INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disorder related to aging and is the most widespread form of dementia. The global prevalence of dementia including AD is predicted to rise from 57.4 million cases in 2019 to 152.8 million cases by 2050 [
1]. With the aging global population, AD is becoming a major healthcare issue worldwide, with an estimated financial burden of $2 trillion by the year 2030 [
2]. Hence, it is crucial to develop effective strategies such as early diagnosis and treatment to improve patient outcomes and reduce costs for patients, caregivers, and the healthcare system.
Because cerebrospinal fluid (CSF) proteins and positron emission tomography (PET) tracers specific to amyloid-β (Aβ) and phosphorylated tau (p-tau) are directly connected to AD, both candidates are excellent sources of information for the detection of biochemical abnormalities within the brain for an AD diagnosis. Nevertheless, the broad application of CSF and imaging-based biomarkers faces limitations due to the invasive nature of lumbar punctures and the high cost and limited availability of PET imaging [
3,
4]. Using blood-based biomarkers for AD diagnosis has been challenging despite the convenience of blood sampling compared to CSF sampling and PET imaging. One reason for this is that only a small proportion of brain proteins are found in the blood, making it difficult to detect AD biomarkers. Additionally, the presence of high levels of plasma proteins, such as albumin and immunoglobulin G in blood samples, can cause analytical interference when measuring AD biomarkers [
5]. The release of brain proteins into the bloodstream may also cause degradation by proteases, leading to metabolism by the liver or elimination by the kidneys. This results in unpredictable changes that are not related to brain function, making it hard to find consistent blood markers for AD [
6]. Despite these challenges, technical advancements such as ultrasensitive immunoassays, mass spectrometrybased proteomic analyses, and bead-based multiplex assays offer renewed optimism [
7,
8]. In particular, blood biomarkers have recently demonstrated an ability to significantly change the diagnostic and prognostic approaches for AD and improve the planning of interventional trials [
9-
11].
We previously developed a bead-based multiple biomarker diagnostic tool named the QPLEX™ Alz plus assay kit (QM-Alz), which can predict the existence of cerebral Aβ deposition. The kit incorporates four blood biomarkers consisting of Aβ40, a galectin-3-binding protein (LGALS3BP), an angiotensin-converting enzyme (ACE), and periostin (POSTN), which have been employed in a previous study [
9-
11] and can distinguish between individuals who test positive or negative for PET imaging [
8,
12]. These four biomarkers are unique to this platform and have shown promising results in identifying cerebral Aβ deposition, and amyloid plaques and neurofibrillary tangles are two diagnostic candidates for AD. Moreover, efforts have been made to improve the performance of QM-Alz by using additional markers.
This study aimed to demonstrate the predictive effectiveness of QM-Alz, a composite of four markers, in differentiating between clinical groups—cognitively normal (CN) and those with AD— using an independent cohort from South Korea. We also aimed to compare the predictive capabilities of Aβ40 alone against those of QM-Alz, both with and without Aβ40 because the diagnostic performance of Aβ for AD may be compared with that of LGALS3BP, ACE, and POSTN, suggested previously as new AD biomarkers. Additionally, we intended to explore the potential improvement in the performance of QM-Alz in terms of clinical diagnosis and PET positivity by incorporating novel factors such as the apolipoprotein E genotype (ApoE) and galectin-3 (Gal-3). We propose a combination of biomarkers that offers the best potential for accurately diagnosing AD, which could be particularly useful in clinical assessments such as the Mini-Mental State Examination (MMSE) and the Clinical Dementia Rating (CDR).
MATERIALS AND METHODS
1. Participants
We recruited participants over the age of 40 who voluntarily decided to participate in the study after hearing the detailed explanation and fully understanding it from 15 referral hospitals in South Korea. We excluded participants who had other psychiatric diseases or specific surgical history. Detailed exclusion criteria are described in the
Supplementary File. Finally, a QM-Alz assay was performed on 128 participants. Most participants were recruited from the Samsung Medical Center (N=47) and Soonchunhyang University Bucheon Hospital (N=45). This project was part of a nationwide multicenter consortium named the Precision Medicine Platform for Mild Cognitive Impairment, based on Multiomics, Imaging, Evidence-based R&BD [
11]. This study was conducted following the Declaration of Helsinki, and approved by the Institutional Review Board of the Samsung Medical Center (IRB No. 2020-01-024[M1]) and each center. Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients for the publication of this paper.
2. Clinical diagnosis
Experienced neurologists diagnosed the participants based on general diagnostic criteria. The criteria for AD dementia are based on the proposal by the National Institute on Aging-Alzheimer’s Association Research Framework. The patients with AD exhibited CDR scores ranging from 0.5 to 3, and their MMSE scores were 10 or higher, indicating their suitability for Seoul neuropsychological screening battery-II (SNSB-II) testing. The SNSB-II evaluates many cognitive factors including verbal and visual memory, visuo-constructive function, language, praxis, components of Gerstmann syndrome (acalculia, agraphia, right/left disorientation, finger agnosia), and frontal/executive functions [
13].
3. Amyloid PET imaging and analysis
All participants underwent either F-florbetaben or F-flutemetamol PET scanning at each center using a Discovery Ste. PET/computed tomography scanner (GE Medical Systems, Milwaukee, USA). Briefly, mean doses of 311.5 MBq F-florbetaben or 185 MBq F-flutemetamol were injected into each individual and, 90 minutes later, an emission PET scan with dynamic mode (4×5-minute frames) was performed. Three-dimensional (3D) PET images were reconstructed using the ordered-subsets expectation maximization algorithm (iterations=4, subset=20). We performed image processing as in the previous studies to obtain a direct comparison Centiloid. The cut-off value of the direct comparison Centiloid was derived from receiver operating characteristic (ROC) curve analysis, which was previously described and calculated as 25.11 [
11].
4. Blood sampling and storage
Blood samples were collected in dipotassium ethylene-diamine-tetraacetic acid (K2 EDTA) tubes (BD Vacutainer Systems, Plymouth, UK) and centrifuged at 700×g for 5 minutes at 20°C. The plasma supernatants were stored at -80°C [
8,
12].
5. QM-Alz assay protocol
Aβ40, LGALS3BP, ACE, and POSTN were quantified using QM-Alz as it is. Gal-3 (R&D Systems, Minneapolis, USA) was coupled to Quantamatrix microdisks, the raw material used to manufacture QM-Alz. The coupling protocol was as follows: we chose a microdisk with a different code from the codes already used in QM-Alz, washed the microdisk with 50 mM MES, pH 5.0 (Sigma-Aldrich, Milwaukee, USA), activated the carboxylic acid on the surface of the microdisk with 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Hydrochloride (ThermoFisher, Waltham, USA) and N-Hydroxysulfosuccinimide sodium salt (ThermoFisher), incubated the microdisk for two hours with Gal-3 in MES, and washed it thrice with 1% bovine serum albumin (BSA).
Microdisks were placed in 96-well plates and 35 μL of diluted human plasma samples and equal amounts of biotin-conjugated detection antibodies were incubated for 90 minutes at room temperature. The microdisks were washed with a 0.1% BSA buffer and incubated with 2 μg/mL of R-phycoerythrin-conjugated streptavidin for 15 minutes at room temperature. The microdisks were washed, re-suspended in 0.1% BSA buffer, and analyzed using the Quantamatrix multiplex assay platform (Quantamatrix, Seoul, Korea). All reagents, microdisks, and 96-well plates that were used were included in QM-Alz.
6. ApoE genotyping
ApoE genotyping was performed as previously described. Briefly, the DNA of the patients was extracted from the blood samples using the QIAamp DNA Blood Mini Kit (QIAGEN GmbH, Hilden, Germany). The genotypes of rs7412 and rs429358 were analyzed by allele-specific real-time polymerase chain reaction (PCR). Depending on the genotypes of rs7412 and rs429358, variants of the
ApoE gene were classified as ε1, ε2, ε3, or ε4. The detailed genotype results are included in the supplementary data (
Supplementry Table 1), and participants with the ε4 gene were classified as
ApoE-positive [
13].
7. Data analysis
Statistical analyses were performed using MedCalc 20.115 (Ostend, Belgium). Several algorithms were generated using logistic regression to discriminate between CN and AD groups for each biomarker combination. The algorithms for distinguishing PET positivity were also separately generated through logistic regression. The basic equation of algorithm value was as follows:
Pi, algorithm values; an, coefficient values for each biomarker; mn, measured concentration for each biomarker; C, constant. Each biomarker concentration of the samples obtained with QM-Alz was multiplied by the coefficient values and Pi was calculated. To confirm the discrimination performance, area under the curve (AUC), ROC curve analysis, independent t-test, sensitivity, specificity, and correlation were calculated to evaluate the discriminatory power of the algorithms. The cut-off value was set to the value where the sum of sensitivity and specificity is maximized. Analysis of covariance (ANCOVA) was performed to investigate the effect of age on the overall analysis.
DISCUSSION
We aimed to evaluate the diagnostic ability of QM-Alz in detecting AD using blood samples. The QPLEX™ platform is a system with strengths in multiplexing, capable of measuring multiple biomarkers simultaneously. Furthermore, it is a bead-based 3D suspension array system that enhances reactivity and sensitivity. This advanced system enables the analysis of rare or volume-limited samples; it requires only 20 μL of undiluted human plasma to detect multiple biomarkers. QM-Alz simultaneously measures four blood biomarkers, Aβ40, ACE, POSTN, and LGALS3BP, and integrates the measurement results into an algorithmic framework. Previous studies have established the association of these four biomarkers with AD [
9-
11]. Notably, Aβ40 emerges as a prominent biomarker for AD, reflecting its significance in disease pathology [
9]. ACE, primarily recognized for its involvement in blood pressure regulation [
14], has also exhibited inhibitory effects on Aβ aggregation [
15]. Reduced ACE activity levels have been observed in patients with AD compared to cognitively normal individuals [
16]. POSTN, implicated in inflammatory diseases, has been identified within the cerebral cortex of patients with AD [
8], suggesting its potential role in the activated inflammatory response during AD pathogenesis. LGALS3BP functions as a receptor for Gal-3 and its interaction with ligands contributes to the inhibition of neutrophil activation [
17]. Our primary findings indicate that QM-Alz can differentiate not only PET positivity but also clinical AD diagnosis. To validate the results of previous studies, we confirmed the reliability and efficacy of QM-Alz to identify individuals with AD symptoms. This was consistent with our previous studies that demonstrated the efficacy of the kit as a bloodbased diagnostic tool for AD in an alternative cohort [
8,
12].
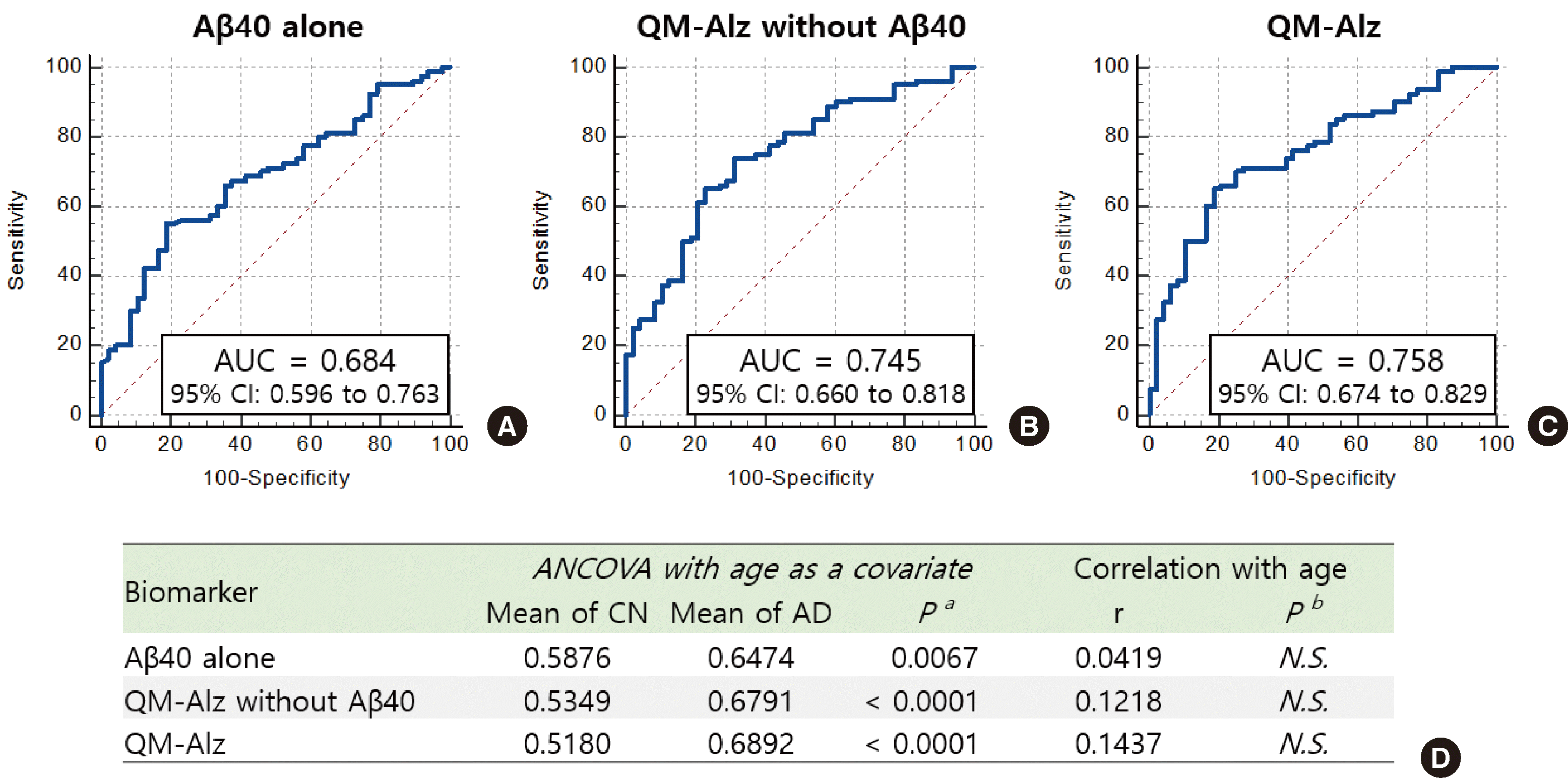
Furthermore, we demonstrated that the application of a multiple-marker approach may yield superior diagnostic results for AD compared to relying on Aβ alone. In agreement with our results, earlier studies reported that the AUC for blood Aβ in isolation was not as high as that for CSF Aβ, particularly for Aβ40, with an AUC ranging from 0.51 to 0.69 [
18,
19]. Our data suggest that QM-Alz with and without Aβ40 results in a higher AUC compared to Aβ40 alone (
Fig. 1), endorsing the use of multiple markers to enhance diagnostic precision.
Using biomarker combinations has advantages as a diagnostic tool. First, due to the heterogeneous nature of AD, a combination of markers appears to perform better than individual biomarkers in diagnosing and predicting the disease. For instance, a diagnostic prediction model based on a combination of plasma Aβ42/Aβ40 ratio, p-tau181, and neurofilament light chain (NFL) exhibited higher diagnostic value than each factor alone [
20]. Based on the complexity of AD pathogenesis, multivariate biomarker panels associated with various biological pathways may provide a more accurate diagnosis than individual markers. Second, bloodbased biomarker panels have been designed to estimate diseaserelated phenotypes such as cognitive decline, brain atrophy, and neocortical Aβ deposition beyond case-control studies [
21]. The combination of plasma biomarkers, namely P-tau217, the Aβ42/Aβ40 ratio, and NFL, provided the most robust results to predict cognitive decline in individuals with normal cognition [
22]. Furthermore, the addition of plasma P-tau181 to factors such as demographics, genetics, and clinical information significantly improved the prediction of memory decline in individuals with normal cognition and mild cognitive impairment [
4].
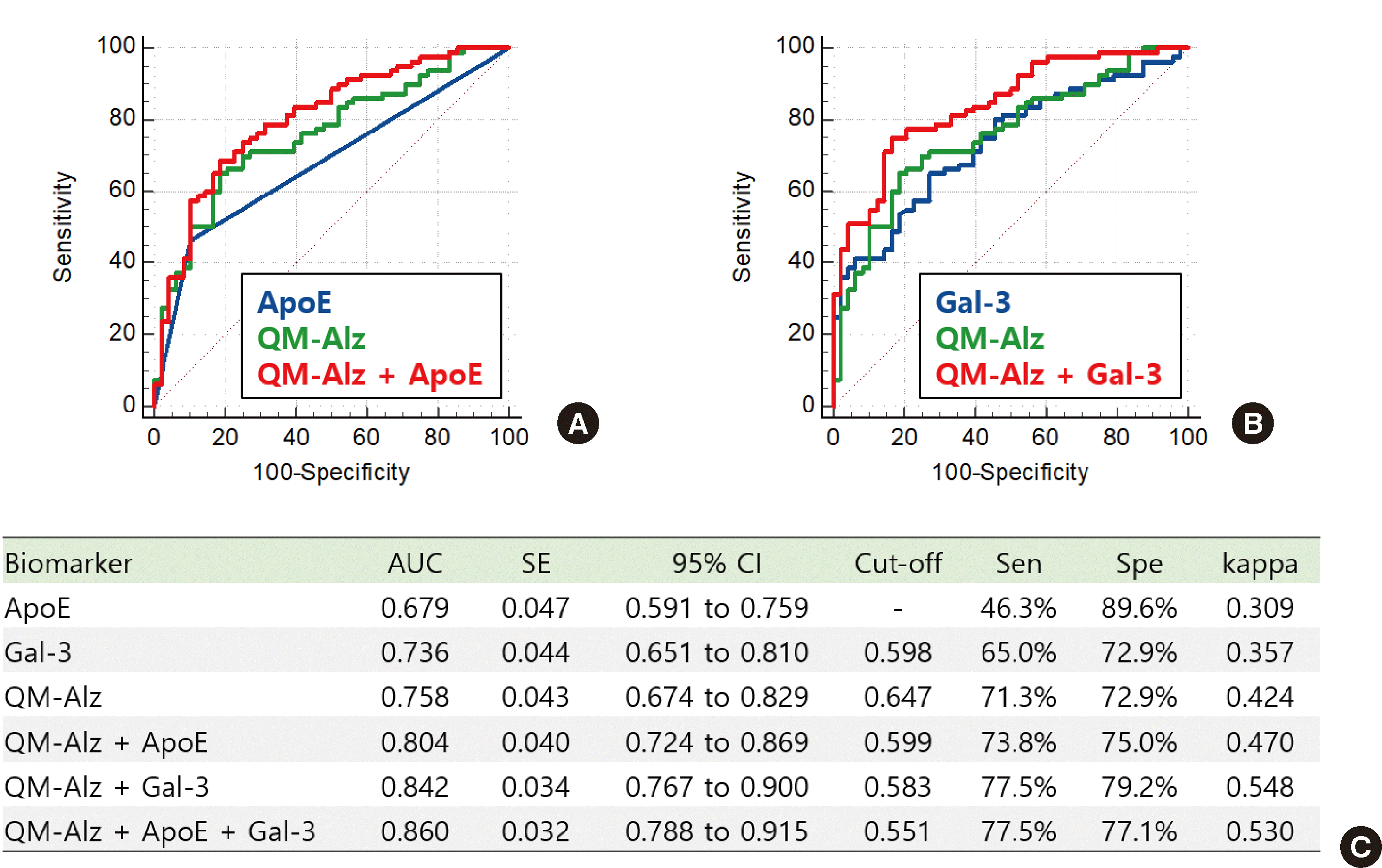
In our secondary analysis, we observed that the performance of QM-Alz could be improved in clinical diagnosis (
Fig. 2) and PET positivity (
Table 3) by including
ApoE and an additional blood protein, Gal-3. The ε4 allele of
ApoE remains the most potent genetic risk factor for sporadic AD [
23].
ApoE, a 34 kDa glycoprotein consisting of 299 amino acids, is co-deposited with Aβ in amyloid plaques. The interaction between
ApoE and pathological Aβ deposition appears to be the central mechanism through which
ApoE influences the risk [
24]. Recently, a combination of
ApoE and biomarkers has been employed in the development of diagnostic tools for AD. For instance, combining the plasma Aβ42/40 ratio with
ApoE and age improved the accuracy of identifying amyloid positivity compared to using
ApoE and age alone [
25]. Moreover, Gal-3 is a member of the galectin protein family known for its binding affinity toward β-galactoside molecules. Through its carbohydrate recognition domain, it identifies proteins with β-galactoside modifications, initiating a range of biological responses [
26]. Elevated Gal-3 levels in the bloodstream could be linked to AD, potentially serving as an early biomarker for disease detection [
27]. This connection may be attributed to the activation of pathways promoting apoptosis, inflammation, and compromised neurodegeneration, which are commonly observed in individuals with AD. Additionally, there is evidence suggesting a relationship between serum Gal-3 levels and cognitive status, observed both in patients with AD and those with normal cognitive function [
28]. We believe that the diagnostic accuracy of
QM-Alz can be improved by including
ApoE and Gal-3. QM-Alz, either alone or in combination with
ApoE and Gal-3, exhibited some correlation with clinical tests, such as the MMSE and CDR (
Table 2). The MMSE is a well-known and frequently employed cognitive screening tool to detect AD that offers a concise method for the evaluation of overall cognitive function in clinical, research, and community environments [
29]. The CDR is a widely used clinical scale with proven diagnostic and severity ranking values and has been extensively applied in international epidemiological studies, case series, and clinical trials [
30]. Therefore, these new combinations, as well as our kit, likely have the potential to serve as useful tools for cognitive impairment without a long survey time.
This study had some limitations. First, although the cohort used here was new and independent from the cohort that was used for the development and validation of the algorithm, it was still limited to East Asians, especially Koreans. Verification with other countries and ethnicities is needed for wider application. Second, we excluded behavioral variant frontotemporal dementia (bvFTD), nonfluent/agrammatic variant primary progressive aphasia (nfvPPA), and semantic variant primary progressive aphasia (svPPA) because their numbers were too small to make a comparison. As it is essential to investigate the performance of the kit in other types of dementia, such research will be necessary for future advancement. Third, we hoped that QM-Alz would be able to distinguish between CN and AD, as well as AD severity. However, there was no significant difference in the algorithm values between AD severities based on CDR scores. Fourth, an AUC of 0.8 is not a satisfactory accuracy. Ongoing research aims to improve this accuracy by identifying and incorporating additional biomarkers by leveraging the ability of the QPLEX™ platform to easily integrate new biomarkers. In particular, Aβ42 is well-known as an effective biomarker for AD diagnosis, but it has not been included in QM-Alz because an antibody exhibiting sufficient performance has not yet been secured. A follow-up study is underway to include Aβ42 in the kit.
In conclusion, we suggest that QM-Alz shows promising potential for the differentiation of clinical conditions and PET positivity using blood samples, with the addition of ApoE and Gal-3 further enhancing its performance. Future research should incorporate larger sample sizes and extended follow-up periods to better evaluate the performance of the kit in real-world clinical settings. The successful validation of this diagnostic tool could have significant implications for early detection and intervention in AD, ultimately leading to improved patient outcomes and enhanced quality of life. These findings hold considerable importance, as they offer a noninvasive and readily accessible method to predict AD development.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download