Abstract
Background/Aims
Pancreatic cancer poses significant challenges due to its tendency for late-stage diagnosis and high mortality rates. Cryoablation, a technique used to treat various types of cancer, has shown potential in enhancing the prognosis of pancreatic cancer when combined with other therapies. However, its implementation is often limited by the need for lengthy procedures and specialized equipment. This study aims to develop a cryoablation needle optimized for endoscopic ultrasonography to simplify its application in treating pancreatic cancer.
Methods
The study involved conducting cryoablation experiments on swine liver tissue. It utilized cryo-needles to evaluate the extent of cell death across various temperatures and durations of cryoablation.
Results
The cryoablation system, which employed liquid carbon dioxide, achieved rapid cooling, reaching temperatures below –60 °C within 30 seconds and maintained the cryoablation process for 200 seconds. These conditions resulted in necrosis of the liver tissue. Notable cellular changes were observed up to 15 mm away from the cryoablation needle.
Conclusions
This experimental study successfully demonstrated the efficacy of using a cryo-needle for cryoablation in swine liver tissue. Further trials involving pancreatic tissue are expected to verify its effectiveness, underscoring the importance of continued research to establish its role as a complementary therapy in pancreatic cancer treatment.
The high mortality rate of pancreatic cancer underscores the urgent need for innovative and effective treatments. In the United States, pancreatic cancer ranks as the third leading cause of cancer-related deaths, following lung and colon cancers.1 Typically diagnosed at advanced, unresectable stages, late detection is a major contributor to its high mortality rate. Systemic chemotherapy plays a critical role in extending the survival of patients with advanced pancreatic cancer. Consequently, new treatment approaches, including immune and targeted therapies, are being explored; however, they have yet to show sufficient effectiveness. Thus, alternative treatments like radiofrequency ablation (RFA) and the combined use of chemotherapy and cryoablation are under investigation, as they show promise in improving survival in clinical trials.2 Moreover, advancements in ‘microwave ablation’ and ‘irreversible electroporation’ are actively pursued as innovative ablation therapies for pancreatic cancer.3,4 Despite these efforts, most of these novel treatments have not achieved widespread use due to limited research.
Cryoablation emerges as a promising method to enhance survival rates in pancreatic cancer treatment. Commonly employed in treating skin and cervical cancers, cryoablation is now being explored for its potential in kidney, liver, lung, and prostate cancers. Recent studies investigating the application of cryoablation in pancreatic cancer suggest that it may be more effective when combined with standard treatments like chemotherapy, radiation, or immunomodulation.5-9 Despite its potential, the use of cryoablation is severely constrained by its lengthy procedure times and the necessity for surgical or percutaneous equipment. If cryoablation could be effectively administered via endoscopic ultrasound, it could become a superior treatment option. Thus, our study aimed to develop a cryoablation needle suitable for endoscopic ultrasound application. Building on the reduced complications seen in endoscopic ultrasonography (EUS)-guided biopsies compared to traditional laparotomies, we anticipate that the EUS method, being less invasive and involving fewer organs, will similarly reduce complications associated with cryoablation. In this preliminary study, we developed a prototype cryoablation needle to assess cell death related to varying ablation temperatures and durations in porcine liver tissues.
The cryoablation equipment featured a cooling needle system, which included a cooling needle, cooling nozzles, and a refrigerant device. This device consisted of a reducing valve (Model SR4B; DAE-A MACHINERY & ELECTRIC Co., Ltd.) and a solenoid valve (Model C322C1; Parker), which regulated the refrigerant flow to the cooling needle system. In this study, cryoablation was conducted by administering liquid carbon dioxide to the target area via a cooling needle connected to a cooling nozzle. Liquid carbon dioxide was chosen due to its significantly higher Joule-Thomson coefficient compared to other refrigerants at the same temperature, enhancing the efficiency of the cryoablation process. The refrigerant, sourced from a 55-bar liquid carbon dioxide reservoir, was directed through the reducing and solenoid valves to the cooling needle system. In the needle system (Fig. 1A), the cooling nozzle was designed to channel the refrigerant through a pipe-shaped channel with an outer diameter of 1 mm and an inner diameter of 0.7 mm. This setup enabled the induction of the Joule-Thomson effect via an orifice with a diameter of 0.4 mm, allowing effective expulsion of the refrigerant into the cooling needle. The needle was engineered with an outer diameter of 3 mm, suitable for insertion into the target tissue, and an inner diameter of 2 mm to ensure the smooth discharge of the liquid carbon dioxide from the nozzle. A cooling needle with a length of 80 mm was specifically designed to facilitate insertion into the pancreas by 20 mm through a carefully made abdominal wall perforation. The needle featured two holes, each 1.5 mm in diameter and located 60 mm from the needle tip, acting as refrigerant discharge outlets. This design permitted the safe discharge of liquid carbon dioxide into the gastrointestinal tract while minimizing the risk of refrigerant release near the surgical site.
Due to limited research on cryotherapy administered through EUS, we developed and implemented a novel protocol designed to achieve the lowest possible temperature using a thin catheter. The in vivo experiment involved reducing the pressure of the liquid carbon dioxide in the refrigerant control system to 40 bar and conducting a 30-second precooling phase. The cooling conditions were precisely controlled: the solenoid valve opened for 0.2 seconds and closed for 2 seconds, repeating this cycle for a total duration of 210 seconds. After this phase, the cooling needle was removed approximately one minute after thawing at the surgical site was observed.
During the cryoablation process, temperature measurements were taken using custom-made T-type thermocouples, each with a diameter of 70 μm. To accurately monitor the temperature around the cooling needle, thermocouples were positioned at distances of 0, 5, 10, and 15 mm from the needle tip along its surface. To measure the surrounding temperature, thermocouples were inserted inside 22-G EUS needles and positioned at the specified measurement locations. A specially designed jig was utilized to ensure precise placement of the sensors. This jig facilitated the radial alignment of the sensors, allowed for adjustments in radial distance, and enabled modification of the needle sensor length to control depth. In total, temperatures at six different locations were recorded, using combinations of distances 2, 3, and 4 mm radially from the needle wall and 0, 5, 10, and 15 mm vertically from the needle tip.
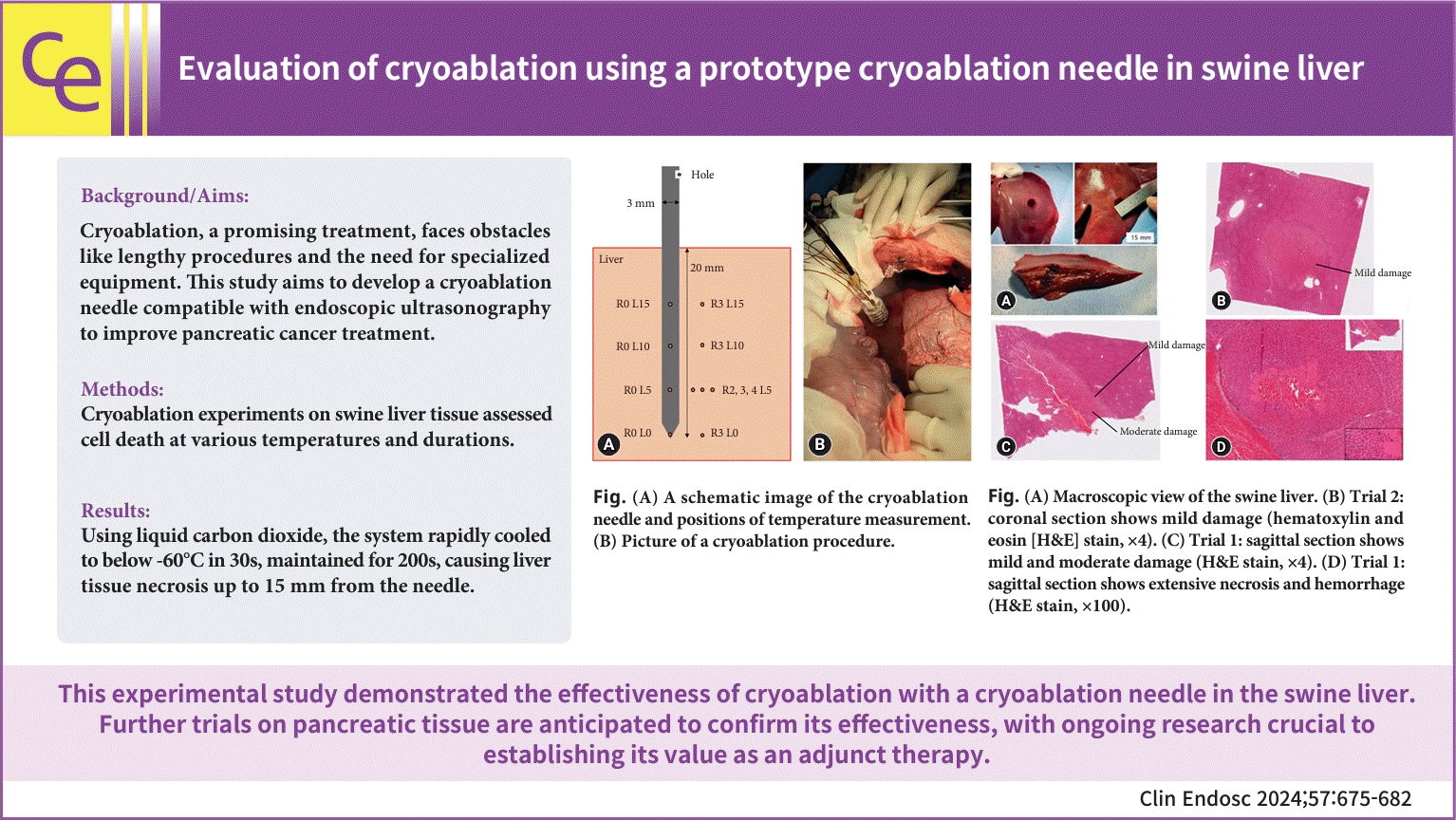
A 30 kg swine was utilized for this cryoablation experiment. Before the procedure, a midline incision was made in the abdomen to fully expose the liver. The needle was inserted into the swine liver to a depth of more than 2 cm (Fig. 1B). Cryoablation was conducted at two different sites, as previously described, in two trials. The sessions were spaced one hour apart to allow for procedural preparation. Following the final procedure, the pigs were euthanized within 30 minutes, and the livers were extracted and preserved in formalin. To assess the extent of liver tissue damage, the livers were sectioned and stained with hematoxylin and eosin. The extent of liver necrosis was evaluated using the Batts-Ludwig system. Necrosis, or cell death, involves the transition of viable cells to a nonviable state, leading to the dissolution of cell contents.10 Scattered necrosis was classified as mild, necrotic clusters as moderate, and prominent diffuse damage as severe.11,12
Figures 1A and B depict a schematic and photograph, respectively, of the cryoablation needle and its insertion into the liver for ablation. Figure 2 presents the temperature readings of the needle throughout the cryoablation process. Trials 1 and 2 yielded identical results. However, after completing Trial 1, the R0 L10 sensor ceased to function. Consequently, we excluded these results from the graphical representation in Trial 2. The R0 L5 sensor recorded a rapid temperature drop, reaching below –60 °C within the first 30 seconds of the procedure, marking the lowest temperature recorded in the needle. At the procedure's end, the temperature returned to 0 °C within one minute. The sensors R0 L10, R0 L15, and R0 L0 demonstrated temperature drops to below –50 °C, –40 °C, and –30 °C, respectively, each within 30 seconds.
Figure 3 illustrates the temperature measurements of the tissue surrounding the needle during the cryoablation process. The graph on the left for each trial displays the temperatures at a distance of 3 mm from the needle at various depths (L0, L5, L10, and L15). Approximately one minute into the procedure, the temperature in these areas fell below 0 °C, with the lowest temperature being reached after two minutes. This temperature, around –10 °C, was maintained for two minutes. Following the conclusion of cryoablation, the temperature returned to near 0 °C within one minute. The graph on the right in each trial shows the temperature changes at distances of 2, 3, and 4 mm from the needle tip at a depth of 5 mm. Here, the temperatures at distances of 2, 3, and 4 mm dropped to –25 °C, –15 °C, and –5 °C, respectively.
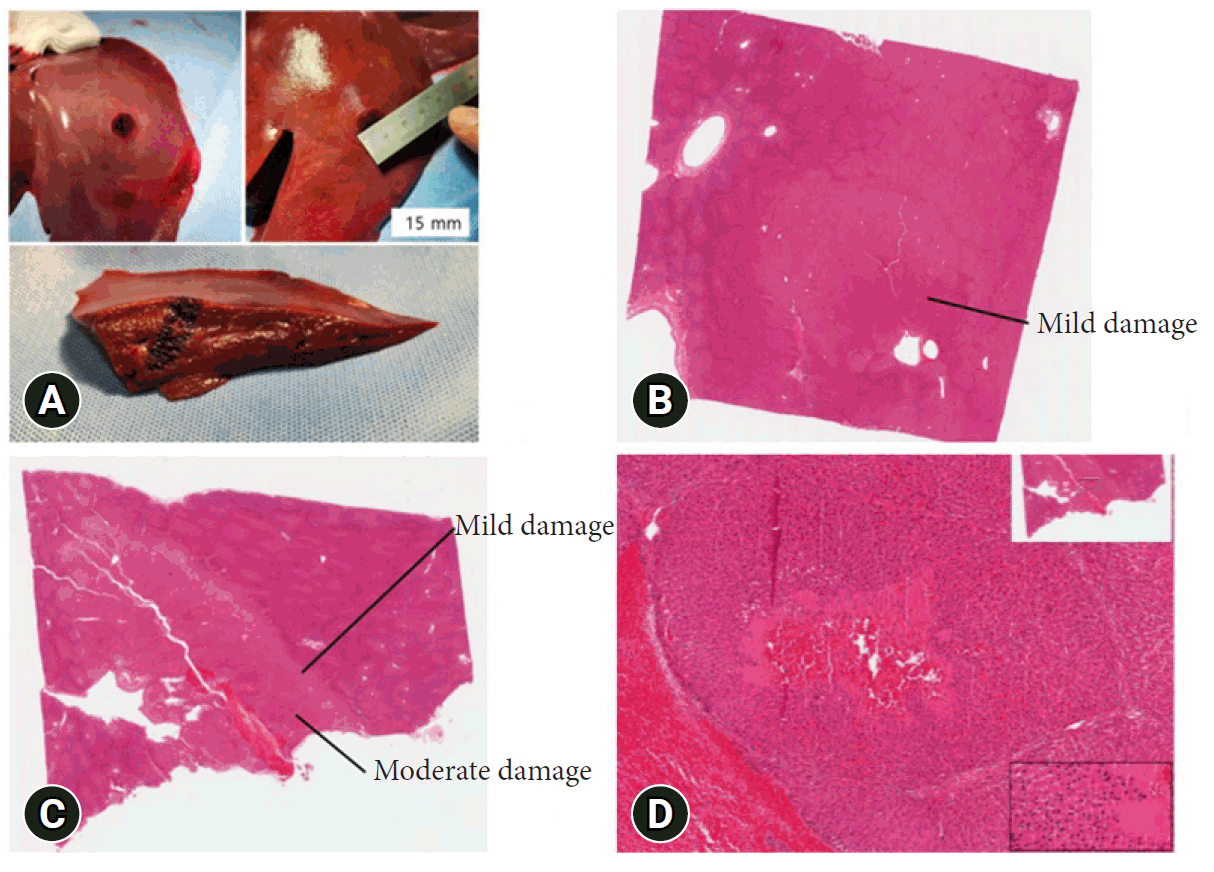
Figure 4A shows the liver of a swine post-sacrifice, displaying blackened areas on the liver's surface that span approximately 15 mm in length. These color changes occurred along the needle's path, visible after a sagittal incision was made in the direction of needle insertion (Trial 2 area). Figure 4B depicts the cryoablation area in a coronal section of the liver (Trial 1), primarily showing regions of mild damage, although some parts of the central area exhibit moderate damage. Figure 4C illustrates the cryoablation area in a sagittal section of the liver (Trial 1), where the extent of damage exceeds moderate levels and covers more than 1 cm, indicating a higher moderate-to-mild damage ratio compared to Trial 2. Finally, Figure 4D presents a high-power field view of the cryoablation area, characterized by extensive necrosis and hemorrhage.
In this preliminary study, we developed a cryoablation system and applied it to swine livers to measure cellular temperatures during the procedure and assess cell death postoperatively. Due to the relatively small size of the pig’s pancreas, accurately measuring the ablation size with our device may prove challenging. Therefore, to assess the control range of temperature and the extent of necrosis more objectively, research was conducted on the liver. Future experiments are planned on the pancreas using a similar experimental framework.
In this research, a specialized cryoablation needle was designed to administer cryoablation in the treatment of pancreatic cancer. To facilitate cryoablation via EUS, the needle was engineered with a reduced diameter. However, reducing the diameter from 3 mm to 2 mm could decrease the cooling range by approximately 18% in heat absorption capacity, potentially reducing the effective treatment range proportionally. Multiple treatment sessions, rather than a single session, could be utilized to address this. Additionally, incorporating insulation outside the treatment area could allow the needle at the target site to maintain a lower temperature and absorb more heat. Future designs will aim to further improve these aspects to overcome these challenges.
Traditional cryoablation methods using Argon gas face limitations in heat exchange efficiency due to their reliance on a single-phase jet for target cooling. This method requires the use of lower refrigerant temperatures and higher pressures or flow rates to achieve comparable cooling effects. Additionally, the use of Argon complicates precision in cryoablation surgeries due to the extremely low temperature of the refrigerant (with a boiling point of –186 °C) and the demanding operational conditions. In contrast, the liquid CO2 used in this study is delivered in a two-phase jet form when directed at the target, significantly enhancing heat exchange efficiency. This efficiency, coupled with a high Joule-Thomson coefficient, allows for finer control over temperature and the extent of the cryoablation process. Our cryoablation needle was able to rapidly cool down to –60 °C within 30 seconds and sustain cryoablation for 200 seconds, successfully inducing necrosis in liver tissue. Additionally, necrosis was observed in liver tissue at cryoablation temperatures around –5 °C to –10 °C for approximately 100 seconds. Significant cellular changes were evident even in areas 3 to 4 mm from the cryoablation needle. When the needle diameter was 3 mm, significant changes were noted up to approximately 10 mm, and with the inclusion of milder changes, effects were observed up to approximately 15 mm. These findings suggest that the treatment can be applied to cancers larger than 1 cm in diameter. Despite nearly identical temperature settings in Trials 1 and 2, the degree of tissue damage varied, likely influenced by a one-hour delay in the sacrifice in Trial 1 in vivo.
In Figure 3, during the analysis of Trial 2, the temperature surrounding the L15 segment of the probe was the highest in comparison with the L10, L5, and L0 segments. However, in Trial 1, the temperature at L15 was recorded as being lower than that at L10. This discrepancy is likely due to the target folding during needle insertion, which is affected by variations in curvature and thickness of the target. Consequently, it is hypothesized that the R3 L15 sensor, in terms of actual positioning within the liver, is located at R2 L15. Supporting this assumption, the temperature trends at R3 L15 in Trial 1 closely resemble those of the adjacent graph at R2 L5, and mirror the trends observed at R2 L5 in Trial 2. In Trial 2, referring to Figure 2 for needle temperatures, the consistent pattern of L5 exhibiting the lowest temperature is aligned. Additionally, R3 L0 and R3 L10 in Trial 2 demonstrate similar cooling temperatures because they conduct heat from R3 L5. The highest temperature observed at L15, the most distant location, aligns with the expected trend.
Recent advancements in chemotherapy and immunotherapy have improved survival rates for various cancers; however, the 5-year survival rate for pancreatic cancer remains significantly lower at approximately 10%. Several factors contribute to this disparity. At the time of diagnosis, only about 15% to 20% of patients are eligible for immediate surgery. Furthermore, nearly 30% of patients are diagnosed at an advanced stage, while the remainder present with metastatic disease.13 Tumor cells frequently demonstrate resistance to existing treatments, thereby diminishing the efficacy of therapeutic drugs.14 The microenvironment of pancreatic cancer also plays a critical role in its poor prognosis, particularly due to the presence of dense desmoplasia—a distinctive feature of these tumors. The accumulation of proteoglycans and hyaluronan increases fluid pressure, causing structural changes in the organ and posing significant challenges for the efficient delivery of therapeutic treatments.2,15 Additionally, the stromal component of pancreatic cancer is characterized by poor vascularity, which further impedes the delivery of therapeutic agents.16 Therefore, combining chemotherapy or immunotherapy with local therapeutic approaches may be essential for the effective management of pancreatic cancer.
Cryoablation employs a mechanism distinct from other localized treatment modalities for pancreatic cancer, such as RFA and irreversible electroporation. Unlike these techniques, which use heat energy or high-voltage electricity, cryoablation utilizes a different approach.17,18 It involves both direct and indirect mechanisms: during the direct mechanism, the freezing phase causes ice formation in the extracellular space, increasing extracellular osmolarity. This draws water out of the cells, leading to cellular shrinkage. As temperatures drop further, intracellular ice forms, disrupting cell membrane functionality. During the thawing phase, extracellular ice melts first, moving into the intracellular space, causing cell swelling and rupture. In the delayed phase, cells that are damaged but not yet dead undergo apoptosis through indirect mechanisms, such as thrombosis of blood vessels, which induces tissue ischemia and hampers recovery. Macrophages and neutrophils aid in removing damaged cells.19
Based on these facts, we believe that cryoablation could be a valuable therapy for managing pancreatic cancer. The cryoablation probe extensively researched in the commercial sector is a hybrid of cryo and RFA, known as cryothermal ablation/HTP (ERBE Elektromedizin). Various studies have been conducted using this probe.20,21 However, research on devices performing cryoablation alone directly via EUS has been limited. Recently, there has been increasing interest in this area, with animal experiments being conducted in the United States using a cryoablation probe named FrostBite.22 Thus, the development and research of this catheter are considered highly significant.
Although the number of studies specifically demonstrating the effects of cryoablation on pancreatic cancer is limited, its application in other cancer types has yielded many positive results. In vitro studies have shown that a single exposure to –25 °C can cause irreversible cell death in PANC-1 cells, a pancreatic cancer cell line. Furthermore, repeated cryoablation cycles can achieve similar effects, even at higher temperatures.23,24 Additionally, when PANC-1 cells are treated with chemotherapeutic agents, a single exposure to –15 °C is sufficient to induce irreversible cell death.23 Xu et al.5 demonstrated that combining 125I seeding therapy with cryoablation for treating pancreatic cancer resulted in a tumor control rate of nearly 90%, even in cases involving metastatic disease. Although the study involved a limited number of patients, it underscores the potential benefits of integrating cryoablation with other therapies. Recently, Som et al.25 reported using an immunoadjuvant gel that enhanced the abscopal effect of cryoablation, showing promising results for cancers resistant to checkpoint inhibitors. These findings suggest that combination therapy, including cryoablation, has potential efficacy in treating pancreatic cancer.
This study has several limitations. Primarily, the cryo-needle, in its current form, reaches temperatures below –40 °C along its entire length, which may inadvertently harm healthy tissues near cancer sites, particularly when the cancer cells are located deep within the body. To address this issue, it is essential to enhance the heat exchange efficiency at the needle's tip, develop a system for localized cooling, and incorporate a thermal insulation barrier at least 10 mm from the needle tip to protect surrounding normal cells. Additionally, for the insertion of the cryo-needle into an endoscope for procedural use, it is crucial to reduce the diameter of the hose-coupling part to less than 3.7 mm. Optionally, the needle design should be modified to increase the procedure’s flexibility. Moreover, there is a need to adjust the structure of the needle to either increase the temperature of the internally expelled carbon dioxide gas or to design it in a way that enables the external expulsion of the gas.
Based on our findings from studies conducted on swine liver, we anticipate observing significant cell death progression in treated cells one hour after cryoablation under similar conditions. Existing cryo-needle designs are not yet suitable for endoscopic procedures; however, we believe that cryo-needles can be adapted for endoscopic use in the near future. Further trials on pancreatic tissue are anticipated to confirm its effectiveness, with ongoing research being crucial to establishing its value as an adjunct therapy.
Notes
Funding
This study was supported by a 2022 Weolbong grant from the Korean Gastrointestinal Endoscopy Research Foundation.
Author Contributions
Conceptualization: SYH, GHK; Data curation: HS, JL, DK; Formal analysis: HS; Funding acquisition: SYH; Investigation: HS; Methodology: GHK; Project administration: SYH, GHK; Resources: TIK, DUK; Software: TIK, DUK; Supervision: SYH, GHK; Validation: JL; Visualization: HS; Writing–original draft: HS, JL, TIK, DUK, DK; Writing–review & editing: SYH, GHK.
REFERENCES
1. Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023; 73:17–48.
2. De Grandis MC, Ascenti V, Lanza C, et al. Locoregional therapies and remodeling of tumor microenvironment in pancreatic cancer. Int J Mol Sci. 2023; 24:12681.
3. Carrafiello G, Ierardi AM, Fontana F, et al. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013; 24:1513–1520.
4. Gajewska-Naryniecka A, Szwedowicz U, Łapińska Z, et al. Irreversible electroporation in pancreatic cancer: an evolving experimental and clinical method. Int J Mol Sci. 2023; 24:4381.
5. Xu KC, Niu LZ, Hu YZ, et al. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol. 2008; 14:1603–1611.
6. Niu L, Chen J, He L, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013; 42:1143–1149.
7. He L, Niu L, Korpan NN, et al. Clinical practice guidelines for cryosurgery of pancreatic cancer: a consensus statement from the China Cooperative Group of Cryosurgery on Pancreatic Cancer, International Society of Cryosurgery, and Asian Society of Cryosurgery. Pancreas. 2017; 46:967–972.
8. Luo XM, Niu LZ, Chen JB, et al. Advances in cryoablation for pancreatic cancer. World J Gastroenterol. 2016; 22:790–800.
9. Kovach SJ, Hendrickson RJ, Cappadona CR, et al. Cryoablation of unresectable pancreatic cancer. Surgery. 2002; 131:463–464.
10. Krishna M. Patterns of necrosis in liver disease. Clin Liver Dis (Hoboken). 2017; 10:53–56.
11. Batts KP, Ludwig J. Chronic hepatitis: an update on terminology and reporting. Am J Surg Pathol. 1995; 19:1409–1417.
12. Chowdhury AB, Mehta KJ. Liver biopsy for assessment of chronic liver diseases: a synopsis. Clin Exp Med. 2023; 23:273–285.
13. Shinde RS, Bhandare M, Chaudhari V, et al. Cutting-edge strategies for borderline resectable pancreatic cancer. Ann Gastroenterol Surg. 2019; 3:368–372.
14. Quiñonero F, Mesas C, Doello Ket al. The challenge of drug resistance in pancreatic ductal adenocarcinoma: a current overview. Cancer Biol Med. 2019; 16:688–699.
15. Thomas D, Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer. 2019; 18:14.
16. Katsuta E, Qi Q, Peng X, et al. Pancreatic adenocarcinomas with mature blood vessels have better overall survival. Sci Rep. 2019; 9:1310.
17. Peng H, Shen J, Long X, et al. Local release of TGF-β inhibitor modulates tumor-associated neutrophils and enhances pancreatic cancer response to combined irreversible electroporation and immunotherapy. Adv Sci (Weinh). 2022; 9:e2105240.
18. Yousaf MN, Ehsan H, Muneeb A, et al. Role of radiofrequency ablation in the management of unresectable pancreatic cancer. Front Med (Lausanne). 2021; 7:624997.
19. Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. 2010; 21(8 Suppl):S187–S191.
20. Testoni SG, Petrone MC, Reni M, et al. EUS-guided ablation with the HybridTherm Probe as second-line treatment in patients with locally advanced pancreatic ductal adenocarcinoma: a case-control study. Endosc Ultrasound. 2022; 11:383–392.
21. Testoni SG, Petrone MC, Reni M, et al. Efficacy of endoscopic ultrasound-guided ablation with the HybridTherm probe in locally advanced or borderline resectable pancreatic cancer: a phase II randomized controlled trial. Cancers (Basel). 2021; 13:4512.
22. Baust JM, Robilotto A, Raijman I, et al. The assessment of a novel endoscopic ultrasound-compatible cryocatheter to ablate pancreatic cancer. Biomedicines. 2024; 12:507.
23. Baust JM, Santucci KL, Van Buskirk RG, et al. An in vitro investigation into cryoablation and adjunctive cryoablation/chemotherapy combination therapy for the treatment of pancreatic cancer using the PANC-1 cell line. Biomedicines. 2022; 10:450.
24. Baumann KW, Baust JM, Snyder KK, et al. Characterization of pancreatic cancer cell thermal response to heat ablation or cryoablation. Technol Cancer Res Treat. 2017; 16:393–405.
25. Som A, Rosenboom JG, Wehrenberg-Klee E, et al. Percutaneous intratumoral immunoadjuvant gel increases the abscopal effect of cryoablation for checkpoint inhibitor resistant cancer. Adv Healthc Mater. 2024; 13:e2301848.
Fig. 1.
(A) A schematic image of the cryoablation needle and positions of temperature measurement. (B) Picture of a cryoablation procedure.
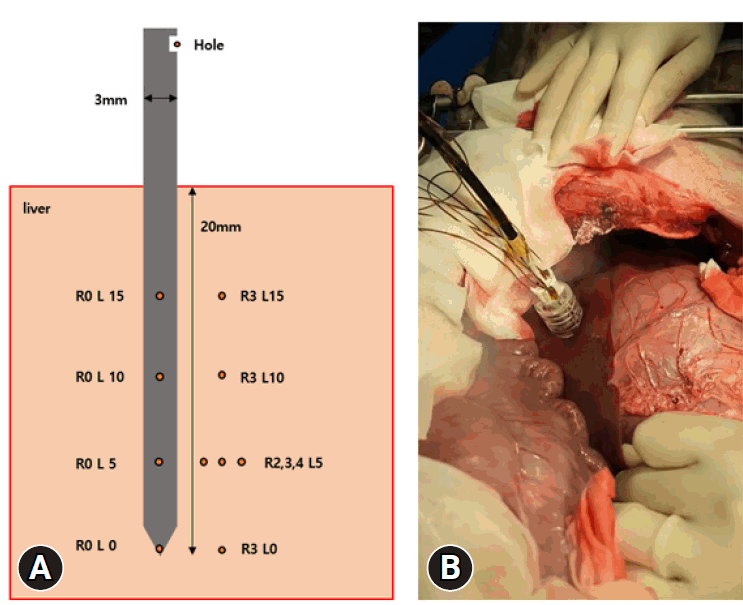
Fig. 2.
Temperature curve during cryoablation of the cryo-needle in Trials 1 and 2. (A) A schematic diagram of the cryo-needle and temperature measurement locations. (B) Temperature curve in Trial 1. (C) Temperature curve in Trial 2.
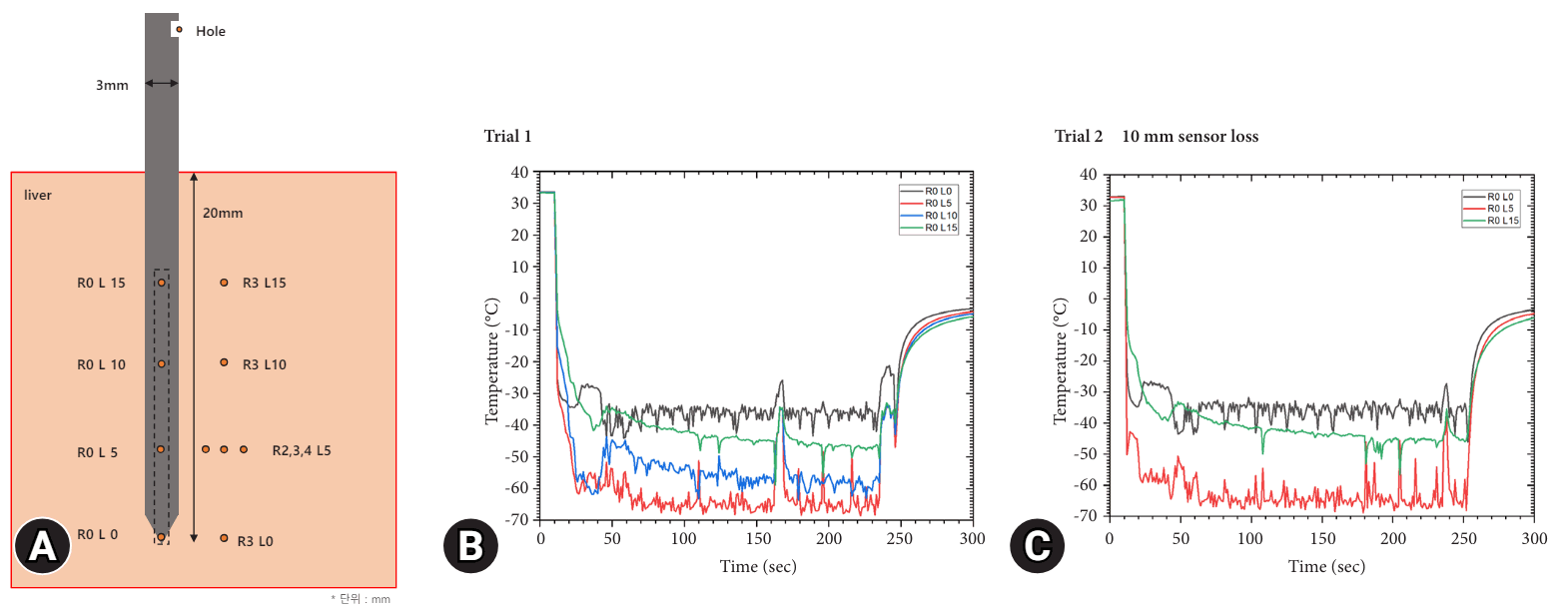
Fig. 3.
Temperature curve during cryoablation around the needle in Trial 1 and 2. (A) Schematic diagram of temperature measurement locations around the needle. (B) Temperature curve in Trial 1. (C) Temperature curve in Trial 2.
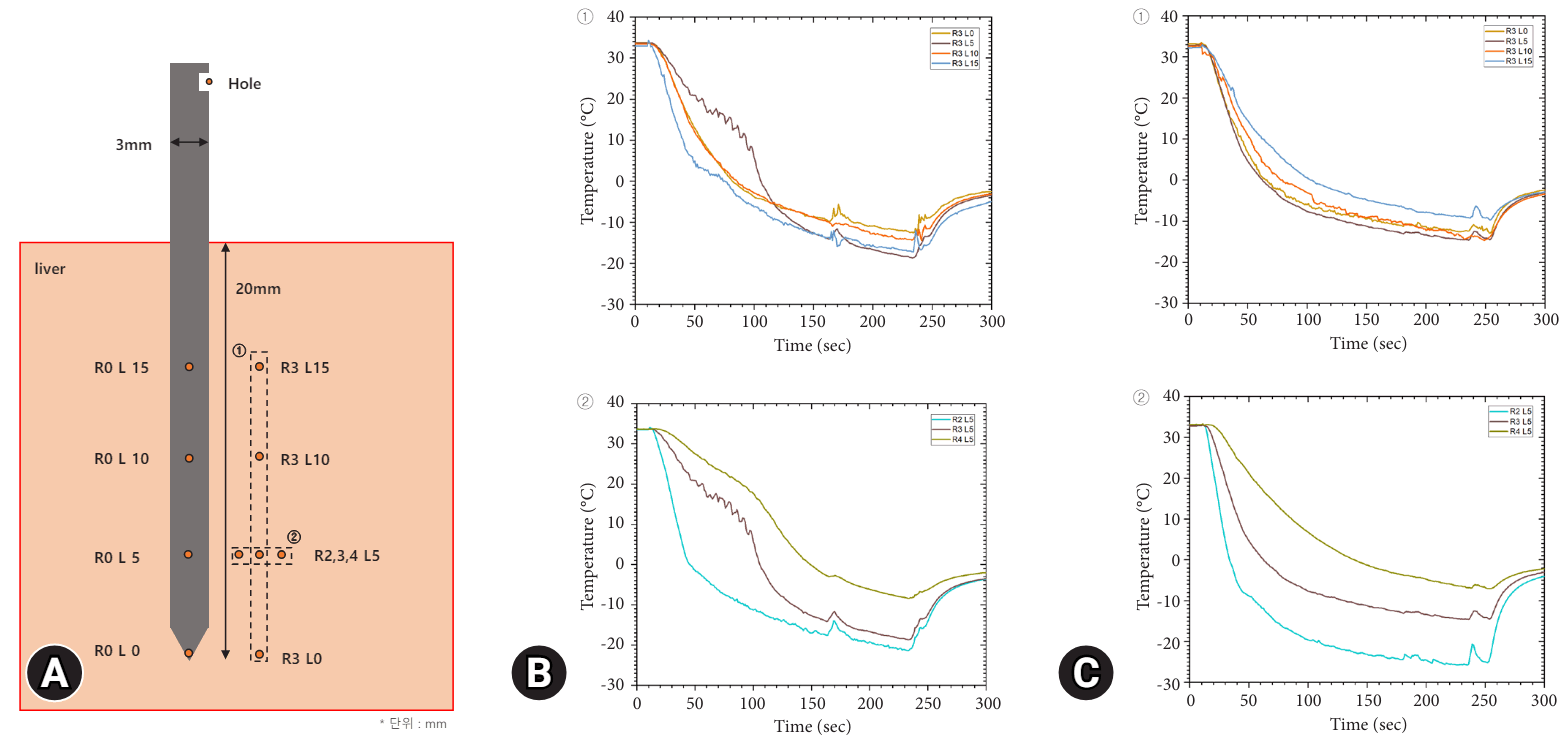
Fig. 4.
(A) Macroscopic view of the swine liver. (B) Trial 2: coronal section shows mild damage (hematoxylin and eosin [H&E] stain, ×4). (C) Trial 1: sagittal section shows mild and moderate damage (H&E stain, ×4). (D) Trial 1: sagittal section shows extensive necrosis and hemorrhage (H&E stain, ×100).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download