INTRODUCTION
Fibromyalgia (FM) is a rheumatological condition characterized by widespread and generalized musculoskeletal pain lasting for a minimum of 3 months [
1]. FM symptoms include muscle stiffness especially in the morning, tenderness, fatigue, joint pain, headaches, back pain, vulvodynia, menstrual pain, and tingling sensations in the extremities [
1]. This condition frequently results in sleep difficulties, cognitive impairments, anxiety, and depression, ultimately diminishing the patient's quality of life [
1]. The frequency of FM in the general population varies significantly by region, with the lowest rate in Venezuela at 0.2% and the highest prevalence in the United States at 6.4% [
2]. This condition is more prevalent in females than in males, with a prevalence increase of up to 9 times [
3].
The presence of pain in FM is linked to alterations in brain areas responsible for pain processing, reduced function of pain-inhibiting pathways, and heightened function of pain-promoting pathways [
4,
5]. However, the clear pathogenetic mechanism that underlies pain in FM is not fully understood and is believed to involve multiple central and peripheral pathways [
4,
5]. Duloxetine, milnacipran, and pregabalin are recommended by several guidelines for the pharmacological therapy of FM [
6]. Regrettably, these medications exhibit a poor level of response and a high frequency of discontinuation due to adverse effects such as irregular heart rhythms, impaired heart function, mental disorders, serotonin-related syndrome, and increased complications after surgery [
7–
9]. The European Medicines Agency also withholds approval for the use of these medications due to their unfavorable risk-benefit profile [
7–
9].
Naltrexone is classified as a non-selective opioid receptor antagonist medication [
10,
11]. This medication was initially launched in the 1980s as an adjunctive treatment to deter relapse in patients with a history of opiate or alcohol dependence [
10,
11]. Low-dose naltrexone (LDN) typically refers to doses ranging from 1 to 6 mg [
10,
11]. However, in clinical settings, doses as high as 9 mg of naltrexone have been administered for the treatment of FM [
10,
11]. It is important to note that there is currently insufficient evidence from large randomized controlled trials (RCTs) to support the use of these lower doses [
10,
11]. Given the recent publication of multiple RCTs, it is necessary to perform a meta-analysis in order to consolidate and analyze the evidence regarding the use of LDN for treating FM. The objective of our study is to assess the efficacy and safety of LDN in treating FM.
MATERIALS AND METHODS
1. Eligibility criteria
The systematic review and meta-analysis were conducted in accordance with established criteria, namely the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [
12]. The protocol of this review has been registered in PROSPERO (CRD42024553767). PICOS-based inclusion criteria must be satisfied in order to be qualified for inclusion in this research:
(1) Population = adult (≥ 18 years) patients with the diagnosis of FM.
(2) Intervention = received medication in the form of LDN (doses ranging from 1 to 6 mg daily).
(3) Control = received only a placebo for the treatment of FM.
(4) Outcome = have data on the:
- Efficacy = change in the pain score from baseline (primary outcome); change in the Revised Fibromyalgia Impact Questionnaire (FIQR) from baseline; change in the pressure pain threshold (PPT) from baseline; change in the pain catastrophizing scale (PCS)-R (rumination), M (magnification), H (helplessness) from baseline; and proportion of patients with at least a 30% improvement in pain.
- Safety = headache, vivid dreams, nausea, diarrhea, dizziness, and serious adverse events (SAEs).
(5) Study design = RCTs.
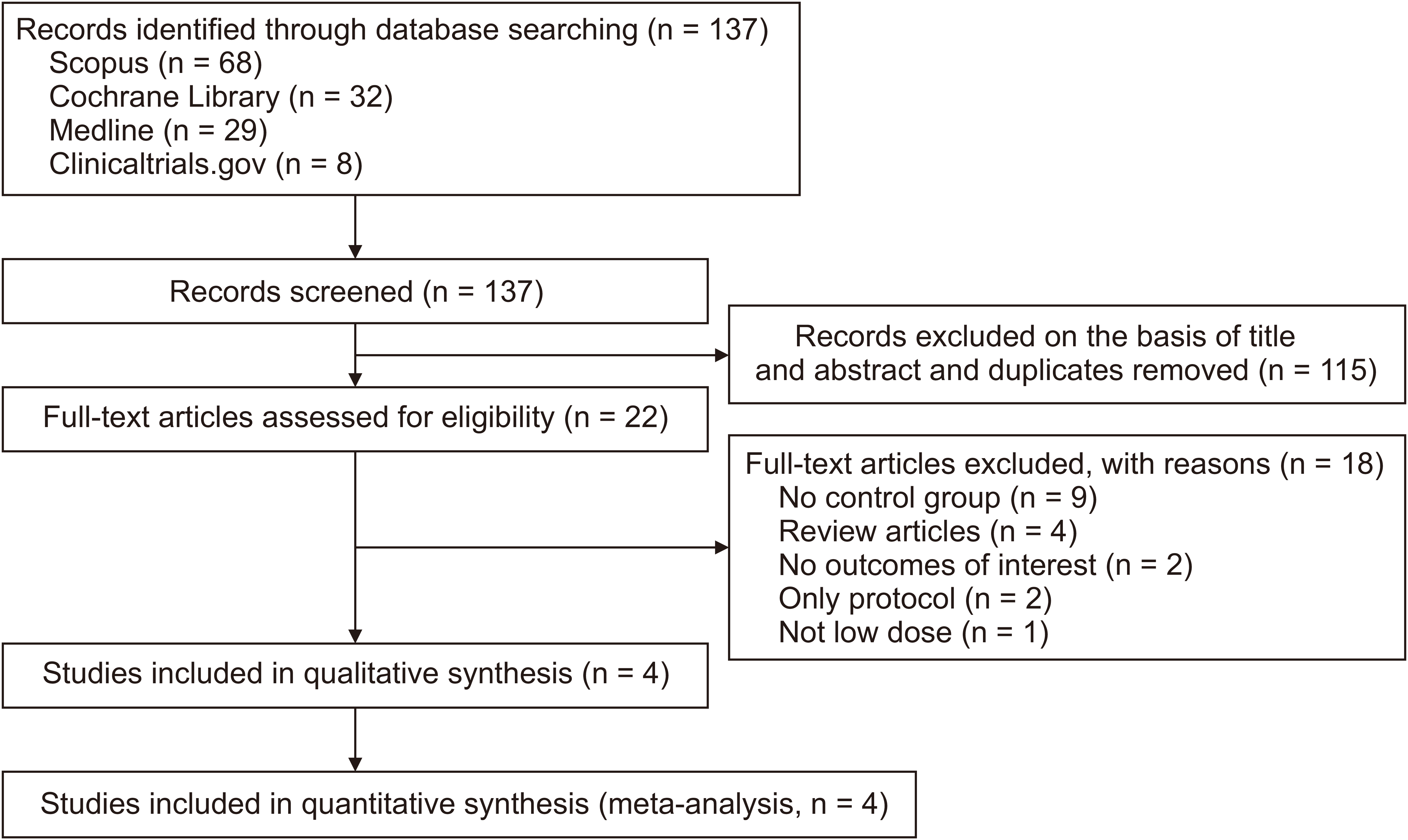
Meanwhile, the following studies were excluded: (1) population of interest consists of patients with chronic pain syndromes without a clear diagnosis of FM; (2) used a standard dose of naltrexone as an intervention; (3) compare the use of LDN with other interventions besides a placebo; (4) insufficient data to enable calculation of the outcome of interest; (5) studies lacking appropriate comparison group; (6) protocol, letter to editor, case-report, case-series, cross-sectional studies, observational studies, and non-primary research; (7) scholarly articles that are not readily available in their complete text or studies that have not yet undergone the publication process.
2. Search strategy and study selection
We conducted an extensive literature search in scientific databases such as Scopus, Medline, Cochrane Library, and ClinicalTrials.gov through May 20th, 2024. There were no restrictions on the length of time or language of publications. The combination of MeSH and non-MeSH terms were used for the search, as follows: "(naltrexone OR low-dose naltrexone OR LDN) AND (fibromyalgia OR fibromyalgia syndrome OR fibromyositis OR fibrositis OR FM OR FMS)". Additional information regarding the search methods employed for each database is provided in
Supplementary Table 1. During the initial search, the title and abstract of each paper were evaluated. If any primary research papers, which were cited in the systematic reviews or meta-analyses but were not initially found during the search process, met the established criteria for inclusion and exclusion, they would be incorporated into the study. The redundant articles were eliminated. If they pass the initial screening, the resulting full text was evaluated based on the given eligibility criteria. The screening was manually done by two of the authors. In instances where disagreements arose throughout the screening process, we endeavored to achieve resolution by soliciting the perspectives of an additional reviewer.
3. Data extraction
The procedure of data gathering was conducted autonomously by two reviewers. The retrieved data was presented in the following format: the study's author, publication year, study design, country of origin, sample size, LDN regimen, study duration, mean age, sex distribution, body mass index, and the desired outcomes.
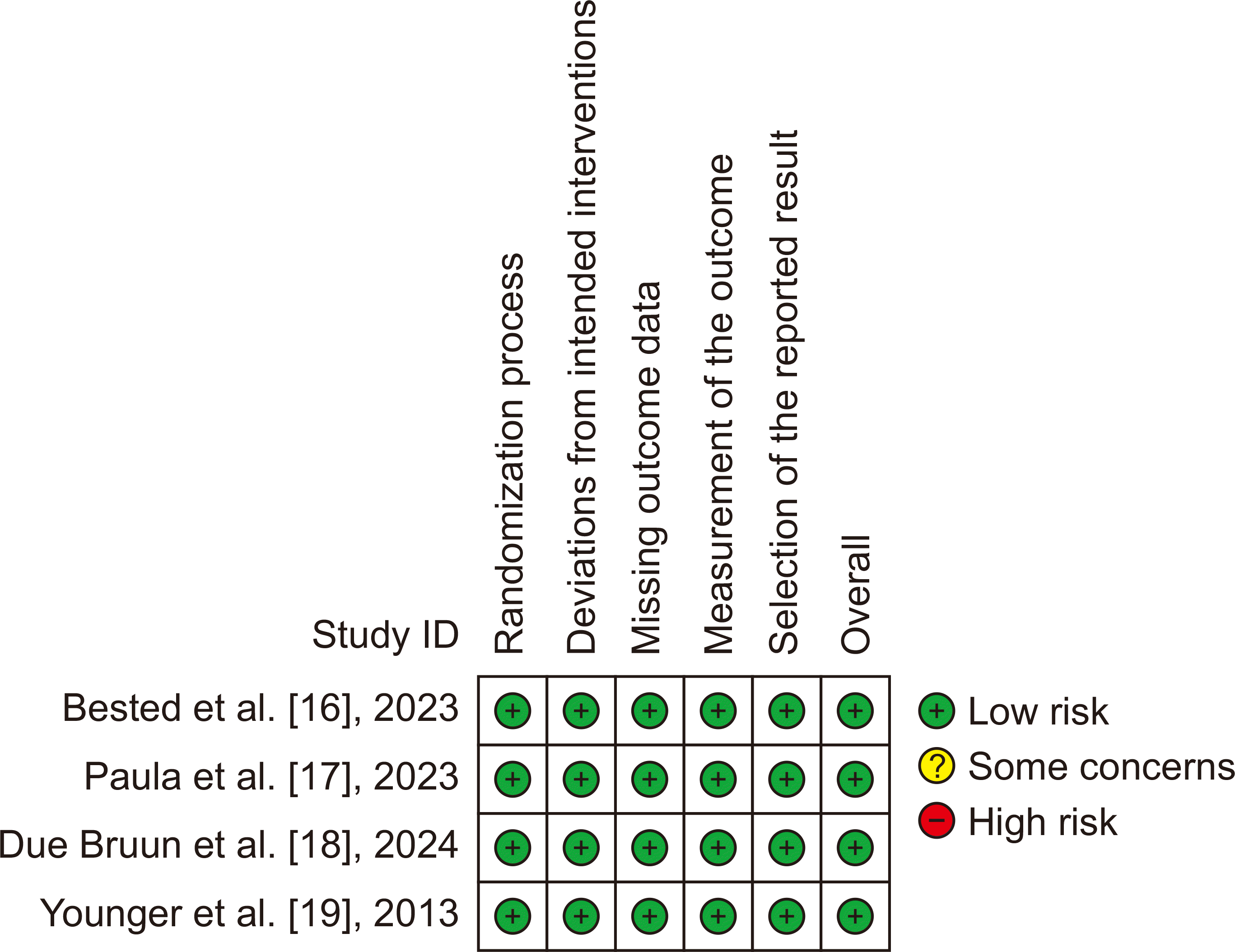
4. Risk of bias assessment
Two independent reviewers utilized standardized assessment tools to evaluate potential bias in each study. The researchers utilized Risk of Bias version 2 (RoB v2) to evaluate the quality of each RCT [
13]. This scale encompasses assessments of the randomization of study participants, deviations from intended interventions, absence of outcome data, measurement of the outcome, and selection of the stated results of the studies [
13]. The evaluations made by the authors were classified into three categories: "low risk," "high risk," or "some concerns" regarding bias [
13].
5. Statistical analysis
The Inverse-Variance technique was used to compute the mean difference (MD) and its corresponding 95% confidence interval (95% CI) for examining continuous variable outcomes. Meanwhile, Mantel-Haenszel technique was used to compute the odds ratio (OR) and its corresponding 95% CI across multiple studies examining a dichotomous outcome. The incorporation of a diverse array of participant characteristics necessitated a meticulous analysis of a substantial level of heterogeneity. The issue was addressed using random-effect models. The researchers employed the I-squared (I2) statistic to assess the level of heterogeneity seen across the research papers [
14]. Values exceeding 50% were deemed to signify a substantial or noteworthy degree of heterogeneity [
14]. In order to facilitate computational analysis, we transformed the data, which was originally presented as median (interquartile range) or median (minimum–maximum), into mean (standard deviation [SD]) using the formula developed by Wan et al. [
15]. When the number of included research studies is less than 10, the ability to detect publication bias through funnel plots or statistical tests is reduced compared to when the number of included studies exceeds 10. Therefore, only when the meta-analysis includes a number of studies over 10, the utilization of a funnel plot can be justified for the purpose of evaluating the presence of publication bias. The analysis in this study only employed Review Manager 5.4, a software program developed by the Cochrane Collaborations. A trial sequential analysis (TSA) was performed using TSA software version 0.9.5.5 Beta from the Copenhagen Trial Unit Centre for Clinical Intervention Research (
www.ctu.dk/tsa) to determine if the necessary information size was achieved for the evaluated outcomes from RCTs. We only performed TSA for the primary outcome of this study (change in the pain scores) and an additional two efficacy outcomes (change in the FIQR scores and the proportion of patients with at least 30% improvement in pain). The other efficacy outcomes cannot be analyzed in the TSA due to insufficient data to enable analysis (lack of mean ± SD from the included studies). Our analysis employed a random-effects model with a standard test threshold of
P < 0.05, a presumed two-sided test of significance, a power level of 80%, and 5% type 1 error for estimating information size. These calculations were adjusted for heterogeneity between studies.
DISCUSSION
The results of this comprehensive review and meta-analysis indicate that LDN is more effective than a placebo in lowering pain and improving the PPT in patients diagnosed with FM. Nevertheless, we observed no substantial disparity between LDN and a placebo for the improvement of FIQR or overall PCS scores. From a safety standpoint, the administration of LDN is linked to a higher occurrence of vivid dreams and feelings of nausea. Nevertheless, there were no notable disparities observed in relation to SAEs or other unfavorable events, such as headaches, diarrhea, and dizziness.
Naltrexone's active metabolite, belonging to the pure opioid antagonist class, has the ability to reversibly and competitively inhibit mu-opioid, delta-opioid, and kappa-opioid receptors [
22]. These receptors are involved in modulating pain [
22]. Naltrexone administration can diminish pain through beta-endorphin activity at mu-opioid receptors, which is involved in the endogenous analgesic process [
22].
Furthermore, naltrexone exerts an antagonistic influence on non-opioid receptors, specifically toll-like receptor 4, which is present on macrophages like microglia [
23]. Microglia are immune cells located in the central nervous system (CNS) that undergo activation in response to diverse stimuli [
24]. Upon activation, microglia release inflammatory and excitatory substances that induce symptoms of illness behavior, including heightened pain sensitivity, exhaustion, cognitive impairment, sleep disturbances, mood deviations, and overall discomfort [
24]. When consistently stimulated, the subsequent proinflammatory sequence can become harmful to the nervous system, leading to many negative consequences [
24]. Due to the diverse array of inflammatory substances released by activated microglia, such as proinflammatory cytokines, substance P, nitric oxide, and excitatory amino acids, various symptoms and medical consequences may be linked to the pathophysiological process of central inflammation [
23,
25]. Conditions like FM can result in persistent stimulation of glial cells and the subsequent release of proinflammatory substances [
23,
25]. Naltrexone decreases the generation of reactive oxygen species and other substances that can stimulate and cause damage to the nervous system by inhibiting the activation of microglia [
26,
27]. Opioid antagonists like naltrexone have been found to have an anti-inflammatory effect not only in the CNS but also in the peripheral tissues [
26,
27]. This is supported by the observation that these antagonists can reduce the production of tumor necrosis factor-alpha, interleukin-6, monocyte chemoattractant protein-1, and other inflammatory chemicals in macrophages located in the peripheral tissues [
26,
27]. It is postulated that naltrexone can enhance pain relief in individuals with FM through this mechanism [
26,
27].
The results of the authors’ present analysis align with the prior review conducted by Yang et al. [
28]. The researchers reported that overall, the administration of LDN was beneficial in treating the symptoms of FM patients, with no significant adverse effects [
28]. Nevertheless, the current review exhibits numerous distinctions from the prior review conducted by Yang et al. [
28].
First, Yang et al. [
28] only conducted a systematic review without including a meta-analysis. A systematic review provides a summary of the findings from the included research without conducting any additional data analysis on the results of each individual study [
29]. Therefore, it is not possible to obtain a more comprehensive understanding of the efficacy or safety of an intervention [
29]. By conducting a meta-analysis, we can extract summary effect sizes (such as OR, risk ratio, MD, standardized mean difference) from the available data of each study included [
29]. This will provide a comprehensive understanding of whether an intervention has a significant impact on an outcome and whether it is actually more effective than the control [
29]. The current review is enhanced by a meta-analysis that combines data from 4 RCTs to show the efficacy of LDN in lowering pain and improving PPT in patients with FM.
Second, though Yang et al. [
28] incorporated a greater number of studies, namely a total of 9 publications, it is important to note that out of these nine studies, only 1 study was an RCT. Meanwhile, the remaining 8 publications consist of 2 case reports, 2 case series, and 4 pilot trials [
28]. The Cochrane Collaborations, in its online guidelines, explicitly advises that research included in a systematic review and meta-analysis must conform to a homogeneous study design [
30]. This is due to variations in the risk of bias and confounding factors among different study designs [
31–
34]. Observational or case-series studies that lack randomization or blinding methods are susceptible to several biases, such as selection bias and information bias, which have the potential to impact the research conclusions [
31,
32]. Moreover, observational studies frequently lack the capacity to anticipate the existence of additional confounding variables that could also impact research conclusions [
31,
32]. RCTs can reduce the impact of confounding factors by using participant randomization [
33,
34]. Allocation concealment and blinding techniques can significantly mitigate biases, encompassing selection bias and information bias, for both participants and outcome assessors [
33,
34]. In addition, out of the nine papers analyzed by Yang et al. [
28], only one study included a placebo comparison group, while the other eight studies did not compare LDN with any control group. In studies examining interventions, the presence of a comparison group is crucial for obtaining a comprehensive understanding of the intervention's efficacy and safety [
35]. An intervention may demonstrate efficacy in improving an outcome, but upon comparison with a placebo, it is revealed that the intervention lacks a statistically significant distinction [
35]. Hence, it is crucial to incorporate research that involves control groups, especially in the context of an interventional study [
35]. The current evaluation exclusively incorporates RCTs that have control groups in the analysis to ensure more robust and reliable outcomes.
Finally, the prior review conducted by Yang et al. [
28] failed to perform a risk of bias assessment on the studies that were included. This goes against the PRISMA recommendations, which explicitly state that every study included in a systematic review or meta-analysis must be evaluated for bias using suitable approaches [
12]. The inclusion of studies with a significant risk of bias can potentially impact the findings of the investigations. Consequently, we performed a risk of bias evaluation using the RoB v2 methodology developed by the Cochrane Collaborations for each RCT included in this study.
The present investigation is not devoid of limitations. First, our analysis is solely derived from a restricted number of RCTs with a relatively small sample size of less than 100 individuals. Consequently, the extent to which the conclusions of this study may be applied to a broader population may be constrained. The possibility of type 1 error from the analytical results also cannot be neglected, therefore our results should be interpreted with a cautious mind. Second, this study revealed substantial heterogeneity in several outcomes of interest, possibly because of the distinct features of the population and the various lengths of follow-up periods. Finally, the included studies lacked data on functional impact, such as anxiety, depression, and decreased quality of life. As a result, additional analysis on this aspect is not possible. However, we believe that the findings derived from our comprehensive examination and meta-analysis can offer valuable perspectives on enhancing the management of FM patients. To validate the results of our investigation, additional multicenter RCTs with substantial sample numbers and extensive long-term data are required.
The systematic review and meta-analysis demonstrated that the utilization of LDN can significantly decrease pain ratings and enhance PPT in individuals diagnosed with FM. Nevertheless, the administration of LDN did not result in substantial changes in both FIQR and PCS. Additionally, it was linked to a higher occurrence of vivid dreams and nausea when compared to the placebo. Even so, LDN remains safe to administer since there was no observed escalation in SAEs or other side effects such as headache, dizziness, and diarrhea. The results of our study endorse the utilization of LDN in clinical practice as a viable therapeutic option for FM, aiding in the alleviation of pain.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download