INTRODUCTION
Herpes zoster (HZ) usually presents as a unilateral bandlike vesicular rash in the dermatome corresponding to the affected nerve caused by a reactivation of varicella zoster viruses (VZV) latent in the sensory ganglion [
1]. The crude prevalence of HZ in the general population is from 20% to 30%, with an increasing incidence over 50 years of age, with an approximate occurrence of 50% in those aged 85 years [
2]. During the acute episode, the treatment is focused on decreasing the intensity and duration of symptom and preventing complications. Postherpetic neuralgia (PHN), defined as acute zoster associated pain (ZAP) sustained for at least 90 days after the rash, is a debilitating complication of HZ. PHN becomes more common with increasing age, affecting about 5% of those younger than 60 years, increasing to 20% of those 80 years and older, according to a large population-based study [
3]. Unfortunately, there is still no reliable intervention that relieves the pain of PHN [
4]. Therefore, effective treatments to prevent PHN have become an important focal point in current research. Epidemiological research reported that interventions aimed at reducing the inflammation and repetitive painful stimuli during acute zoster might attenuate central sensitization, and consequently reduce the prevalence of PHN [
5]. In this respect, ultrasound (US)-guided paravertebral block (PVB) is effective in resolving pain in acute HZ and appears capable of preventing the incidence of PHN [
6–
8]. Compared with PVB technique, intercostal nerve block (ICNB) under US guidance is an easier superficial block with a very low incidence of complications for different surgeries involving the chest wall and for rib fractures [
9]. However, to the authors’ knowledge, there was only one comparative trial with a small sample, estimating the effect of ICNB for acute HZ [
10]. Therefore, it was hypothesized that the application of repetitive ICNBs technique under US guidance during the acute phase of HZ could significantly reduce the HZ-related burden of illness (HZ-BOI) over 30 days (HZ-BOI-AUC30 scores). It might be an alternative to the conventional thoracic paravertebral blocks (TPVBs) in providing acute pain management and possible prophylaxis for PHN in patients with thoracic HZ. Furthermore, it was a more time-efficient approach and had a better side effect profile compared to TPVB.
Go to :

RESULTS
A total of 169 patients were assessed for eligibility, but 41 cases were excluded due to given reasons in
Fig. 1, hence, 128 patients were included in the final analysis. There were no differences in demographic characteristics at baseline among the three groups (
Table 1).
Table 1
Baseline characteristics of participants in three groups
|
Variables |
Control group
(n = 26) |
TPVB group
(n = 49) |
ICNB group
(n = 53) |
F/χ2
|
P value |
|
Age (yr) |
64.15 ± 8.38 |
65.49 ± 8.06 |
66.10 ± 7.53 |
0.470 |
0.628 |
|
Female sex |
12 (46.2) |
26 (53.1) |
23 (43.4) |
0.983 |
0.612 |
|
Prodromal duration (day) |
11.70 ± 1.26 |
10.90 ± 1.33 |
10.65 ± 1.50 |
0.840 |
0.437 |
|
ZBPI: Baseline average pain score |
8 (4, 10) |
8 (6, 10) |
8 (7, 10) |
0.779 |
0.677 |
|
Distribution of pain |
|
|
|
1.978 |
0.740 |
|
Single thoracic dermatomal |
16 (55.2) |
33 (67.3) |
29 (54.7) |
|
|
|
2–3 thoracic dermatomal |
7 (26.9) |
11 (22.4) |
15 (28.3) |
|
|
|
≥ 4 thoracic dermatomal |
3 (11.5) |
5 (10.2) |
9 (17.0) |
|
|
|
Affected side |
|
|
|
1.113 |
0.573 |
|
Left |
15 (57.7) |
22 (44.9) |
26 (49.1) |
|
|
|
Right |
11 (42.3) |
27 (55.1) |
27 (50.9) |
|
|
|
Rash severity |
|
|
|
0.956 |
0.620 |
|
Number of lesions < 50 |
18 (69.2) |
37 (75.5) |
42 (79.2) |
|
|
|
Number of lesions ≥ 50 |
8 (30.8) |
12 (24.5) |
11 (20.8) |
|
|
|
Haemorrhagic lesion |
2 (7.7) |
6 (12.2) |
5 (9.4) |
0.438 |
0.804 |
|
Concomitant disease |
|
|
|
|
|
|
Hypertension |
11 (42.3) |
18 (36.7) |
16 (30.2) |
1.211 |
0.546 |
|
Diabetes mellitus |
6 (23.1) |
14 (28.6) |
17 (32.1) |
0.692 |
0.708 |
|
History of previous analgesic use |
|
|
|
1.551 |
0.818 |
|
None |
3 (11.1) |
5 (10.2) |
8 (15.1) |
|
|
|
NSAID |
15 (57.7) |
30 (61.2) |
34 (64.2) |
|
|
|
Anti-epileptic or week opioid |
8 (30.8) |
14 (28.6) |
11 (20.8) |
|
|

As shown in
Table 2, there was a significant decrease in HZ-BOI-AUC30 scores in both the TPVB and ICNB groups, in comparison to the control group. However, no significant difference was found between the TPVB and ICNB groups. More specifically, the mean in the control group was 152.2 (95% confidence interval [CI]: 124.7, 179.7), 94.7 (95% CI: 81.5, 107.8) in TPVB group, and 111.9 (95% CI: 97.4, 126.4) in the control group. The mean of BOI-AUC90 and BOI-AUC180 were comparable between the TPVB and ICNB groups, while they were significantly lower than those of the control group. The percentage of cases using rescue analgesics was lower in the TPVB and ICNB groups than the control group, but the difference was statistically significant only at D30 between the two intervention groups (celecoxib: 39.9%
vs. 11.0%
vs. 14.0%,
P < 0.001 at D30; 26.1%
vs. 9.6%
vs. 10.3%,
P = 0.037 at D
90; 20.3%
vs. 4.4%
vs. 8.1%,
P = 0.013 at D
180 and oxycodone & acetaminophen: 22.2%
vs. 9.6%
vs. 12.5%,
P = 0.202 at D30; 17.0%
vs. 5.9%
vs. 7.4%,
P = 0.039 at D
90; 10.5%
vs. 2.2%
vs. 2.9%,
P = 0.032 at D
180,
Fig. 3).
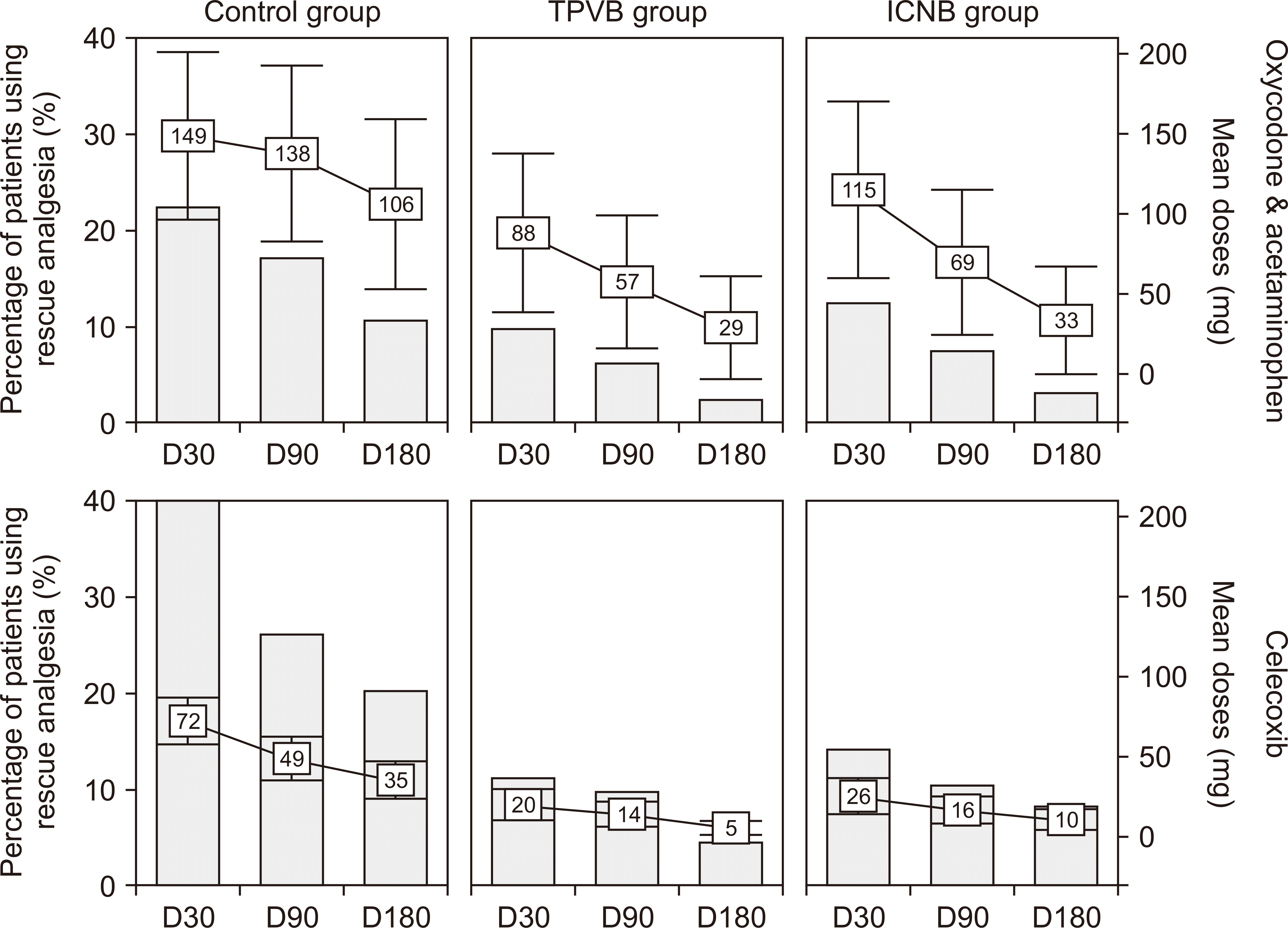 | Fig. 3Consumption of rescue analgesics in patients experiencing pain that may not be sufficiently controlled during the follow-up period. TPVB: thoracic paravertebral block, ICNB: intercostal nerve block. 
|
Table 2
HZ-BOI scores of three groups during study days 0–30, 30–90, and 90–180
|
Group |
BOI-30AUC |
|
BOI-30-90AUC |
|
BOI-90-180AUC |
|
M |
95% CI |
F |
P value |
M |
95% CI |
F |
P value |
M |
95% CI |
F |
P value |
|
Control (n = 26) |
152.2 |
124.7–179.7 |
9.052 |
< 0.001 |
|
129.5 |
106.1–152.9 |
10.704 |
< 0.001 |
|
117.9 |
96.5–139.2 |
24.062 |
< 0.001 |
|
TPVB (n = 49) |
94.7 |
81.5–107.8 |
|
|
|
82.3 |
69.1–95.4 |
|
|
|
57.9 |
50.8–65.0 |
|
|
|
ICNB (n = 53) |
111.9 |
97.4–126.4 |
|
|
|
79.9 |
70.1–89.7 |
|
|
|
62.6 |
52.6–72.6 |
|
|
|
|
Post-hot analysis |
MD |
95% CI |
P value |
|
MD |
95% CI |
P value |
|
MD |
95% CI |
P value |
|
|
Control vs. TPVB |
57.5 |
30.8–84.3 |
< 0.001 |
|
47.3 |
25.1–69.4 |
< 0.001 |
|
59.9 |
41.8–78.1 |
< 0.001 |
|
Control vs. ICNB |
40.3 |
14.3–66.2 |
0.003 |
|
49.6 |
26.7–72.4 |
< 0.001 |
|
55.3 |
37.6–72.9 |
< 0.001 |
|
TPVB vs. ICNB |
17.3 |
–3.4–38.0 |
0.101 |
|
2.3 |
–15.4–20.0 |
0.795 |
|
4.7 |
–9.3–18.8 |
0.507 |

Compared with the control group, the incidence of PHN was significantly lower in the TPVB and ICNB groups across all follow-up time points. However, no differences were found at D
90 and D
180 between the two intervention groups with respect to PHN incidence (45.4%
vs. 18.6%
vs. 20.9%,
P = 0.044 at D
90 and 36.4%
vs. 9.3%
vs. 14.0%,
P = 0.018 at D
180) (
Table 3).
Table 3
PHN incidence for three groups
|
Content |
Post-hoc analysis |
Difference in incidence
(95% CI) |
Rate ratio
(95% CI) |
χ2 value |
P value |
|
PHN incidence at D90
|
Control vs. TPVB |
11/26 (42.3%) |
9/49 (18.4%) |
23.9% (2.1%, 45.8%) |
3.259 (1.127, 9.428) |
4.979 |
0.032 |
|
Control vs. ICNB |
11/26 (42.3%) |
11/53 (20.8%) |
21.6% (–0.4%, 43.5%) |
2.800 (1.007, 4.070) |
4.033 |
0.045 |
|
TPVB vs. ICNB |
9/49 (18.4%) |
11/53 (20.8%) |
–2.4% (–17.8%, 13.0%) |
0.859 (0.322, 2.293) |
0.092 |
0.807 |
|
PHN incidence at D180
|
Control vs. TPVB |
8/26 (30.8%) |
4/49 (8.2%) |
22.6% (3.3%, 41.9%) |
5.000 (1.337, 18.695) |
6.459 |
0.019 |
|
Control vs. ICNB |
8/26 (30.8%) |
6/53 (11.3%) |
19.4% (–0.2%, 39.1%) |
4.267 (1.233, 14.770) |
5.775 |
0.024 |
|
TPVB vs. ICNB |
4/49 (8.2%) |
6/53 (9.4%) |
–3.2% (–14.6%, 8.3%) |
0.853 (0.215, 3.379) |
0.051 |
0.821 |

Patients among the three groups demonstrated a greater improvement in HR-QoL after 30, 90, and 180 days, as compared to their baseline value. However, the effects at D30, 90, and 180 were significantly more apparent in the two intervention groups. Differences between the TPVB and ICNB groups were not significant at D30 or at other follow-up time points. According to the EQ-5D-3L, significant improvements at all time points within the 6-month follow-up period were observed in two intervention groups regarding the domains of pain/discomfort (
P < 0.001 at D30,
P = 0.017 at D
90,
P < 0.001 at D
180), usual activities (
P < 0.001 at D30,
P < 0.001 at D
90,
P = 0.025 at D
180), mobility (
P = 0.029 at D30,
P = 0.042 at D
90,
P < 0.001 at D
180), symptom of anxiety/depression (
P = 0.037 at D30,
P < 0.001 at D
90,
P < 0.001 at D
180) and self-care (
P = 0.163 at D30,
P = 0.210 at D
90,
P < 0.001 at D
180), when compared with the control group (
Fig. 4).
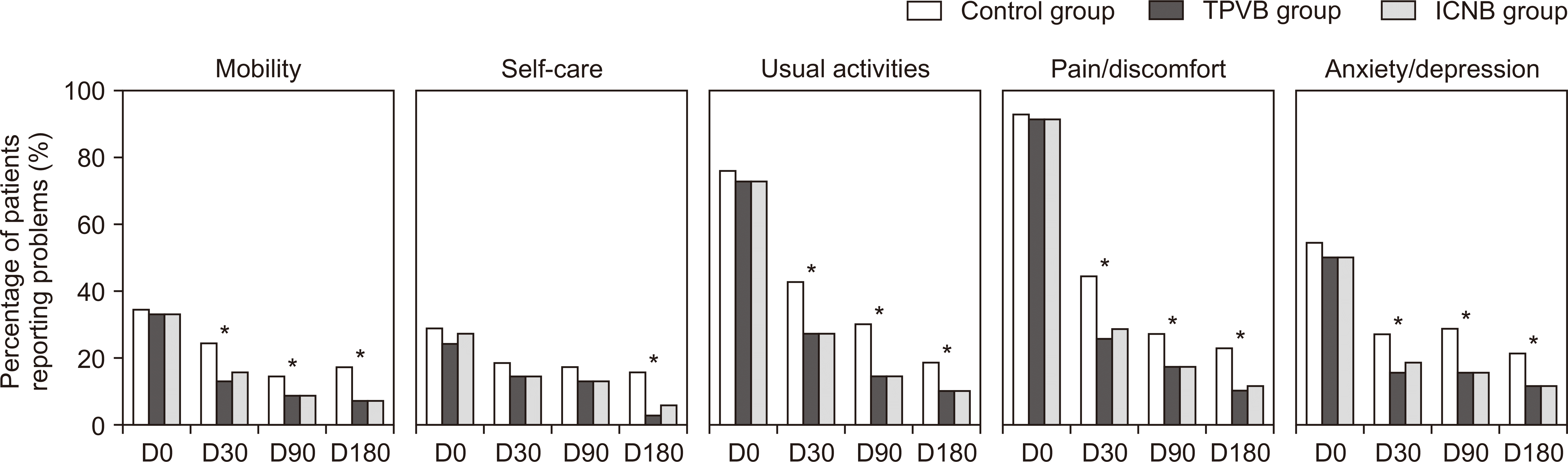 | Fig. 4The percentage of patients who reported problems in the five domains of EuroQol EQ-5D at the time of Day 0, 30, 90 and 180. TPVB: thoracic paravertebral block, ICNB: intercostal nerve block. *P < 0.05 when compared to control group. 
|
No serious adverse events were observed in the study. There was no serious intravascular injection in either the TPVB or ICNB group. While 11.6% and 7.0% of cases in the TPVB and ICNB groups experienced dizziness within 15 minutes after injection, respectively, the difference did not reach the level of statistical significance (P = 0.713). However, the incidence of patients complaining of insufferable pain during puncture was significantly higher in the TPVB group than in the ICNB group (67.4% vs. 23.3%, P < 0.001). Moreover, the ICNB approach was also associated with a significantly shorter procedure time as compared to the conventional TPVB (16.47 ± 3.39 vs. 11.69 ± 2.58, P < 0.001).
Go to :

DISCUSSION
The findings of this retrospective study illustrated that US-guided repetitive ICNBs for acute thoracic HZ significantly decreased illness burden over 30, 90, and 180 days. It was attributable to better analgesia in terms of reduced incidence of PHN, less rescue analgesic consumption, and greater improvements of HR-QoL between ICNB and AVT alone. In addition, the ICNB approach was more time-efficient than the conventional PVB.
Usually, AVT is recommended within 72 hours at the initial diagnosis of HZ. A considerable amount of evidence has demonstrated that, although antiviral agents and rescue analgesics as the current standard treatment for acute HZ can accelerate the healing of lesions and decrease acute pain, nevertheless, high quality evidence showed that oral antiviral drugs do not reduce the incidence of PHN significantly [
17]. During the acute phase of HZ, the reactive VZV replicates in the dorsal root ganglion (DRG), and transports to the peripheral nerve leading to an inflammation of the sensory ganglion and adjacent nerve as well as causing tissue damage, which mainly accounts for ZAP. Continuous infiltration of inflammation results in an abnormal expression of ion channels and consequently promoted the release of neuro-transmitters, up-regulated nociceptor excitability that leads to central sensitization, and makes the disease course persistent [
18]. Whereas, once the most common and difficult-to-cure complication of PHN develops, it not only decreases HR-QoL in patients but also significantly increases the healthcare burden at both the individual and societal levels. As a result, several supplemental interventional procedures have been tried according to the hypothesis that inhibition of the inflammatory process and sustained peripheral stimuli reaching the central nervous system throughout the acute phase not only alleviate central sensitization but also lower the occurrence of PHN, especially for those with risk factors including older age and greater severity of the prodrome, rash, and ZAP [
19]. Studies have shown that the administration of epidural corticosteroids is associated with a reduced PLA2 activity level within injured nerves to produce a direct anti-inflammation effect by preventing prostaglandin generation. It is also suggested that besides an anti-inflammation action, a corticosteroid was able to stabilize neural membranes, thus suppressing ectopic neural discharges with nerve injury to decrease nociceptive input. Local anesthetic (LA) may offer a therapeutic effect by improving intra-radicular blood flow to reduce neural dysfunction [
20–
22]. Therefore, early recognition and prompt management of ZAP with interventional treatment should be emphasized for the possible prevention of PHN.
Neuraxial and sympathetic administration of LA and corticosteroids including intrathecal, epidural, and sympathetic blocks have been reported to be effective in controlling pain caused by HZ and PHN. Although the beneficial effect appears to be consistent, this can be challenging when a neuraxial blockade is performed in the thoracic region as a result of the risk of serious complications including instability of hemodynamic, spinal hematoma, urinary retention, intractable headaches, as well as contraindicated conditions such as coagulopathy [
23]. Conversely, the PVB is one of the most common interventions used for managing pain associated with acute HZ. The PVS accommodates LA plus steroid spreading into the cephalad, caudal, intercostal, interpleural, epidural, and prevertebral spaces to generate block effect in the unilateral spinal nerve together with the rami communicants and the dorsal ramus, as well as the sympathetic chain [
24]. Although serious PVB-related adverse events are relatively rare, the most commonly occurring are inadvertent vascular puncture, followed by hypotension, haematoma, pleural puncture, and pneumothorax [
25]. In recent years, US guidance has been considered a standard localization technique for peripheral nerve block owing to visualization of the muscles, fascia, nerve, needle, and LA injectate without use of a contrast agent [
26]. Theoretically, the application of US technique to PVB can capture the direct visualization of the entire needle, while simultaneously confirming proper LA injection with the anterior displacement of the pleura to reduce the risk of adverse effects. Therefore, US-guided PVB is generally associated with a high success rate, with few adverse effects [
27]. Liu et al. [
6] estimated the efficacy of US-guided PVB intervention for the treatment of ZAP using a different course. They found that the best efficacy was achieved in the acute group. Several randomized controlled studies confirmed that lower BOI caused by acute pain and incidence of PHN during the entire 6-month follow-up were obtained after receiving US-guided repetitive thoracic PVB during the acute phase as opposed to the standard AVT [
7,
8]. These results are consistent with the present study, in that the early use of repetitive thoracic PVB under US guidance was more effective than antiviral medications alone in reducing HZ-related BOI and improving QoL at 30, 90, and 180 days after inclusion, on the basis of the authors’ experience, this technique remains the preferred strategy for inhibiting inflammation, facilitating nerve healing and suppressing the development of PHN, because lower occurrence of PHN was observed at D
90 and D
180 post-therapy according to the present study.
Considering that the risk of intravascular puncture and pneumothorax were increased by repeated injection even under US guidance, as well as the injury tendency due to the deeply located targeted nerve structure in thoracic PVB technique, the authors estimated the efficacy of the ICNB, a more lateral approach, in terms of assuring efficacy and decreasing complications in the current study. On the basis of US guidance, ICNB has been clinically used as an alternative to PVB to provide effective analgesia in a diversity of cases including mastectomy, cardiac, thoracic, and abdominal surgery [
28]. According to the anatomy, the intercostal space between the adjacent ribs is usually shallower and wider than that between the two thoracic TP, which allows a less steep needle angle trajectory and consequently results in a better visualization of the needle puncture under real-time guidance. In addition, this block technique can also reduce the risk of inadvertent neuraxial block and hematoma owing to the more lateral approach as compared to the conventional PVB [
29]. In accordance with what was expected, patients in the ICNB group showed less illness burden related to HZ at all time points during the follow-up period as compared to TPVB group. And significantly lower incidence of PHN at D
90 and D
180 were also observed in the ICNB group as opposed to the control group. There was no significant difference between the two intervention groups. Furthermore, the same better trend in improved QoL during the follow-up period was observed in the ICNB group and TPVB group as opposed to the control group. Importantly, less procedure time with a lower incidence of insufferable pain during puncture was observed in patients receiving ICNB demonstrating that the US-guided ICNB was an easier and more time-efficient approach than the conventional TPVB. However, providing complete pain relief, as perceived by patients during puncture, might be very challenging, especially when the ICNB procedures needs to be repeated. These findings were consistent with that of a previous comparative study, their results showed comparable data in the pain reduction, treatment duration, and injection frequency in both US-guided ICNB and the fluoroscopy (FL)-assisted epidural nerve block. But the ICNB is more accessible than the epidural block under FL guidance, which was recommended as an alternative option for thoracic HZ [
30]. Increasing evidence shows that perforation of pleura was one of the most serious complications of TPVB technique, however, no serious adverse events were observed in the study. This would be benefit from the measurement of pleura depth from entry point before puncture and the real-time guidance during puncture using ultrasonography. Nevertheless, unlike the TPVB, ICNB only targets the peripheral branches of the thoracic nerve roots, not the DRG itself, which primarily accounts for ZAP. Consequently, the positive results in comparing the effectiveness of PVB and ICNB in preventing PHN or other second outcomes might be understated due to the sample size being kept small by adopting HZ-BOI as the primary outcome.
The study did have some limitations. Firstly, although increasing evidence supported the use of US guidance technique in the peripheral nerve block, it was necessary to admit that this technique was highly experience dependent. Secondly, patients were allowed to use rescue analgesics in this study, which would be a confounding factor in the analysis of procedural efficacy. Thirdly, incidence of serious adverse events including inadvertent vascular puncture and pneumothorax was not significantly different between the two intervention groups, which might be due to the limited sample size, therefore, a well-designed randomized study with a large sample to investigate the safety of US-guided ICNB technique in acute HZ is needed.
In conclusion, both US-guided repetitive TPVBs and ICNBs were effective for acute HZ in thoracic dermatomes as compared to AVT alone, and accounted for the possible prophylaxis for PHN. Additionally, the ICNB approach was a time-efficient approach with a lower risk of side effects as opposed to conventional TPVB technique, which might be encouraged as an alternative to conventional TPVB.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download