD | 55 | 79% (3 mo); 74% (6 mo) | Hospitalized refractory UC or first line salvage for ASUC due to prior biologic experience |
| Berinstein et al. (2021) [21] | TOF 10 mg BD or 10 mg TDS | 40 | 85% (3 mo) | First line salvage for ASUC due to prior biologic experience |
| Eqbal et al. (2023) [22] | TOF 10 mg TDS | 11 | 82% (3 mo); 82% (12 mo) | Sequential therapy after IFX failure |
| Constant et al. (2022) [23] | TOF 10 mg BD or 10 mg TDS | 11 | 73% (3 mo) | First line salvage for ASUC due to prior biologic experience or sequential therapy after IFX failure |
| Xiao et al. (2022) [24] | TOF 10 mg BD or 10 mg TDS | 8 | 63% (3 mo) | First line salvage for ASUC due to prior biologic experience or sequential therapy after IFX failure |
| Komeda et al. (2023) [25] | TOF 10 mg BD | 8 | 88% (unclear timing) | First line salvage in biologic naïve ASUC |
| Malakar et al. (2023) [26] | TOF 10 mg BD | 8 | 88% (3 mo) | First line salvage for ASUC due to prior biologic experience |
| Honap et al. (2020) [27] | TOF 10 mg BD | 7 | 43% (3 mo) | First line salvage for ASUC due to prior biologic experience or sequential therapy after IFX failure |
| Gilmore et al. (2022) [28] | TOF 10 mg TDS | 5 | 80% (3 mo) | Sequential therapy after IFX failure or early post-IFX induction (ASUC < 7 day completing IFX induction) |
| Kotwani et al. (2020) [29] | TOF 10 mg BD | 4 | 100% (3 mo) | First line salvage for ASUC due to prior biologic experience |
| Gilmore et al. (2023) [6] | UPA 45 mg OD | 6 | 100% (3 mo); 83% (4 mo) | First line salvage for ASUC due to prior biologic experience |
| Zinger et al. (2023) [8] | UPA 45 mg OD | 4 | 75% (3 mo) | Sequential therapy after IFX failure |
| Ali et al. (2023) [30] | UPA 45 mg OD | 1 | 100% (6 mo) | First line salvage for ASUC due to prior biologic experience |
Indication for TOF and UPA included a combination of first line salvage (in both biologic experienced and naïve patients) as well as sequential (second line salvage) therapy after IFX failure (total 168 patients with a crude combined 3-month colectomy-free rate of approximately 80%).
JAK, Janus kinase inhibitor; ASUC, acute severe ulcerative colitis; TOF, tofacitinib; UPA, upadacitinib; BD, twice daily; OD, once daily; TDS, three times daily; UC, ulcerative colitis; IFX, infliximab.
Table 3.
Table 4.
The numbers of observed colectomies per group are presented as median (95% CI) and the colectomy rates are calculated as a percentage using the median value. Risk stratification models and treatment strategies are each analyzed with 1,000 simulations.
Response defined as proportion of patients avoiding colectomy by 3 months. Standard IFX induction with assumed overall 3-month colectomy-free rate of 77%; accelerated IFX response rate tested at 80%, 85%, and 90%, using the same propensity of response as standard IFX; JAK inhibitor response rate tested 60%, 70%, and 80%, where propensity to respond to JAK is assumed to be independent of propensity to respond to IFX.
ASUC, acute severe ulcerative colitis; AUC, area under the curve; CI, confidence interval; IFX, infliximab; JAK, Janus kinase inhibitor.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download