Abstract
Background/Aims
Methods
Results
Notes
Funding Source
This work was supported by Kitasato University Kitasato Institute Hospital, Tokyo, Japan (Grant No. 19003).
Conflict of Interest
Sagami S served as a speaker for AbbVie, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Gilead Sciences, Janssen Pharmaceutical, Nippon Kayaku and Zeria Pharmaceutical and as an endowed chair for AbbVie, JIMRO, Zeria Pharmaceutical, Kyorin Pharmaceutical, Mochida Pharmaceutical and EA Pharma. Maeda M served as an endowed chair of AbbVie GK, EA Pharma, Zeria Pharmaceutical, JIMRO, Kyorin Pharmaceutical, and Mochida Pharmaceutical. Miyatani Y has served as a speaker of AbbVie; received research funding from Japan Foundation for Applied Enzymology; and as an endowed chair of AbbVie, JIMRO, Zeria Pharmaceutical, Kyorin Pharmaceutical, Mochida Pharmaceutical, Otsuka Holdings, and EA Pharma. Nakano M has served as a speaker or a consultant in Covidien, Mochida Pharmaceutical, Takeda Pharmaceutical, Zeria Pharmaceutical, Kyorin Pharmaceutical, and Nippon Kayaku; received research funding from Mitsubishi Tanabe Pharma and the Japanese foundation for research and promotion of endoscopy. Hibi T has received Lecture fees from Aspen Japan K.K, AbbVie GK, Ferring, Gilead Sciences, Janssen, JIMRO, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Pfizer, Takeda Pharmaceutical, Advisory/consultancy fees from Apo Puls Station, AbbVie GK, Bristol-Myers Squibb, Celltrion, EA Pharma, Eli Lilly, Gilead Sciences, Janssen, Kyorin, Mitsubishi Tanabe Pharma., Nichi-Iko Pharmaceutical, Pfizer, Takeda Pharmaceutical, Zeria Pharmaceutical and research grants from AbbVie GK, Activaid, Alfresa Pharma Corporation, JMDC Inc., Gilead Sciences, Inc., Nippon Kayaku Co., Ltd., Eli Lilly Japan K.K., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Ferring Pharmaceuticals and Bristol-Myers Squibb; received scholarship contributions from Mitsubishi Tanabe Pharma Corporation, Zeria Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd.; Pfizer Japan Inc. belonged to study group sponsorship by Otsuka Holdings, AbbVie GK, EA Pharma Co., Ltd., Zeria Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Kyorin Pharmaceutical Co., Ltd., and Mochida Pharmaceutical Co., Ltd. Grants or fund. And he is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. Kobayashi T received Lecture fees from Takeda Pharmaceutical Co., Ltd., Activaid, Alfresa Pharma Corporation, Zeria Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Abbie GK, Pfizer Japan Inc., Janssen Pharmaceutical K.K., Thermo Fisher Diagnostics K.K., JIMRO Co., Ltd. research grants from AbbVie GK, Activaid, Alfresa Pharma Corporation, JMDC Inc., Gilead Sciences, Inc., Nippon Kayaku Co., Ltd., Eli Lilly Japan K.K., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Ferring Pharmaceuticals and Bristol-Myers Squibb; received scholarship contributions from Mitsubishi Tanabe Pharma Corporation, Zeria Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., and belonged to study group sponsorship by Otsuka Holdings, AbbVie GK, EA Pharma Co., Ltd., Zeria Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Kyorin Pharmaceutical Co., Ltd., and Mochida Pharmaceutical Co., Ltd. Grants or funds Advisory/consultancy fees from Janssen Pharmaceutical K.K., EA Pharma Co., Ltd., KISSEI Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Activaid, Pfizer Japan Inc., Nippon Kayaku Co., Ltd., Alfresa Pharma Corporation, Kyorin Pharmaceutical Co., Ltd., Abbie GK, Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation. None of the funding received for this work was from any of the above organizations. The authors not listed have no conflicts of interest to declare.
Data Availability Statement
Data supporting the results are available from the corresponding author (Kobayashi T) upon reasonable request.
Author Contributions
Conceptualization: Sagami S, Kobayashi T. Data curation: Karashima R, Sagami S. Formal analysis: Karashima R, Sagami S. Funding acquisition: Sagami S. Investigation: Karashima R. Methodology: Karashima R, Sagami S, Kobayashi T. Project administration: Kobayashi T. Resources: Sagami S. Supervision: Sagami S, Kobayashi T. Visualization: Karashima R, Sagami S, Maeda M, Hojo A, Kobayashi T. Writing - original draft: Karashima R. Writing - review & editing: all authors. Approval of final manuscript: all authors.
Additional Contributions
The authors are grateful for assisting with this study to Kunio Asonuma, Tadae Mori, Toyomi Ishibashi, Kayo Sakata, and Yuki Watanabe. The authors thank Satoshi Kuronuma and Osamu Takeuchi for measuring fecal calprotectin (FC) levels, and the Department of Clinical Laboratory for their expertise in measuring leucine-rich α-2-glycoprotein (LRG). The study is partly supported by Alfresa Pharma Corporation for measuring FC.
REFERENCES
Fig. 2.
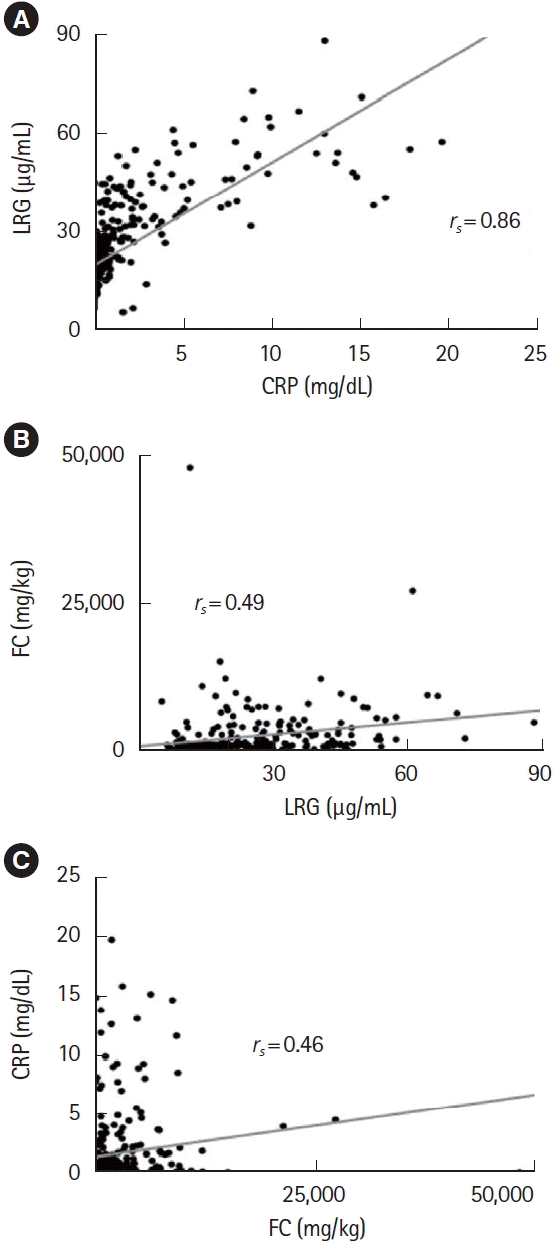
Fig. 3.
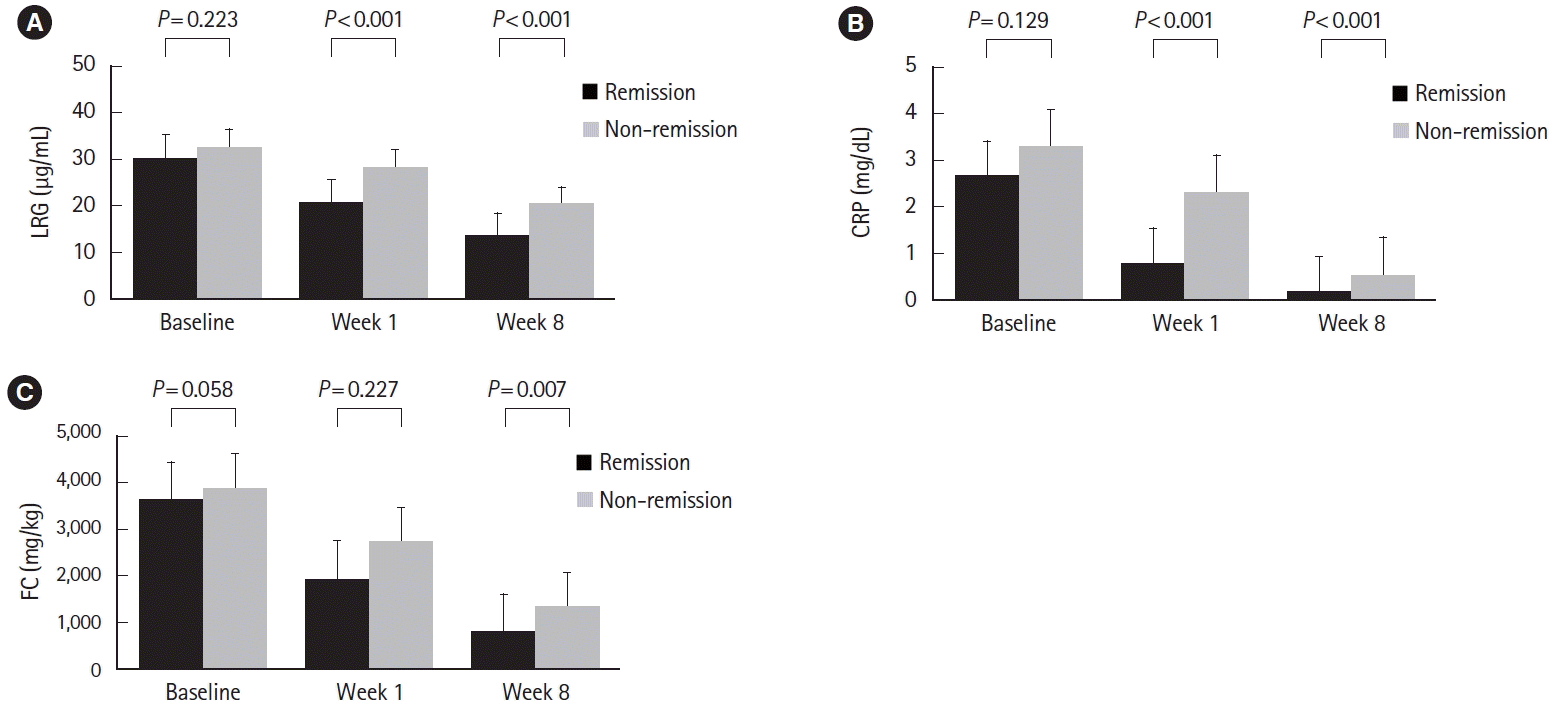
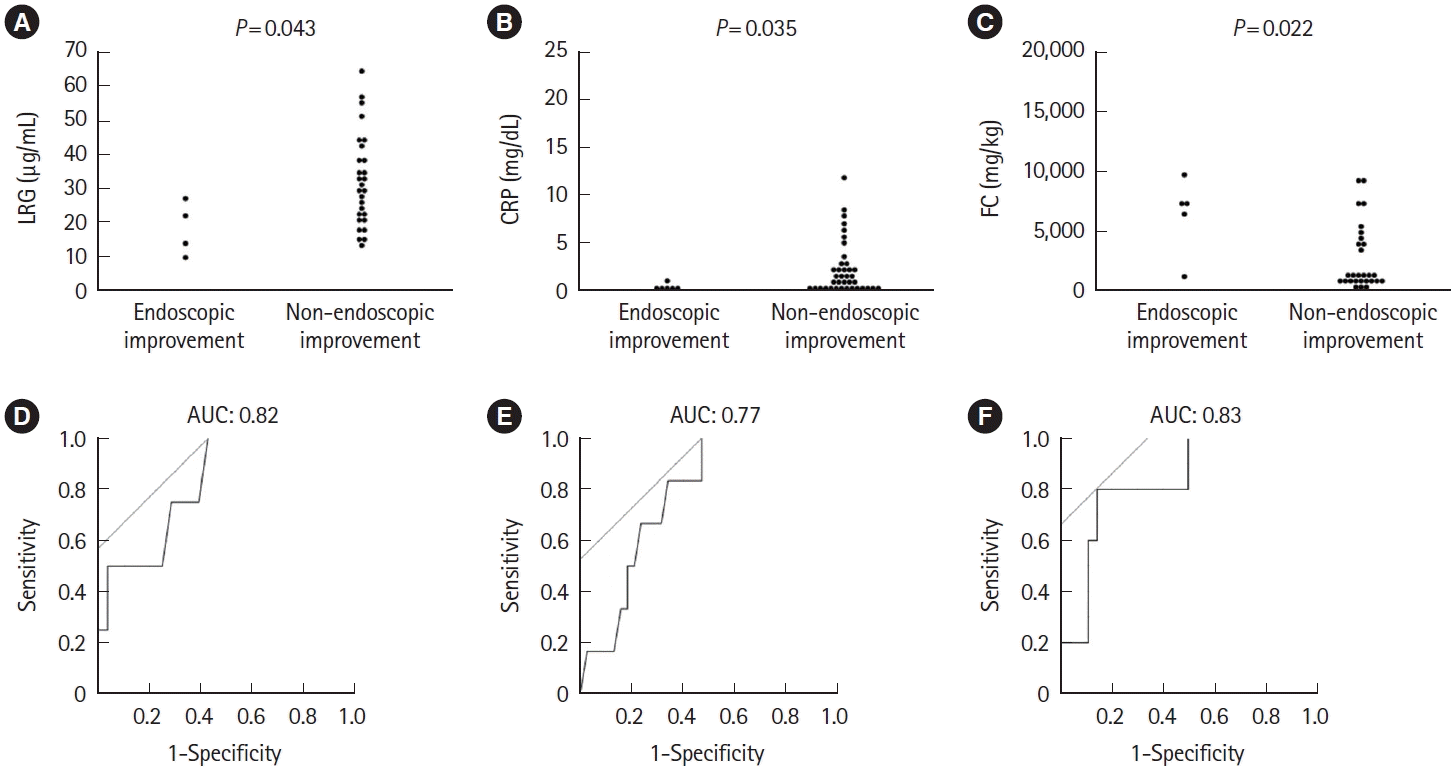
Fig. 4.
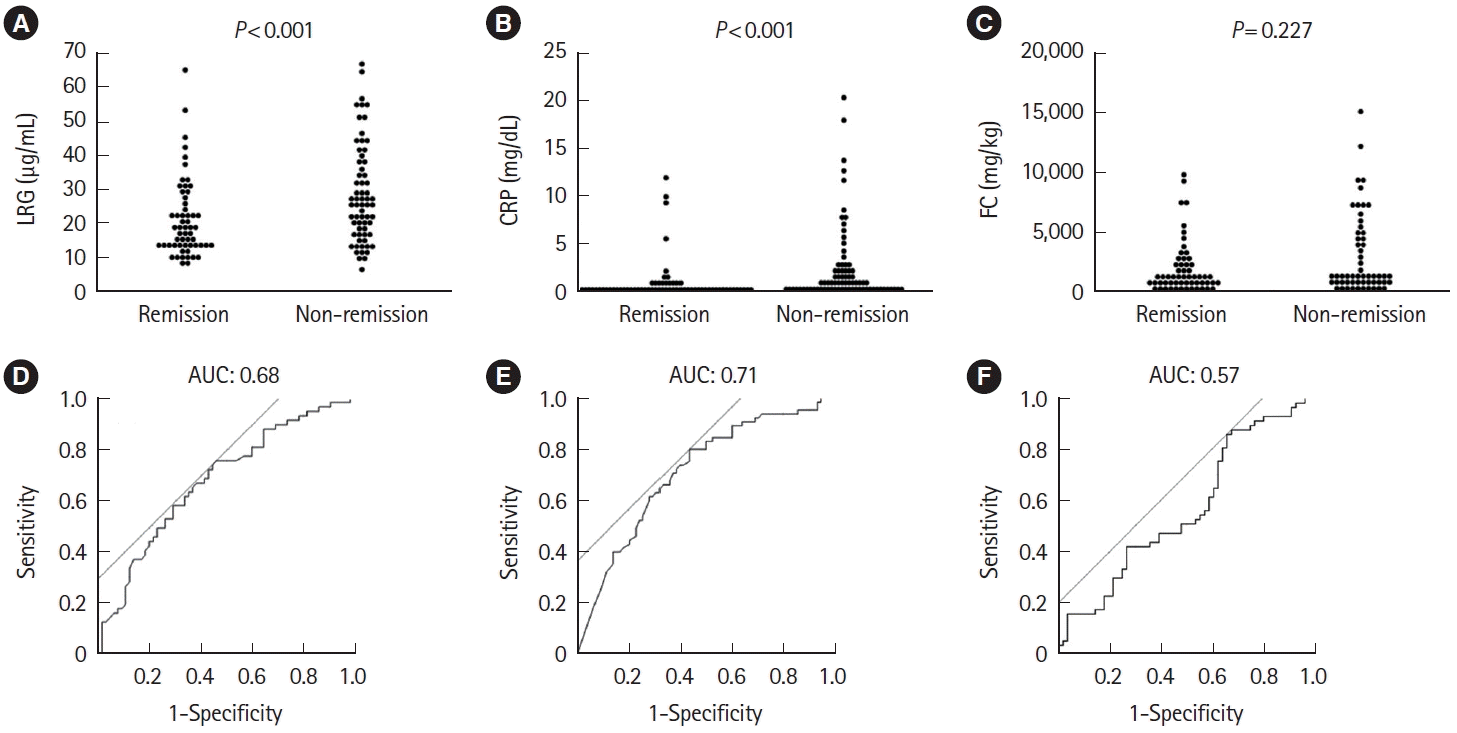
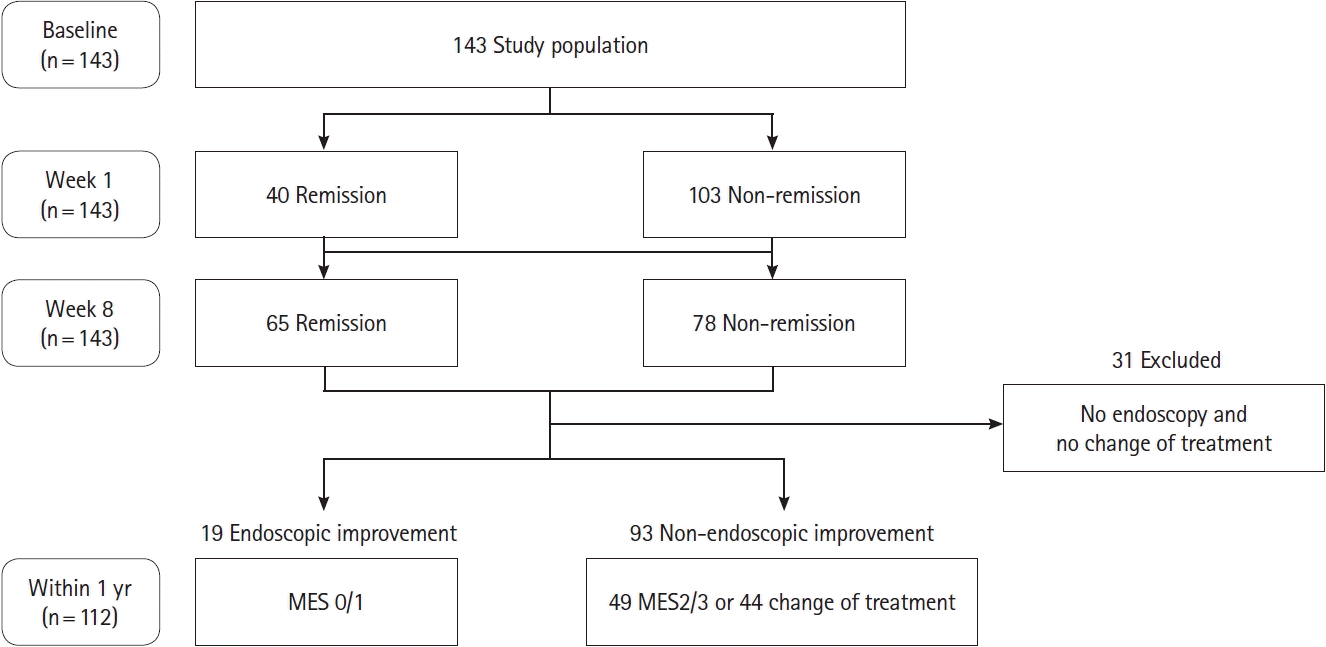
Fig. 5.
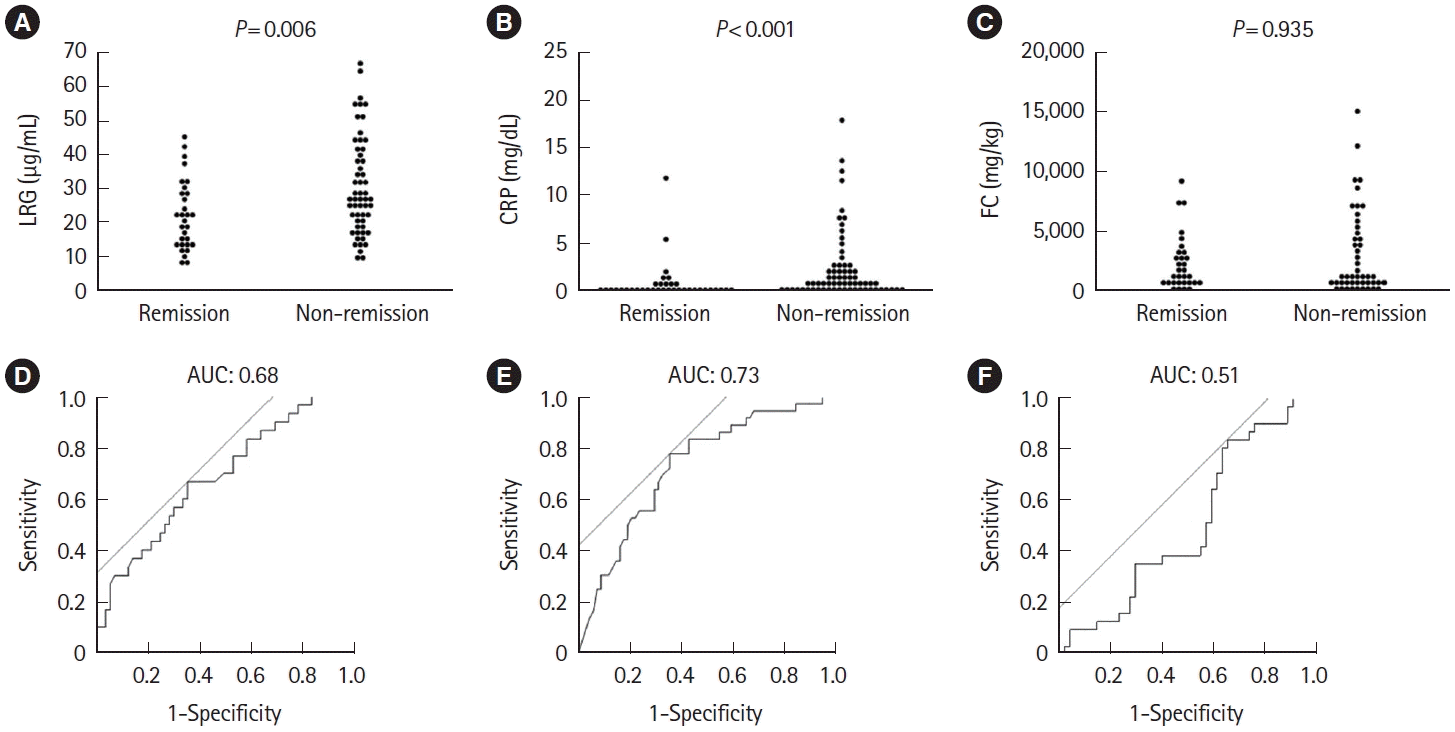
Fig. 6.
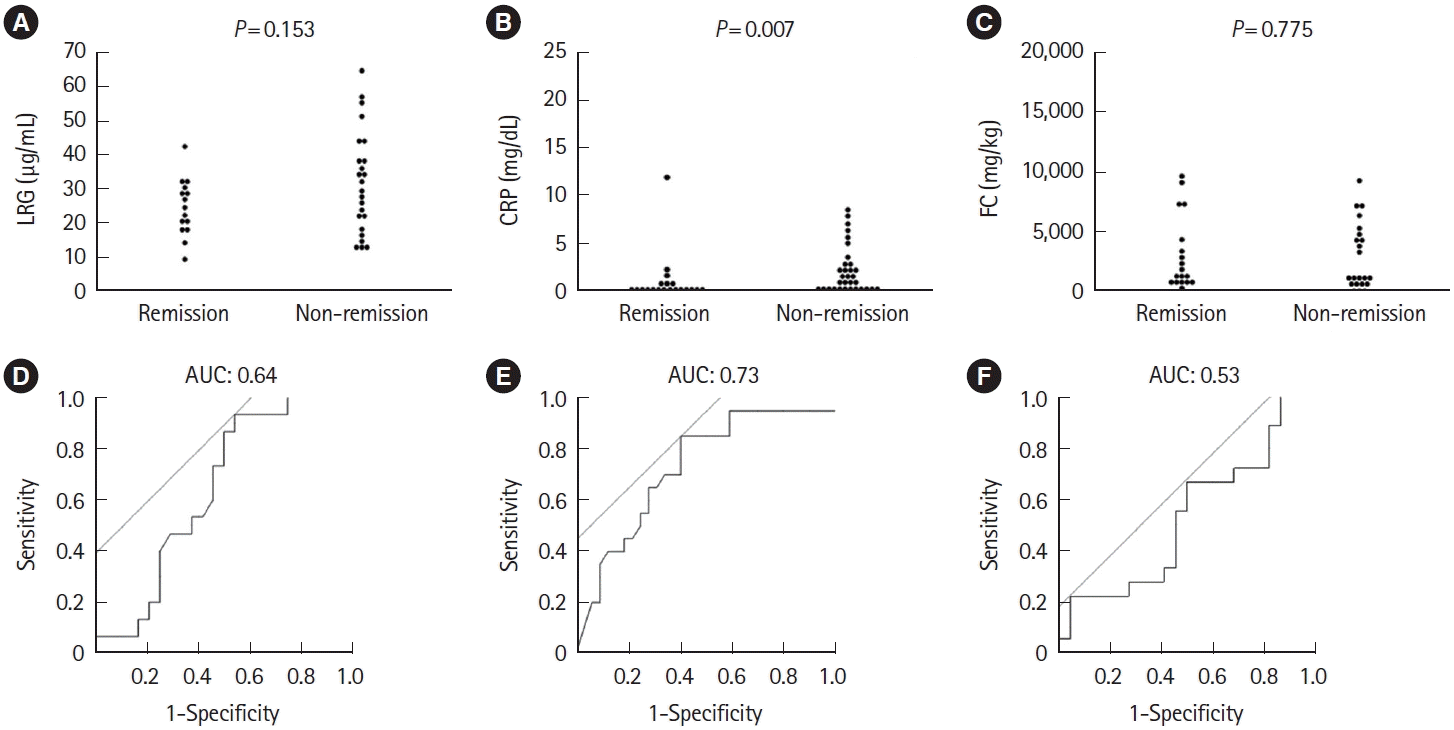
Fig. 7.
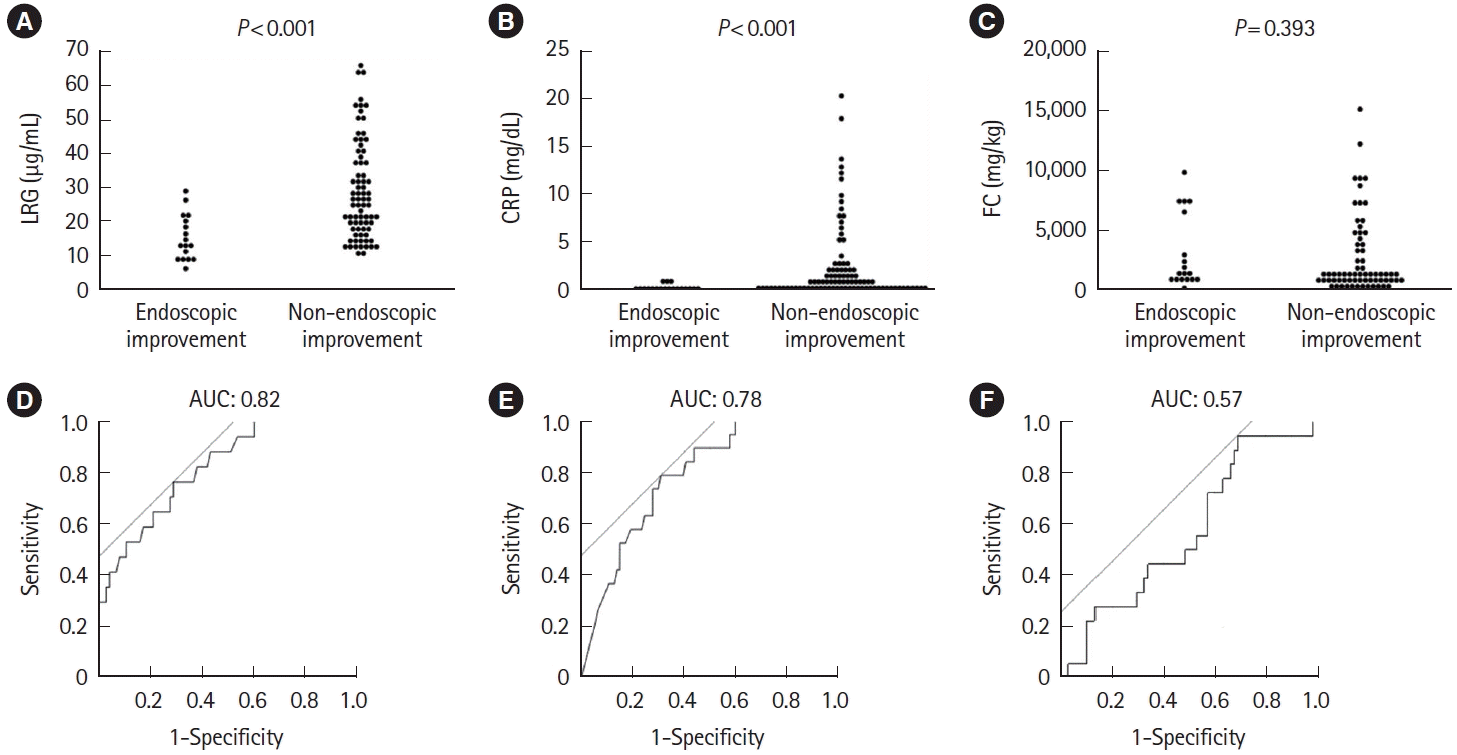
Fig. 8.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download