INTRODUCTION
Natural killer (NK) cells are pivotal to innate immunity. Direct cytotoxicity and cytokine secretion during infection, tissue injury, and inflammation are major functions of NK cells [
1]. They consist of various heterogeneous subpopulations, each with distinct immunophenotypes and functions [
2-
4]. Bright or dim CD56 expression, with or without CD16 expression, is immunophenotypically the hallmark of NK cells, as well as the variable expression levels of CD94
-NKG2 heterodimers, members of the killer immunoglobulin-like receptor (KIR) family, and members of the natural cytotoxicity receptor (NCR) family. Classically, NK cells are classified into immature (CD56
bright, CD94
hi, CD16
-, CD57
-), mature (CD-56
dim-to-med, CD94
med, CD16
+, CD57
-), and hypermature (CD56
dim-to-med, CD94
low, CD16
+, CD57
+) based on their expression levels of CD16, CD56, CD57, and CD94 [
2,
3]. Through recent studies, the diversity of NK cell subpopulations has been expanded to a remarkable degree [
5,
6]. Cytometry by time-of-flight (CyTOF) analyses assessing 37 parameters simultaneously have unveiled the immense diversity within NK cell subpopulations, identifying up to 30,000 phenotypic variations per individual, and more than 100,000 phenotypic populations within all subjects [
5].
CD19, a 95 kD transmembrane glycoprotein, is one of the specific biomarkers for normal and neoplastic B cells [
7]. It is expressed from the pre-B cell stage until terminal differentiation into plasma cells, and it forms a signal transduction complex along with CD21, CD81, and CD225 [
8]. CD19 is used in clinical laboratories as a crucial marker in identifying the presence of normal B cells, confirming the B cell origin of neoplastic cells using immunohistochemistry staining, and detecting minimal residual disease (MRD) of Blymphoblastic leukemia (B-ALL) using flow cytometry [
9]. Immunotherapies targeting CD19 on B cells, such as bispecific T cell engager or CAR-T cell therapy, are now widely used in clinical practice [
10,
11].
NK cells usually do not express CD19, however, a few reports have confirmed the rare existence of NK cells with a CD19
dim phenotype on flow cytometry [
12-
14], although their characteristics and roles remain to be elucidated. We observed the presence of CD56
+CD19
dim cells during lymphocyte subset analysis by flow cytometry.
In this study, we further examined the functional and clinical characteristics of CD56+CD19dim cells through extensive phenotype and functional profiles using fresh samples from patients and through retrospective investigation of lymphocyte subset analysis data and clinical features of individuals who presented with CD56+CD19dim cells.
Go to :

MATERIALS AND METHODS
1. Subjects
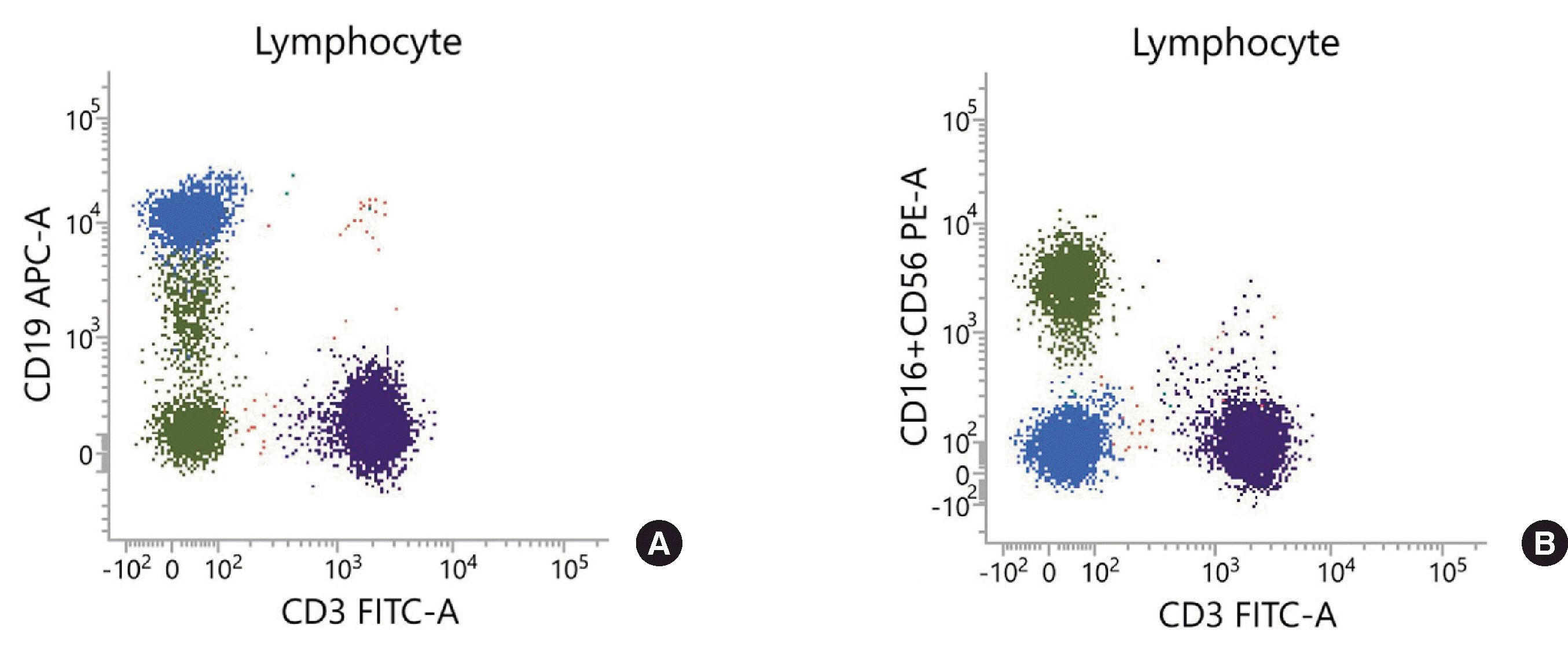
Peripheral blood lymphocyte subset data generated by flow cytometry from 2020 to 2022 at Samsung Medical Center (Seoul, Korea) were analyzed retrospectively. The presence of the distinctive CD56+CD19dim NK cells expressing CD56 with a comparable fluorescence intensity to that of the CD56+CD19– NK cell population, and expressing CD19, but with a lower intensity than that of the CD56–CD19+ B cell population, were defined as those constituting more than 1.0% of total lymphocytes. Information about the clinical diagnosis, medical conditions, and treatment were obtained for the subjects. From the subjects showing CD56+CD19dim NK cells, two were selected for in-depth immunophenotyping and functional profiling based on the availability of fresh blood samples. The experiments were carried out under the approval of the Institutional Review Board of Samsung Medical Center (IRB No. 2022-09-113) and written informed consent was obtained from all patients.
2. Flow cytometry analysis
Further immunophenotyping and functional profiling were performed by flow cytometry using peripheral blood samples. Samples were prepared by surface staining and intracellular staining, with or without stimulation. For surface staining, Brilliant Stain Buffer (BD Biosciences, San Jose, CA, USA) and antibody cocktails were added to peripheral blood samples, followed by incubation for 30 minutes in the dark at room temperature (RT). The red blood cells were then lysed with BD FACS Lysing Solution (BD Biosciences) and incubated for 10 minutes in the dark at RT. Finally, the samples were washed with Dulbecco’s phosphatebuffered saline (DPBS) (Welgene, Gyeongsan, Korea). For testing CD107a, granzyme B, IFN-γ, and MIP-1β, peripheral blood mononuclear cells (PBMCs) were isolated prior to cell stimulation and intracellular staining. An anti-CD107a antibody and cell stimulation cocktail containing phorbol 12-myristate 13-acetate (PMA) with ionomycin were added to the tube with suspended PBMCs. The cells were incubated for 1 hour, followed by 4 hours of incubation with monensin (GolgiStop, BD Biosciences), which blocks intracellular transport. After washing with DPBS containing 3% fetal bovine serum (Gibco, Grand Island, NY, USA), antibody staining for surface markers and intracellular markers were performed in sequence after 30 minutes of fixation/permeabilization. Meanwhile, when testing MIP-1β, cells were simulated with IL-2 and IL-15 instead of PMA and ionomycin, without monensin, and were stained with relevant antibodies after fixation/permeabilization for 30 minutes. Using each prepared sample, flow cytometry analyses were performed and data were acquired. The expression patterns of each marker in CD56+CD19dim cells were compared with those in CD56+CD19– NK cells and CD56–CD19+ B cells.
3. Reagents and instrument
The samples were stained with the following anti-human monoclonal antibodies: anti-CD3-PerCP-Cy5.5 (UCHT1), anti-NKp30-PE (AF29-4D12), anti-NKp44-PerCP-Cy5.5 (P44-8) antibodies from BioLegend (San Diego, CA, USA); anti-CD3-BV510 (UCHT1), anti-CD3-BV605 (SK7), anti-CD8-APC-Cy7 (SK1), anti-CD8-PerCP-Cy5.5 (SK1), anti-CD56-APC (NCAM16.2), anti-CD14-FITC (MφP9), anti-CD16-PerCP-Cy5.5 (3G8), anti-CD16-V450 (3G8), anti-CD19-APC-R700 (HIB19), anti-CD20-BV605 (L27), anti-CD21-PE-Cy5 (B-ly4), anti-CD22-PE (S-HCL-1), anti-CD94-APC (HP-3D9), anti-CD57-FITC (HNK-1), anti-CD158a-FITC (HP-3E4), anti-CD27-BV510 (L128), anti-CD38-FITC (HB-7), anti-IgD-APC (IA6-2), anti-HLA-DR-V450 (L243), anti-7-AAD, anti-perforin-FITC (δG9), anti-CD107a-PE (H4A3), anti-granzyme B-FITC (GB11), anti-IFN-γ-PE (4S.B3), and anti-MIP-1β-PE-Cy7 (D21-1351) antibodies from BD Biosciences; anti-CD16-Super Bright 645 (eBioCB16), anti-CD19-PE-Cy7 (HIB19), anti-CD19-FITC (HIB19), anti-CD27-PerCP (O323), and anti-NKp46-BV421 (29A1.4) antibodies from Invitrogen (Waltham, MA, USA); and anti-CD56-PECy7 (N901), anti-CD158b-PerCP-Cy5.5 (GL183), and anti-CD158a,h-PE (EB6B) antibodies from Beckman Coulter (Indianapolis, IN, USA). Flow cytometry analyses were conducted using a FACSLyric ™ instrument (BD Biosciences) and data were analyzed using Flowjo software (Flowjo, LLC, Ashland, OR, USA).
Go to :

DISCUSSION
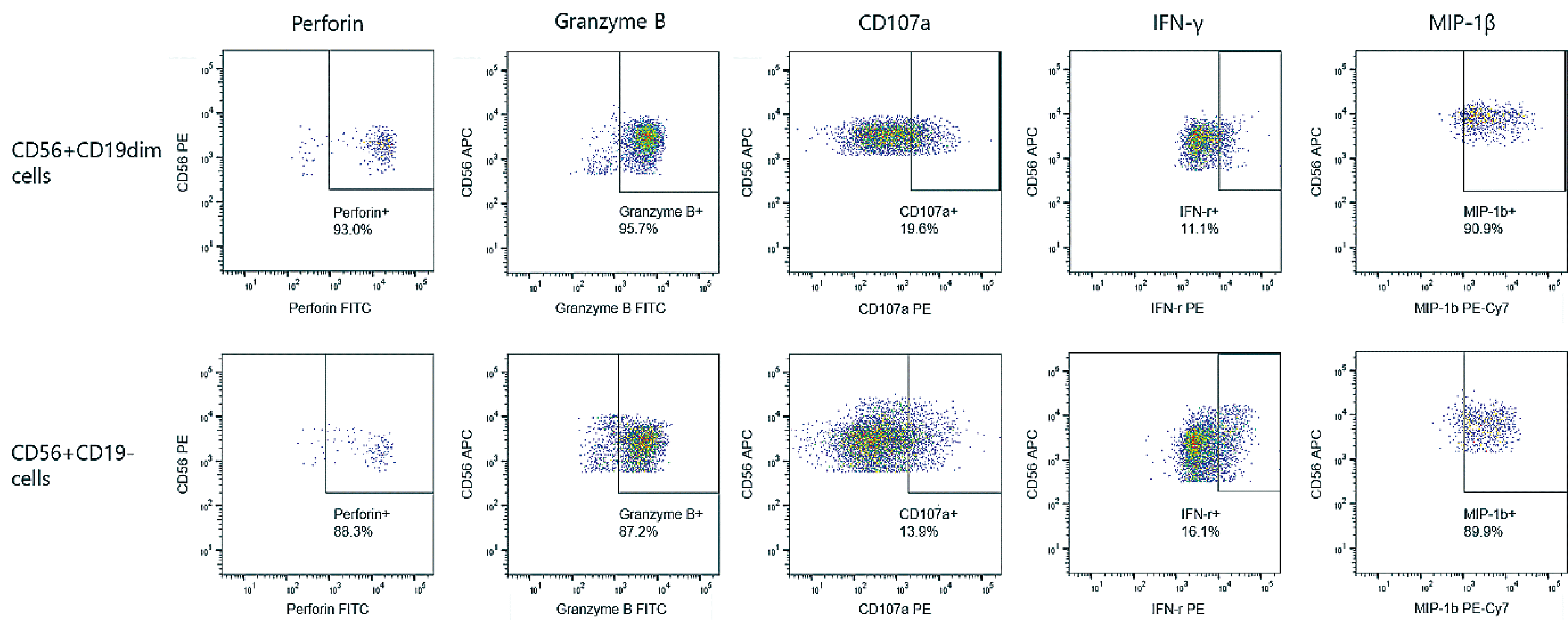
Through extensive lineage and functional analysis using flow cytometry, and the investigation of the clinical features of individuals harboring CD56+CD19dim cells, we revealed that CD56+CD19dim cells constitute a subpopulation of NK cells expressing various NK cell markers (CD16, CD57, CD94, NCR family members, and KIR family members), and are positive for markers representing cytolytic function (perforin, intracellular granzyme B and CD107a) and cytokine-secreting function (intracellular IFN-γ and MIP-1β), but are negative for other B cell-related markers (CD20, CD21, CD22, and IgD).
It is unusual for CD19 to be expressed in NK cells; however, such cases have been reported previously [
12-
14]. Soma et al. revealed the presence of CD19
+ NK cells for the first time in 0.2% of clinical samples subjected for MRD analysis of B-ALL using flow cytometry. CD19
+ NK cells were reported to account for 1.3–24.0% of total NK cells, and 0.04–0.9% of total white blood cells (WBCs) [
12]. Subsequently, Korol et al. reported the existence of NK cells with CD19 expression at a frequency of 4.4% from the 1,002 samples submitted for lymphocyte subset study, and their proportions were 0.19–2.56% of total WBCs and 0.53–4.95% of total lymphocytes [
13]. In addition, they excluded the possibility of CD19 expression mediated by trogocytosis, by demonstrating that CD21, a B cell receptor that complexes with CD19 [
8], was not expressed on CD19
+ NK cells. Li et al. also reported that they detected NK cells expressing CD19 during the flow cytometry analyses for blast screening, at a frequency of 0.04–0.69% in 13% (4/32) of peripheral blood samples, and at a frequency of 0.02–0.05% from 9% (2/22) of bone marrow samples [
14]. We identified CD19
dim NK cells at a frequency of 1.68% from the 1,011 subjects for whom lymphocyte subset studies were performed in the 3-year study period. Follow-up analysis of the subjects’ lymphocyte subsets over the 3 years, the average proportions of CD19
dim NK cells in the total lymphocytes for each subject were 0.70–12.31%. However, the frequencies in clinical samples between studies varied [
12-
14], which might be due to the differences in the characteristics of the patients enrolled in each study and whether the proportions reported were of patients or of samples. One of the reasons that the frequency of CD19
dim NK cells was relatively lower in our study than in previous reports may be due to the exclusion of cases with the frequency of CD19
dim NK cells less than 1.0% of total lymphocytes to avoid false-positivity due to non-specific binding of anti-CD19 antibodies. We also reaffirmed that CD19 expressed on NK cells was not induced through trogocytosis, since CD21 was negative in our study, as reported in previous studies [
13].
The NCR family, which comprises the main activating receptors along with the Fc receptor, CD16, includes NKp30, NKp44, and NKp46 [
15-
19]. The acquisition of NCRs during the maturation of NK cells is associated with the gain of cytolytic activity against tumor cells [
20]. Both NKp30 and NKp46 are expressed regardless of the activation status of the NK cell; however, the expression of NKp44 is up-regulated when NK cells are in an activated state, induced by stimulation with cytokines, such as IL-2, IL-1β, and IFN-β [
16-
18,
21]. CD94, which is expressed on NK cells and a part of CD8
-positive T cells, forms a heterodimer with members of the NKG2 family, and acts as an activating receptor or an inhibitory receptor depending on the type of NKG2 molecule. Among them, CD94/NKG2A, an inhibitory receptor, has a higher affinity for its ligand, HLA-E, resulting in inhibition of the cytotoxicity of CD94
hi NK cells [
22,
23]. Members of the KIR family, the main inhibitory receptors of NK cells, bind to self-MHC class I ligands on healthy cells, preventing NK cells from provoking an autoimmune response [
19]. We found that CD19
dim NK cells have increased expression levels of CD94 and CD158b, which inhibit their cytotoxicity function, and decreased expression levels of the activating receptor NKp30, compared to their levels in conventional CD19
-negative NK cells. These findings suggest that cytotoxic activity is down-regulated in CD19
dim NK cells.
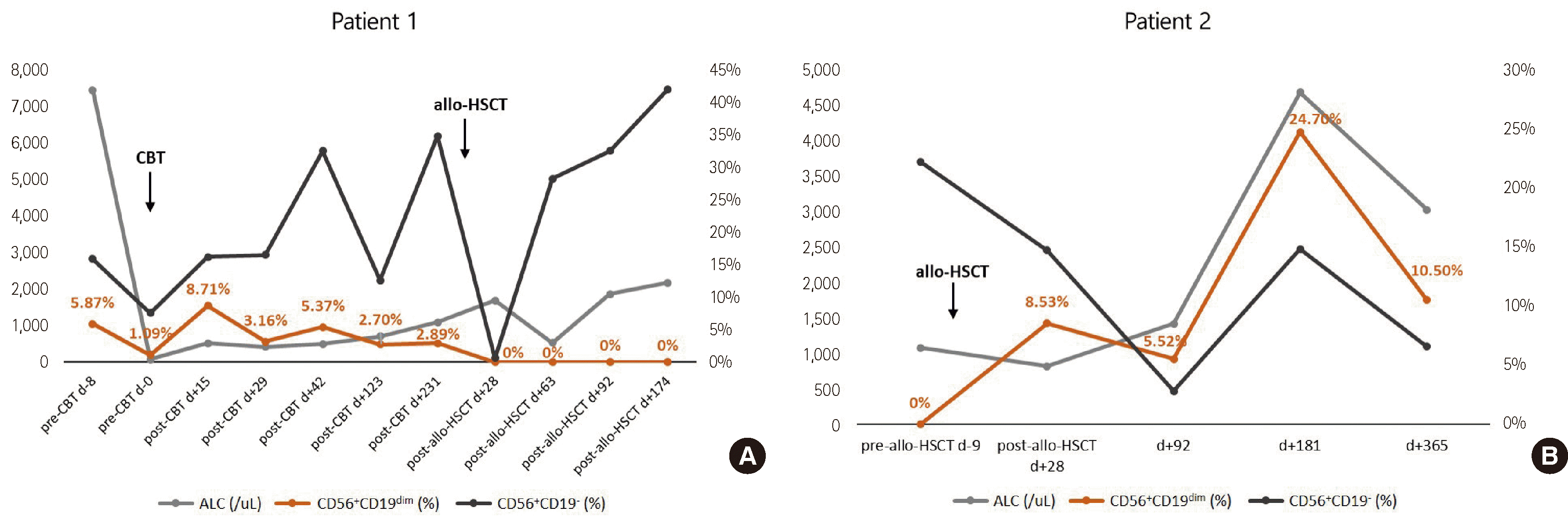
Of the 17 patients we identified, 6 were healthy HSCT donors, and 2 of the remaining 11 patients had received HSCT from donors with confirmed CD19dim NK cells. In one of them, the presence of CD19dim NK cells were observed on lymphocyte subset studies since receiving HSCT, but were absent before HSCT, suggesting that they originated from the donor. For the other patient, it could not be confirmed whether the CD19dim NK cells originated from the HSCT donor or were presented even before receiving HSCT, since a lymphocyte subset study was not performed prior to HSCT. Thus, in 7 of 17 patients, CD19dim NK cells were derived from a healthy donor, implying less relevance of the existence of CD19dim NK cells to the patient’s disease status. Furthermore, it was considerable that the presence of CD19dim NK cells persisted in every subject except one in whom they disappeared after receiving HSCT from a donor without CD19dim NK cells, and that the CD19dim NK cells that disappeared were not detected again in the subject who had received HSCT from a donor without CD19dim NK cells. The expression levels of CD19 in NK cells are suspected to be associated with unknown intrinsic factors, rather than with environmental factors or effects of medical treatments, but this requires further studies to investigate the possibilities.
The proportions of the CD19
dim NK cell population among the total lymphocyte population appeared to be fluctuating, and not remaining stable, in some patients. However, the relationship between the increase or decrease in the proportion of the CD19
dim NK cell population and specific clinical events, such as infection, was obscure. There were limitations in that the clinical significance and potential role of CD19
dim NK cells could not be determined. CD19 on B cells is involved in the intrinsic signaling pathway along with B cell receptors and other surface proteins [
7]. Consequently, promoting the recruitment and binding of various downstream protein kinases, CD19 plays a role in antigen-induced B cell responses and terminal differentiation to memory B cells or plasma cells [
24]. Further research is required to determine whether CD19 molecules on CD19
dim NK cells also have similar roles.
The presence of CD19
dim NK cells can confound the results when CD19 is used as a B cell-gating marker in flow cytometry. Soma et al. reported that CD19
-positive NK cell populations are a potential cause of misinterpretation in MRD analysis of B-ALL, by wrongly assigning them as an abnormal B cell population [
12]. Moreover, Korol et al. suggested that the existence of CD19
-positive NK cells may induce the over-quantification of B cell subsets, especially non-memory B cells, in lymphocyte subset studies by assigning CD19
-positive NK cells as CD27
-negative B cells [
13]. Furthermore, increased expression levels of cytoplasmic CD3 epsilon chain in CD19
-positive NK cells may mislead their assignment to the T cell lineage. Therefore, one needs to be alert when interpretating MRD analysis results of B-ALL or lymphocyte subset studies using flow cytometry when the presence of CD19
dim NK cells are noted. Another scenario where the existence of CD-19dim NK cells can affect clinical practice is that they are a potential unintended target of CD19
-targeting CAR-T cell therapy. In patients in whom the CD19
dim NK cell population is a component of blood lymphocytes, CD19
-targeting CAR-T cell therapy would result in a reduction in the number of NK cells, which may, paradoxically, interrupt anti-tumor immune responses. Thus, clinicians need to pay more attention when considering the CD19
-targeting CAR-T cell therapy in patients with the confirmed presence of a CD19
dim NK cell population.
In conclusion, the findings of our study imply that the CD19dim NK cell population is a distinctive NK cell subpopulation, rather than a temporary non-specific phenomenon, and it is important to recognize the existence of these cells to avoid the misinterpretation of test results or potential therapeutic misdirection.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download