Abstract
Background
MicroRNA (miRNA) plays a crucial role in neuropathic pain (NP) by targeting mRNAs. This study aims to analyze the regulatory function and mechanism of miR-382-5p/dual specificity phosphatase-1 (DUSP1) axis in NP.
Methods
We utilized rats with chronic constriction injury (CCI) of the sciatic nerve as the NP model. The levels of miR-382-5p and DUSP1 were reduced by intrathecal injection of lentiviral interference vectors targeting miR-382-5p and DUSP1. The mRNA levels of miR-382-5p and DUSP1 in the dorsal root ganglions (DRGs) were measured by RT-qPCR assay. The pain behavior was evaluated by mechanical nociceptive sensitivity and thermal nociceptive sensitivity. The expression levels of interleukin-6 (IL)-6, IL-1β, and tumor necrosis factor-α in the DRGs were analyzed by ELISA assay. The targeting relationship between miR-382-5p and DUSP1 was verified by DLR assay and RIP assay.
Results
Compared to the Sham group, the CCI rats exhibited higher levels of miR-382-5p and lower levels of DUSP1. Overexpression of miR-382-5p significantly decreased DUSP1 levels. Reducing miR-382-5p levels can lower the mechanical nociceptive sensitivity and thermal nociceptive sensitivity of CCI rats and inhibit the over-activation of pro-inflammatory factors. Reduced miR-382-5p levels decreased NP in CCI rats. DUSP1 is the target of miR-382-5p, and down-regulation of DUSP1 reverses the inhibitory effect of reduced miR-382-5p levels on NP.
Neuropathic pain (NP) is a primary type of chronic pain, defined by the International Association for the Study of Pain as pain resulting from lesions of the somatosensory nervous system or diseases [1]. NP is classified into central NP and peripheral NP, depending on the initially affected site [2]. The main characteristics of NP are hyperalgesia, abnormal pain, spontaneous pain and paraesthesia [3]. According to epidemiological studies, NP patients make up 20%–25% of chronic pain patients, and the pain degree of NP is more severe than that of non-NP [4]. This specific type of pain impacts 7%–10% of the global population, compromising patients' quality of life and increasing their financial burden [5]. Currently, the mechanisms behind NP's onset and development remain unclear, and clinical treatments have limited effectiveness. Therefore, a comprehensive understanding of the molecular mechanism of NP's pathogenesis could aid in developing new therapeutic strategies.
MicroRNAs (miRNAs) play a crucial role in gene expression regulation, with over 60% of mRNA being targeted by miRNAs [6]. They are essential for maintaining the nervous system's function and can influence the onset and development of NP by regulating neuroinflammation [7]. As a member of the miRNAs, miR-382-5p has been shown to be associated with nerve damage and inflammatory diseases. For example, during spinal cord injury, the inflammatory response of microglia is linked to increased levels of miR-382-5p, which inhibits spinal cord injury repair [8]. Conversely, down-regulation of miR-382-5p can inhibit neuronal apoptosis and neuroinflammation of human neural precursor cells [9]. Extracorporeal shock waves can facilitate neural function recovery by inhibiting miR-382-5p expression [10]. Furthermore, miR-382-5p levels have also been shown to be elevated in the affected skin of NP patients after shingles [11]. Studies have indicated that promoting the conversion of microglia to an anti-inflammatory phenotype is a potential strategy for treating NP. Dual specificity phosphatase-1 (DUSP1) is a key component in the regulation of anti-inflammatory responses, and overexpression of DUSP1 has been shown to attenuate pain behaviors in NP rats by promoting microglia polarization [12]. Additionally, researchers found that activating DUSP1 plays a neuroprotective role in nerve damage by inhibiting neuronal apoptosis and inflammation of microglia [13]. However, whether miR-382-5p influences NP by regulating the expression of DUSP1 remains to be further studied.
Based on previous studies, it was hypothesized that miR-382-5p plays a role in the onset and progression of NP, and DUSP1 may be the target of miR-382-5p in NP. The miR-382-5p/DUSP1 axis may be a new target for treating NP.
We purchased 120 adult male Sprague-Dawley rats (180–200 g) from SLAC Laboratory Animal Co. The rats were kept at room temperature of 20°C–25°C, relative humidity of 40%–70%, normal 12 hr circadian rhythm, and they were free to eat and drink. The Shengli Oilfield Central Hospital Laboratory Animal Ethics Committee (no. Q/ZXYY-ZY-YWB-LL202247) approved the experimental protocol. All animal experiments adhered to national and international guidelines to minimize animal discomfort.
One hundred twenty adult male Sprague-Dawley rats were divided into two batches (two rats were removed from the study due to death). The first batch of 40 rats was randomly divided into the sham group (n = 20) and the chronic constriction injury (CCI) group (n = 20). The rats were fed in the same environment as before, and behavioral tests were conducted after 2 weeks of acclimatization in the animal house. Behavioral tests were performed at 0, 3, 7, 14, and 21 days after CCI surgery, four rats were euthanized at each time point, and dorsal root ganglions (DRGs) were collected for real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR), Western blot, and enzyme-linked immunosorbent assay (ELISA) experiments. The second batch of 78 rats was randomly divided into the sham group (n = 13), CCI group (n = 13), CCI + LV-anti-NC group (lentivirus negative control was injected before CCI, n = 13), CCI + LV-anti-miR-382-5p group (lentiviral interference vectors targeting miR-382-5p were injected before CCI, n = 13), CCI + LV-anti-miR-382-5p + sh-NC group (lentiviral interference vectors targeting miR-382-5p and lentiviral negative controls were injected before CCI, n = 13), CCI + LV-anti-miR-382-5p + sh-DUSP1 group (lentiviral interference vectors targeting miR-382-5p and DUSP1 were injected before CCI, n = 13). The rats were fed in the same environment as before, and behavioral tests were conducted after 2 weeks of acclimatization in the animal house. Behavioral tests were performed at 0, 3, 7, 14, and 21 days after CCI surgery. All rats were euthanized on day 21, and DRGs were collected for RT-qPCR and ELISA experiments (Fig. 1).
We constructed the NP model through CCI surgery. First, the rats were anesthetized with pentobarbital sodium (40 mg/kg), then were positioned prone to expose the left lower limb. An incision was made in the femoral skin of the left lower limb and separated the tissue layer by layer. The sciatic nerve was then ligated with 4-0 chromium sheep's intestines at 1-mm intervals, four times in total. The ligature strength was controlled to avoid being too loose or too tight, and was optimal when it produced a small, temporary twitch of the thigh. After ligation, the incision was sutured layer by layer. Post-surgery, the rats were placed on a heating pad until they woke up. In the sham group, only the sciatic nerve was exposed without ligation.
The technique of intrathecal injection was used as previously reported [14]. After anesthetizing the rats with pentobarbital sodium (40 mg/kg), The rats are then immobilized in a prone position with their waists elevated. A 2 cm median longitudinal incision was made along the L5-L6 lumbar interspace. The subcutaneous fascia and muscle around the incision were bluntly separated, and the spinous processes were removed using bone-biting forceps to expose the dura mater. Then, the dura mater was gently punctured with a needle (22G). The needle had penetrated the dura mater when the rat's tail flutters or twitches on both sides and emits clear fluid. Subsequently, a PE10 catheter is then inserted about 2 cm into the subarachnoid space and fixed with a 4-0 thread once the cerebrospinal fluid flowed out. After securing the catheter, the wound was sutured and sterilized. At the end of the procedure, 20,000 U of penicillin was injected intraperitoneally into the rat. The rats are observed continuously for four days after the catheterization, excluding any with paralysis, infection, or catheter detachment. In addition, 20 μL of lidocaine was injected into the catheter, and successful intrathecal injection was indicated if the rats were immediately paralyzed in both hind limbs and resumed normal locomotion within 30 min. Next, to reduce miR-382-5p and DUSP1 levels in the rats, lentiviral interference vectors targeting miR-382-5p and DUSP1 (LV-anti-miR-382-5p, sh-DUSP1) and their negative controls (LV-anti-NC, sh-NC) were injected intrathecally into the rats at a volume of 10 μL . This was done 72 hours prior to the sciatic nerve chronic constriction surgery.
Behavioral tests in the rats included both mechanical nociceptive sensitivity and thermal nociceptive sensitivity. These were measured by paw withdrawal threshold (PWT) and paw withdrawal latency (PWL). The mechanical nociceptive sensitivity and thermal nociceptive sensitivity were evaluated on days 0, 3, 7, 14, and 21 of CCI surgery.
The rats were given 30 minutes to acclimate in a glass box prior to the test. After 30 minutes, Von Frey fibers (Stoelting) were utilized to vertically stimulate the left hind foot of the rats. The pressure was recorded when the rat displayed signs of pain, such as foot retraction or licking. Each rat underwent five stimulations, with at least 10 minutes interval between each.
The rats were given 30 minutes to acclimate in a glass box prior to the test. After 30 minutes, the bottom of the left hindfoot of the rats was heated with a thermal radiator (preheated 2–3 minutes in advance) under the glass plate. The latency period from the start of the stimulation to the retraction of the hind foot was recorded. To prevent potential burns to the rat's plantar soft tissue, a maximum exposure limit of 30 seconds was set for the radiant heat. Each rat underwent this test five times, with a minimum of a 10-minute interval between each test.
After the behavioral experiment, the DRGs from each group of rats were collected for follow-up experiments. The collection time and number of DRGs are based on the "experimental grouping" mentioned above. Briefly, after anesthesia, the dorsal skin and fascia of the rats were cut open. The spinous processes and transverse processes from the thoracic to the sacral vertebrae were cut to expose the spinal cord. The L4-L5 DRGs were then removed.
RT-qPCR kits were purchased from TIANGEN. First, the total RNA was extracted from the DRGs using TRNzol Universal Reagent. Next, the RT-PCR was performed utilizing the FastKing gDNA dispelling RT SuperMix Kit and the miRcute miRNA First-Strand cDNA Synthesis Kit. Lastly, the SuperReal PreMix Plus (SYBR) Kit instructions were followed to configure the qPCR reaction system and perform amplification reactions. The primer sequences are displayed in Table 1. U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as internal references for miR-382-5p and DUSP1 normalization, respectively.
Briefly, the DRGs were thawed at room temperature, and then RIPA lysis solution (Beyotime) was added to the DRGs. After sufficient lysis, they were centrifuged (10,000 g, 5 minutes) and the supernatant was collected. Subsequently, the levels of interleukin (IL)-6, IL-1β and tumor necrosis factor-α (TNF-α) were measured according to the instructions of the ELISA assay kit (Invitrogen).
Total Protein Extraction: Frozen DRGs were thawed at room temperature. RIPA lysis solution was added and allowed to sufficiently lyse the tissues. The homogenate was then transferred to a low-temperature centrifuge for centrifugation (10,000 g, 5 minutes). The supernatant was collected at the end of centrifugation. Protein Denaturation: Protein samples were mixed with 5 × SDS buffer (Beyotime) and heated in boiling water for about 10 minutes. After denaturation, the samples were stored in a –20°C refrigerator. Western Blot: Total proteins were separated by 10% SDS-PAGE gel electrophoresis and transferred onto a PVDF membrane (Millipore). The PVDF membrane was incubated with the diluted anti-DUSP1 antibody (ab236501, 1/2,000 dilution, Abcam) and anti-GAPDH antibody (ab8245, 1/1,000 dilution, Abcam) overnight at 4°C. The PVDF membrane was washed with TBST solution 3 times and incubated with the second antibody (ab205719, 1/2,000 dilution, Abcam) for 2 hours. The target proteins on the PVDF membrane were detected by the chemiluminescence detection system (Amersham). The grayscale values of the target bands were analyzed using Image-pro Plus 6.0 software.
ENCORI (https://rnasysu.com/encori/index.php) was used to predict the target genes of miR-382-5p. The authors found that DUSP1 has a potential binding site with miR-382-5p. The pmirGLO dual luciferase reporter plasmid containing DUSP1 wild type (DUSP1-WT) and DUSP1 mutant (DUSP1-MUT) was synthesized. HEK-293T cells (Pricella) were inoculated in 24-well plates, and DEME medium was added. When the cell density reached 50%, the DUSP1-WT and DUSP1-MUT recombinant plasmids, miR-382-5p mimic, miR-382-5p inhibitor, mimic NC and inhibitor NC were transfected into HEK-293T cells using Lipofectamine 2000 (Invitrogen). Luciferase activity was then assayed using a dual luciferase assay kit (Beyotime) after 48 hours.
First, the DRGs were lysed using RIP lysis buffer. Afterwards, the lysate was co-incubated with magnetic beads containing anti-Ago antibody (ab186733, 1/1,000, Abcam) and anti-IgG antibody (ab172730, 1/1,000, Abcam). Next, proteinase K was added to digest proteins and retrieve the immunoprecipitated RNA. Lastly, levels of miR-382-5p and DUSP1 were analyzed in the precipitates using an RT-qPCR assay.
The data was analyzed using GraphPad Prism 9.0 software. All data are expressed as mean ± standard deviation. Statistical analysis was performed using Student's t-test and one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test for multiple-comparison analysis. Repeated measures ANOVA was performed on the data at different time points between the groups. P < 0.05 was considered statistically significant.
To understand the role and molecular mechanism of miR-382-5p in NP, a rat model of NP was constructed using CCI surgery. From day 3 after surgery, there were significant differences in mechanical nociceptive sensitivity and thermal nociceptive sensitivity between the sham and CCI groups. On day 3 after surgery, PWT and PWL in CCI rats were significantly lower than in sham rats (P = 0.003 and P < 0.001, respectively, Fig. 2A, B). This suggests that NP rats are more sensitive to pain. Additionally, the levels of neuroinflammatory factors in the DRGs were analyzed using ELISA assay. On the 21st day after surgery, pro-inflammatory factors (IL-6, IL-1β, TNF-α) were higher in the CCI group compared to the sham group (P < 0.001, Fig. 2C–E). Using an RT-qPCR assay, the level of miR-382-5p was measured in the DRGs. As illustrated in Fig. 2F, miR-382-5p levels in the DRGs of CCI rats were significantly elevated on day 7 after CCI surgery (P = 0.002); on days 14 and 21 after CCI surgery, miR-382-5p levels remained elevated, and both were significantly higher than in the sham group (P < 0.001).
To explore the effects of miR-382-5p on pain behavior in NP rats, lentivirus-mediated miR-382-5p interference vector were injected into CCI rats 3 days before CCI surgery. Intrathecal injection of LV-anti-NC did not significantly alter miR-382-5p levels. However, after intrathecal injection of LV-anti-miR-382-5p, miR-382-5p levels were reduced (P < 0.001, Fig. 3A). Compared to the sham rats, PWT and PWL of CCI rats decreased significantly on day 3 after CCI surgery (P = 0.002 and P < 0.001, respectively, Fig. 3B, C). However, on day 3 after CCI surgery, PWT and PWL in the CCI + LV-anti-miR-382-5p group increased compared to the CCI + LV-anti-NC group (P = 0.004 and P = 0.012, respectively); On day 7, 14, and 21 after CCI surgery, PWT and PWL in the CCI + LV-anti-miR-382-5p group were also significantly increased (P < 0.001, Fig. 3B, C). The down-regulation of miR-382-5p could mitigate the pain behavior in NP rats.
We explored the effect of miR-382-5p on neuroinflammation in NP rats through the intrathecal injection of a lentivirus-mediated miR-382-5p interference vector. As depicted in Fig. 4, compared to the CCI + LV-NC group, the levels of pro-inflammatory factors (IL-6, IL-1β, TNF-α) were lower in the CCI + LV-anti-miR-382-5p group (P < 0.001). Down-regulation of miR-382-5p could alleviate the over-activated neuroinflammation in NP rats.
We found that DUSP1 has a binding site with miR-382-5p (Fig. 5A). DLR and RIA assays further confirmed the targeting relationship between them. The DLR assay showed that miR-382-5p mimic and inhibitor could decrease and increase the luciferase activity of DUSP1-WT, respectively (P < 0.001, Fig. 5B). The RIP assay showed that both miR-382-5p and DUSP1 could be enriched in magnetic beads containing Anti-Ago2 (P < 0.001, Fig. 5C). The DUSP1 mRNA level in the DRGs of CCI rats decreased (P < 0.001, Fig. 5D). The protein level of DUSP1 decreased on days 7, 14 and 21 after surgery compared to day 0 (P = 0.033, P < 0.001 and P < 0.001, respectively, Fig. 5E, F). Moreover, compared with CCI + LV-anti-NC rats, the level of DUSP1 in CCI + LV-anti-miR-382-5p rats increased (P < 0.001, Fig. 5G).
As depicted in Fig. 6A, LV-anti-miR-382-5p elevates the level of DUSP1 in CCI rats (P < 0.001). However, this effect is reversed by sh-DUSP1(P = 0.001). Similarly, sh-DUSP1 also reversed the increase of PWT and PWL and the decrease in pro-inflammatory factors (IL-6, IL-1β, TNF-α) induced by LV-anti-miR-382-5p. As shown in Fig. 6B, C, both PWT and PWL increased after the intrathecal injection of LV-anti-miR-382-5p (P < 0.001). Conversely, PWT and PWL decreased after the injection of LV-anti-miR-382-5p and sh-DUSP1 (P < 0.001 and P = 0.006, respectively). As depicted in Fig. 6D–F, LV-anti-miR-382-5p decreased IL-6, IL-1β, and TNF-α levels in DRGs in rats (P < 0.001), while sh-DUSP1 reversed this phenomenon (P < 0.001, P = 0.007 and P < 0.001, respectively). These results suggest that miR-382-5p regulates pain behavior and neuroinflammation in NP rats by targeting DUSP1.
NP is a common clinical disease with a complex pathogenesis, diverse etiology and symptoms, and lack of effective treatment [15]. NP cannot be simulated in the human body, so choosing an ideal NP model for the basic research is crucial. The CCI model is a commonly used animal model of NP. The CCI model simulates nerve fiber injuries located primarily on the surface of peripheral nerves. This model produces a partially denervated sciatic nerve by ligating the sciatic nerve and thus retains some behavioral responses to peripheral stimuli, such as mechanical nociceptive sensitization and thermal nociceptive sensitization [16–18]. Therefore, the pain behavior of NP rats may be assessed by measuring their mechanical nociceptive sensitivities and thermal nociceptive sensitivities. The authors constructed an NP rat model and measured the PWT and PWL of NP rats at different time points. From day 3, PWT and PWL were significantly reduced in CCI rats. The results indicated that CCI rats showed NP behaviors of mechanical nociceptive sensitivity and thermal nociceptive sensitivity. The NP model was successfully prepared. Considering the effect of the surgical procedure itself on inflammation, the sham group of rats was established and were used as a control group. The sham group underwent the same surgical procedure as the CCI group, with the only difference being that the Sham group only exposed the sciatic nerve without ligation. The experimental results showed that sham rats did not exhibit NP behaviors such as reduced mechanical nociceptive sensitivity and thermal nociceptive sensitivity, which justified the use of sham rats as an appropriate control for the CCI model.
MiRNAs inhibit post-transcriptional gene expression by binding to target mRNA [19] and play a crucial role in regulating cell growth, differentiation, development and apoptosis [20]. Several recent studies suggest that abnormal miRNA expression in NP and propose miRNAs as promising targets for NP therapy [21]. For example, miR-101 was found to be reduced in NP patients and inversely correlated with neuroinflammatory factor levels [22]. Additionally, in NP rats, exosomes carrying miR-181c-5p were observed to alleviate NP [23]. Another miRNA, miR-382-5p, was upregulated in NP patients and associated with neuroinflammation and neuronal apoptosis following spinal cord injury [9]. Therefore, in order to investigate miR-382-5p's role in NP, the authors created a rat model of NP via CCI surgery and injected a lentivirus-mediated miR-382-5p interference vector into the CCI rats. The results revealed that miR-382-5p is up-regulated in the DRGs of CCI rats, and down-regulated miR-382-5p can alleviate the rats' pain behavior and reduce the level of inflammatory factors in the DRGs. Neuroinflammatory responses play a significant role in the development of NP. After nerve injury, macrophages and microglia recruited to the injured site release pro-inflammatory cytokines that can induce chronic ectopic electrical activity of the injured neurons and exacerbate pain [24]. These proinflammatory cytokines are associated with central NP and peripheral NP [25,26]. Therefore, effectively inhibiting neuroinflammatory responses could be a crucial therapeutic strategy to mitigate the progression of NP. Thus, these findings suggest that miR-382-5p is associated with neuroinflammation in NP. By suppressing neuroinflammation, down-regulating miR-382-5p could potentially alleviate NP in NP rats.
We used bioinformatics analysis tools to predict the target genes of miR-382-5p to better understand its molecular mechanism in NP. It was found that DUSP1 has a potential binding site with miR-382-5p. Prior studies have highlighted DUSP1’s vital role in neuroprotection. DUSP1 reduces neuroinflammation and neuronal damage caused by brachial plexus injury [13]. It also plays a neuroprotective role in the striatum of rats with Huntington's disease [27]. Bioinformatics analysis revealed that several differentially expressed genes, including DUSP1, are related biomarkers of NP caused by peripheral nerve injury [28]. More importantly, Wang et al. [12] found that DUSP1 encouraged the polarization response of microglia and lessened CCI-induced NP. In the present study, it was discovered that miR-382-5p could regulate DUSP1 expression, and overexpression of miR-382-5p significantly decreased DUSP1 levels. Studies have shown that DUSP1 can inactivate proteins of the MAPK family through dephosphorylation [29]. MAPK is an important signaling system mediating cell response, and phosphorylation of MAPK can activate the expression of target molecules related to inflammation and apoptosis [30]. Therefore, the authors believe that DUSP1 may play an anti-neuroinflammation and anti-apoptosis role in NP through dephosphorylation of MAPK. However, miR-382-5p directly targets DUSP1's mRNA, causing its degradation or translation inhibition. High levels of miR-382-5p lead to decreased expression of DUSP1, while low levels of DUSP1 may lead to sustained activation of MAPKs, which promote the production of inflammatory factors and exacerbate neuroinflammation.
Unfortunately, this study did not further verify the regulatory relationship between the miR-382-5p/DUSP1 axis and MAPK in NP, which is a major limitation of this study. Additionally, whether the miR-382-5p/DUSP1 axis mediates other biological processes in NP, such as oxidative stress and neuronal apoptosis, also requires further study. In future research, the authors will further explore the mechanism of action of the miR-382-5p/DUSP1 axis in NP and its relationship with MAPK.
In conclusion, the authors have reported for the first time that down-regulated miR-382-5p may reduce hyperactivation of neuroinflammation and alleviate NP in NP rats by targeting DUSP1. Thus, inhibiting the miR-382-5p/DUSP1 axis may be an important therapeutic strategy to alleviate NP. The authors’ study suggests a new target for NP treatment.
Notes
REFERENCES
1. Moini Zanjani T, Ameli H, Labibi F, Sedaghat K, Sabetkasaei M. 2014; The attenuation of pain behavior and serum COX-2 concentration by curcumin in a rat model of neuropathic pain. Korean J Pain. 27:246–52. DOI: 10.3344/kjp.2014.27.3.246. PMID: 25031810. PMCID: PMC4099237.
2. Gilron I, Baron R, Jensen T. 2015; Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 90:532–45. DOI: 10.1016/j.mayocp.2015.01.018. PMID: 25841257.
3. Reisig F, Harnik M. 2020; [Neuropathic pain: pharmacotherapy]. Ther Umsch. 77:274–280. German.DOI: 10.1024/0040-5930/a001191. PMID: 32930074.
4. Elzahaf RA, Johnson MI, Tashani OA. 2016; The epidemiology of chronic pain in Libya: a cross-sectional telephone survey. BMC Public Health. 16:776. DOI: 10.1186/s12889-016-3349-6. PMID: 27514513. PMCID: PMC4982430.
5. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. 2017; Neuropathic pain. Nat Rev Dis Primers. 3:17002. DOI: 10.1038/nrdp.2017.2. PMID: 28205574. PMCID: PMC5371025.
6. Friedman RC, Farh KK, Burge CB, Bartel DP. 2009; Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19:92–105. DOI: 10.1101/gr.082701.108. PMID: 18955434. PMCID: PMC2612969.
7. López-González MJ, Landry M, Favereaux A. 2017; MicroRNA and chronic pain: from mechanisms to therapeutic potential. Pharmacol Ther. 180:1–15. DOI: 10.1016/j.pharmthera.2017.06.001. PMID: 28579386.
8. Xiang W, Jiang L, Zhou Y, Li Z, Zhao Q, Wu T, et al. 2021; The lncRNA Ftx/miR-382-5p/Nrg1 axis improves the inflammation response of microglia and spinal cord injury repair. Neurochem Int. 143:104929. DOI: 10.1016/j.neuint.2020.104929. PMID: 33359189.
9. Yin X, Zheng W, He L, Mu S, Shen Y, Wang J. 2022; CircHIPK3 alleviates inflammatory response and neuronal apoptosis via regulating miR-382-5p/DUSP1 axis in spinal cord injury. Transpl Immunol. 73:101612. DOI: 10.1016/j.trim.2022.101612. PMID: 35500847.
10. Ashmwe M, Posa K, Rührnößl A, Heinzel JC, Heimel P, Mock M, et al. 2022; Effects of extracorporeal shockwave therapy on functional recovery and circulating miR-375 and miR-382-5p after subacute and chronic spinal cord contusion injury in rats. Biomedicines. 10:1630. DOI: 10.3390/biomedicines10071630. PMID: 35884935. PMCID: PMC9313454.
11. Cao S, Zhang D, Yuan J, Liu C, Zhou W, Zhang L, et al. 2019; MicroRNA and circular RNA expression in affected skin of patients with postherpetic neuralgia. J Pain Res. 12:2905–13. DOI: 10.2147/JPR.S221615. PMID: 31695480. PMCID: PMC6802488.
12. Wang X, Jiang Y, Li J, Wang Y, Tian Y, Guo Q, et al. 2021; DUSP1 promotes microglial polarization toward M2 phenotype in the medial prefrontal cortex of neuropathic pain rats via inhibition of MAPK pathway. ACS Chem Neurosci. 12:966–78. DOI: 10.1021/acschemneuro.0c00567. PMID: 33666084.
13. Liu LP, Zhang J, Pu B, Li WQ, Wang YS. 2020; Upregulation of JHDM1D-AS1 alleviates neuroinflammation and neuronal injury via targeting miR-101-3p-DUSP1 in spinal cord after brachial plexus injury. Int Immunopharmacol. 89:106962. DOI: 10.1016/j.intimp.2020.106962. PMID: 33039970.
14. Li Z, Li A, Yan L, Yang T, Xu W, Fan P. 2020; Downregulation of long noncoding RNA DLEU1 attenuates hypersensitivity in chronic constriction injury-induced neuropathic pain in rats by targeting miR-133a-3p/SRPK1 axis. Mol Med. 26:104. DOI: 10.1186/s10020-020-00235-6. PMID: 33167866. PMCID: PMC7653812.
15. Yoo SH, Lee SH, Lee S, Park JH, Lee S, Jin H, et al. 2020; The effect of human mesenchymal stem cell injection on pain behavior in chronic post-ischemia pain mice. Korean J Pain. 33:23–9. DOI: 10.3344/kjp.2020.33.1.23. PMID: 31888314. PMCID: PMC6944374.
16. Bennett GJ, Xie YK. 1988; A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33:87–107. DOI: 10.1016/0304-3959(88)90209-6. PMID: 2837713.
17. Attal N, Jazat F, Kayser V, Guilbaud G. 1990; Further evidence for 'pain-related' behaviours in a model of unilateral peripheral mononeuropathy. Pain. 41:235–51. DOI: 10.1016/0304-3959(90)90022-6.
18. Bakare AO, Owoyele BV. 2020; Antinociceptive and neuroprotective effects of bromelain in chronic constriction injury-induced neuropathic pain in Wistar rats. Korean J Pain. 33:13–22. DOI: 10.3344/kjp.2020.33.1.13. PMID: 31888313. PMCID: PMC6944371.
19. Pasquinelli AE. 2012; MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 13:271–82. DOI: 10.1038/nrg3162. PMID: 22411466.
20. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. 2019; An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 234:5451–65. DOI: 10.1002/jcp.27486. PMID: 30471116.
21. Kovanur Sampath K, Belcher S, Hales J, Thomson OP, Farrell G, Gisselman AS, et al. 2023; The role of micro-RNAs in neuropathic pain-a scoping review. Pain Rep. 8:e1108. DOI: 10.1097/PR9.0000000000001108. PMID: 37928202. PMCID: PMC10624461.
22. Liu JC, Xue DF, Wang XQ, Ai DB, Qin PJ. 2019; MiR-101 relates to chronic peripheral neuropathic pain through targeting KPNB1 and regulating NF-κB signaling. Kaohsiung J Med Sci. 35:139–45. DOI: 10.1002/kjm2.12025. PMID: 30887716.
23. Zhang YU, Ye G, Zhao J, Chen Y, Kong L, Sheng C, et al. 2022; Exosomes carried miR-181c-5p alleviates neuropathic pain in CCI rat models. An Acad Bras Cienc. 94:e20210564. DOI: 10.1590/0001-3765202220210564. PMID: 35976364.
24. Kuffler DP. 2020; Mechanisms for reducing neuropathic pain. Mol Neurobiol. 57:67–87. DOI: 10.1007/s12035-019-01757-9. PMID: 31813127.
25. Woolf CJ. 2011; Central sensitization: implications for the diagnosis and treatment of pain. Pain. 152(3 Suppl):S2–15. DOI: 10.1016/j.pain.2010.09.030. PMID: 20961685. PMCID: PMC3268359.
26. Calvo M, Dawes JM, Bennett DL. 2012; The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 11:629–42. DOI: 10.1016/S1474-4422(12)70134-5. PMID: 22710756.
27. Taylor DM, Moser R, Régulier E, Breuillaud L, Dixon M, Beesen AA, et al. 2013; MAP kinase phosphatase 1 (MKP-1/DUSP1) is neuroprotective in Huntington's disease via additive effects of JNK and p38 inhibition. J Neurosci. 33:2313–25. DOI: 10.1523/JNEUROSCI.4965-11.2013. PMID: 23392662. PMCID: PMC3711389.
28. Zhang CG, Wan HQ, Ma KN, Luan SX, Li H. 2019; Identification of biomarkers related to neuropathic pain induced by peripheral nerve injury. J Mol Neurosci. 69:505–15. DOI: 10.1007/s12031-019-01322-y. PMID: 31352588.
29. Xuan C, Cui H, Jin Z, Yue Y, Cao S, Cui S, et al. 2023; Glutamine ameliorates hyperoxia-induced hippocampal damage by attenuating inflammation and apoptosis via the MKP-1/MAPK signaling pathway in neonatal rats. Front Pharmacol. 14:1096309. DOI: 10.3389/fphar.2023.1096309. PMID: 36817145. PMCID: PMC9932780.
30. Fan M, Wang C, Zhao X, Jiang Y, Wang C. 2023; Parthenolide alleviates microglia-mediated neuroinflammation via MAPK/TRIM31/NLRP3 signaling to ameliorate cognitive disorder. Int Immunopharmacol. 120:110287. DOI: 10.1016/j.intimp.2023.110287. PMID: 37182449.
Fig. 1
Experimental design flow chart. LV-anti-NC: empty lentivirus, LV-anti-miR-382-5p: lentivirus that inhibits miR-382-5p, sh-NC: empty lentivirus, sh-DUSP1: lentivirus that inhibits DUSP1, DUSP1: dual specificity phosphatase-1, CCI: chronic constriction injury, RT-qPCR: real-time quantitative reverse transcription polymerase chain reac-tion, ELISA: enzyme-linked immunosorbent assay.
Fig. 2
Elevated miR-382-5p levels in DRGs of NP rats. (A, B) Mechanical nociceptive sensitivity and thermal nociceptive sensitivity were measured in CCI rats on days 0, 3, 7, 14 and 21 of the CCI surgery. (C–E) On day 21 of CCI surgery, the levels of proinflammatory factors (IL-6, IL-1β, TNF-α) in DRGs were measured by ELISA assay. (F) The level of miR-382-5p in DRGs was measured by RT-qPCR on days 0, 3, 7, 14 and 21 of the CCI surgery. Values are presented as mean ± standard deviation. DRGs: dorsal root ganglions, NP: neuropathic pain, CCI: chronic constriction injury, IL-6: interleukin-6, IL-1β: interleukin-1β, TNF-α: tumor necrosis factor-α, PWT: paw withdrawal threshold, PWL: paw withdrawal latency. **P < 0.01; ***P < 0.001 vs. Sham. n = 4.
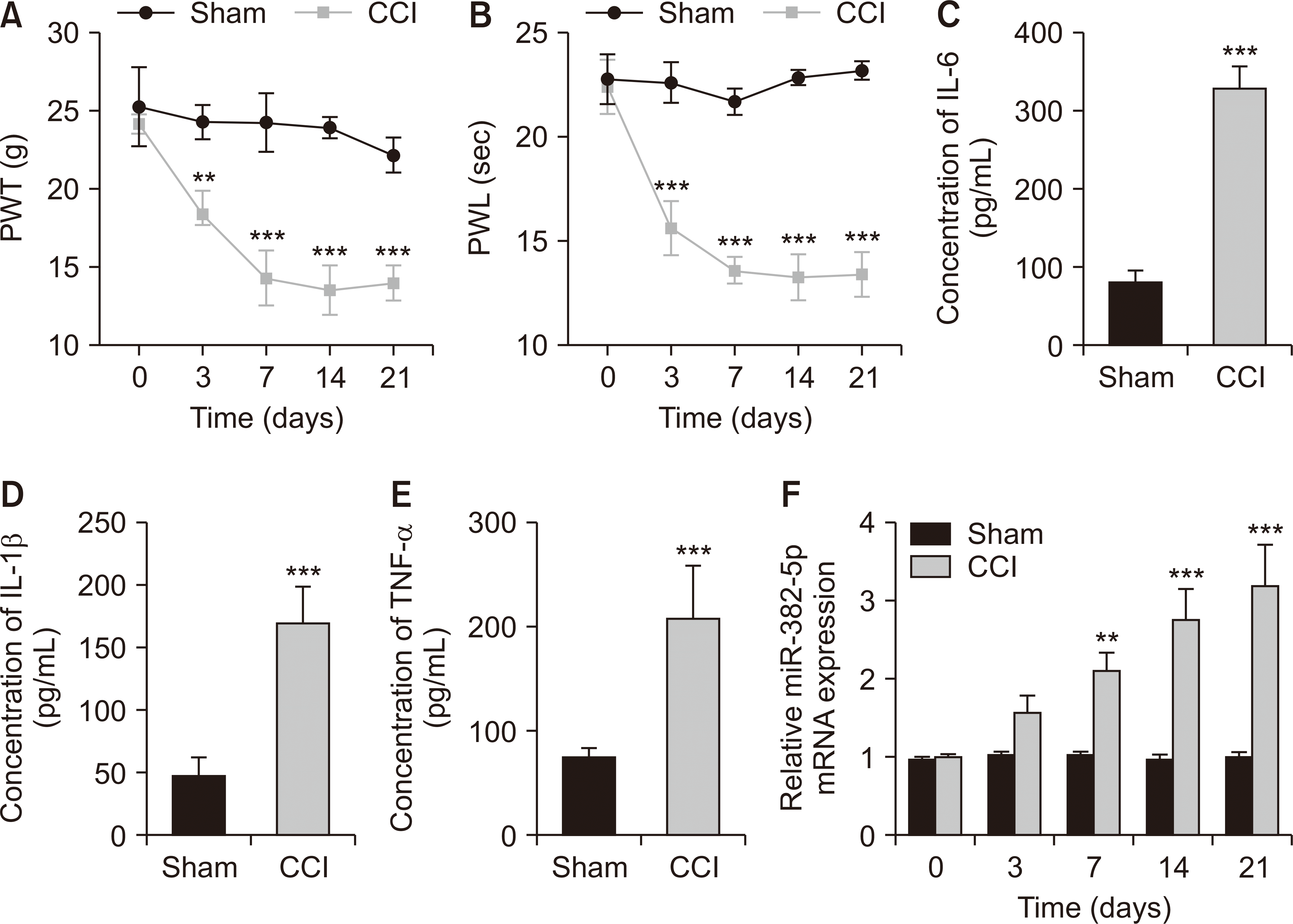
Fig. 3
Intrathecal injection of lentiviral interference vector targeting miR-382-5p (LV-anti-miR-382-5p) alleviated pain behavior in CCI rats. (A) The mRNA level of miR-382-5p in DRGs was detected by RT-qPCR on the day 21 of CCI surgery. (B, C) Mechanical nociceptive sensitivity and thermal nociceptive sensitivity were measured in CCI rats on days 0, 3, 7, 14 and 21 of the CCI surgery. Values are presented as mean ± standard deviation. CCI: chronic constriction injury, DRGs: dorsal root ganglions, RT-qPCR: real-time quantitative reverse transcription polymerase chain reaction, PWT: paw withdrawal threshold, PWL: paw withdrawal latency. **P < 0.01; ***P < 0.001 vs. Sham. #P < 0.05; ##P < 0.01; ###P < 0.001 vs. CCI + LV-anti-NC. n = 13.
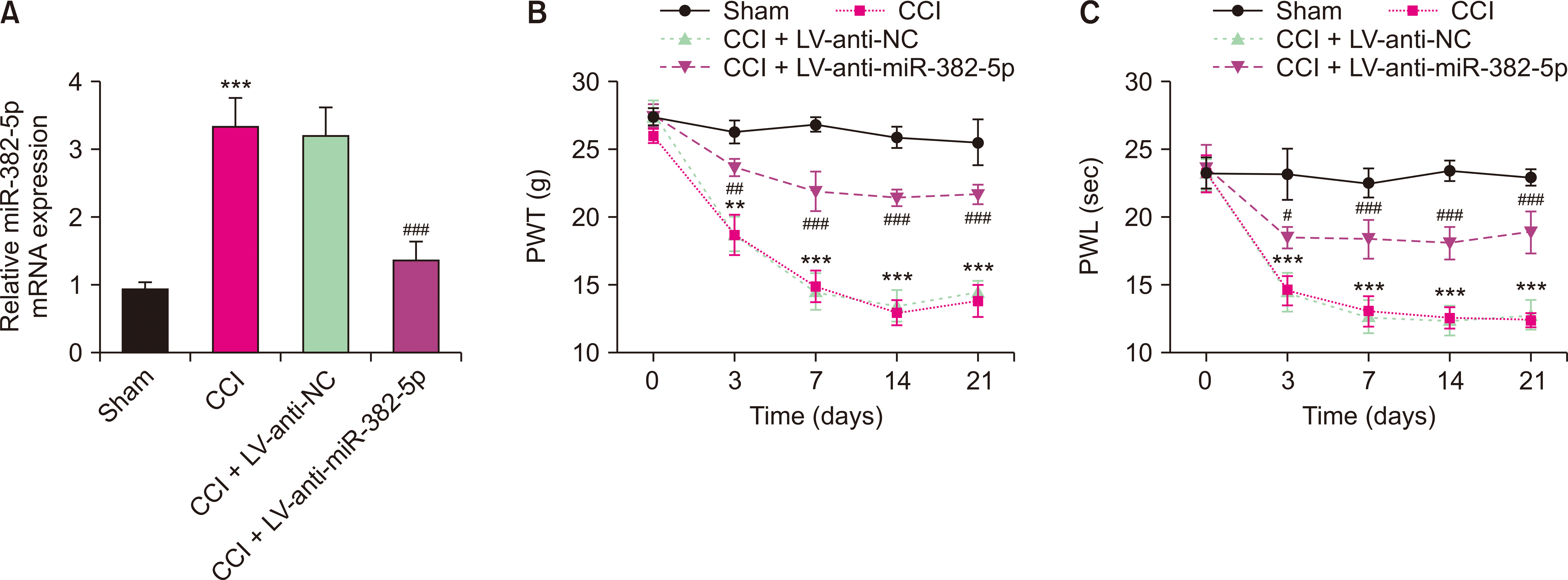
Fig. 4
Intrathecal injection of lentiviral interference vector targeting miR-382-5p (LV-anti-miR-382-5p) inhibited the over-activation of proinflammatory factors in DRGs of CCI rats. (A–C) On day 21 of CCI surgery, the levels of proinflammatory factors (IL-6, IL-1β, TNF-α) in DRGs were measured by ELISA assay. Values are presented as mean ± standard deviation. DRGs: dorsal root ganglions, CCI: chronic constriction injury, IL-6: interleukin-6, IL-1β: interleukin-1β, TNF-α: tumor necrosis factor-α, ELISA: enzyme-linked immunosorbent assay. ***P < 0.001 vs. Sham. ###P < 0.001 vs. CCI + LV-anti-NC. n = 13.
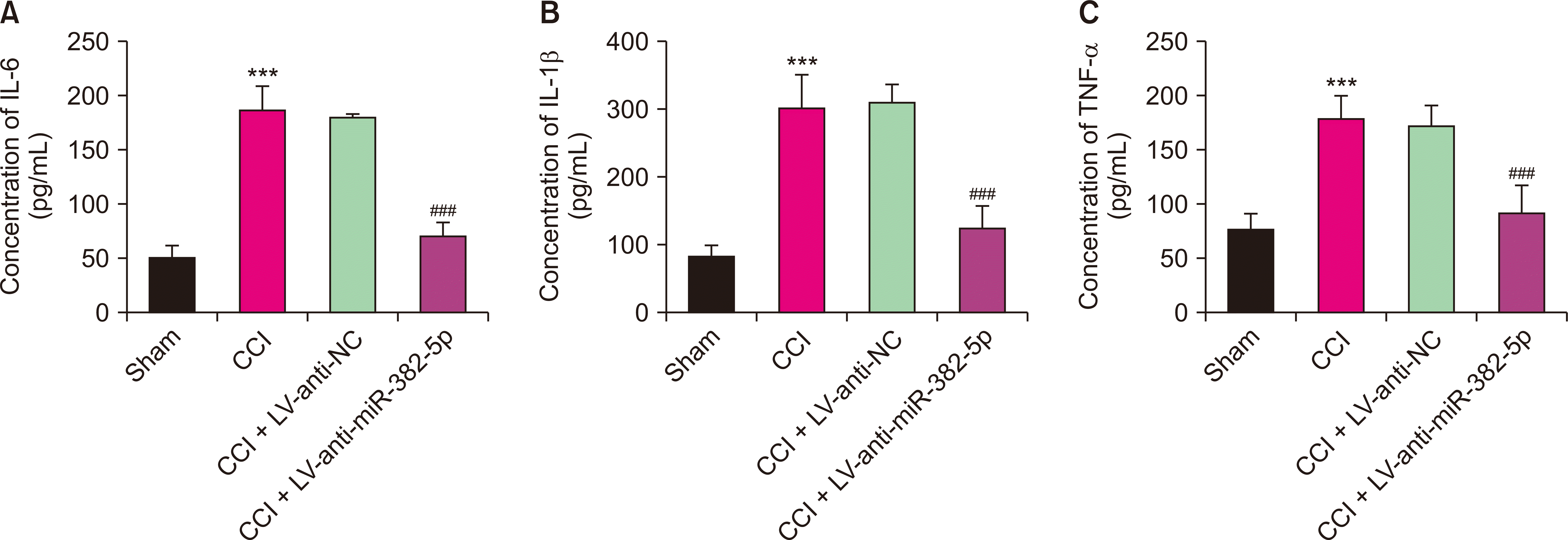
Fig. 5
DUSP1 is a target gene of miR-382-5p. (A) There are complementary binding sites between miR-382-5p and DUSP1. (B) DLR assay confirms the targeting relationship between miR-382-5p and DUSP1. (C) RIP assay shows that both miR-382-5p and DUSP1 could be enriched in immunoprecipitation complexes. (D–F) The mRNA and protein levels of DUSP1 in DRGs were measured by RT-qPCR and ELISA on days 0, 3, 7, 14 and 21 of the CCI surgery. (G) On day 21 of CCI surgery, RT-qPCR was used to detect the effect of lentivirus interfering vector (LV-anti-miR-382-5p) on DUSP1 mRNA levels in DRGs. Values are presented as mean ± standard deviation. DUSP1: dual specificity phosphatase-1, GAPDH: glyceraldehyde 3-phosphate dehydrogenase, DLR: dual-luciferase reporter, RIP: RNA immunoprecipitation, DRGs: dorsal root ganglions, RT-qPCR: real-time quantitative reverse transcription polymerase chain reaction, ELISA: enzyme-linked immunosorbent assay, CCI: chronic constriction injury, WT: wild type, MUT: mutant. *P < 0.05; **P < 0.01; ***P < 0.001 vs. Sham group. ###P < 0.001 vs. CCI + LV-anti-NC. n = 13.
Fig. 6
Down-regulation of DUSP1 inhibited the alleviating effect of down-regulation of miR-382-5p on neuropathic pain. (A) The mRNA level of DUSP1 in DRGs was detected by RT-qPCR on the day 21 of CCI surgery. (B, C) Mechanical nociceptive sensitivity and thermal nociceptive sensitivity were measured in CCI rats on days 0, 3, 7, 14 and 21 of the CCI surgery. (D–F) On day 21 of CCI surgery, the levels of proinflammatory factors (IL-6, IL-1β, TNF-α) in DRGs were measured by ELISA assay. Values are presented as mean ± standard deviation. DUSP1: dual specificity phosphatase-1, DRGs: dorsal root ganglions, RT-qPCR: real-time quantitative reverse transcription polymerase chain reaction, CCI: chronic constriction injury, IL-6: interleukin-6, IL-1β: interleukin-1β, TNF-α: tumor necrosis factor-α, ELISA: enzyme-linked immunosorbent assay, PWT: paw withdrawal threshold, PWL: paw withdrawal latency. ###P < 0.001 vs. CCI + LV-anti-NC. &&P < 0.01; &&&P < 0.001 vs. CCI + LV-anti-miR-382-5p + sh-NC. n = 13.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download