Abstract
The literature pertaining to surveillance following treatment for esophageal squamous cell carcinoma (SCC) was reviewed and summarized, encompassing the current status and future perspectives. Analysis of the standardized mortality and incidence ratios for these cancers indicates an elevated risk of cancer in the oral cavity, pharynx, larynx, and lungs among patients with esophageal SCC compared to the general population. To enhance the efficacy of surveillance for these metachronous cancers, risk stratification is needed. Various factors, including multiple Lugol-voiding lesions, multiple foci of dilated vascular areas, young age, and high mean corpuscular volume, have been identified as predictors of metachronous SCCs. Current practice involves stratifying the risk of metachronous esophageal and head/neck SCCs based on the presence of multiple Lugol-voiding lesions. Endoscopic surveillance, scheduled 6–12 months post-endoscopic resection, has demonstrated effectiveness, with over 90% of metachronous esophageal SCCs treatable through minimally invasive modalities. Narrow-band imaging emerges as the preferred surveillance method for esophageal and head/neck SCC based on comparative studies of various imaging techniques. Innovative approaches, such as artificial intelligence-assisted detection systems and radiofrequency ablation of high-risk background mucosa, may improve outcomes in patients following endoscopic resection.
Esophageal cancer ranks as the seventh most prevalent cancer and the sixth leading cause of cancer-related mortality globally, with 572,000 new cases and 509,000 deaths in 2018.1 Despite a swift rise in the incidence of esophageal adenocarcinoma in Europe and North America, esophageal squamous cell carcinoma (SCC) remains the predominant histological type, constituting 80% of all esophageal cancers worldwide.1
The overall survival rate for patients with advanced esophageal cancer remains low.2 However, when identified at an early stage, esophageal cancer can be treated successfully through endoscopic resection (ER), surgical resection, or chemoradiotherapy.3,4 ER is a curative approach for esophageal cancers with minimal risk of metastasis,3 employing a technique designed to completely remove the cancerous mucosa by deep submucosal resection. The resected specimen is subsequently used for pathologic diagnosis to stratify the patient’s risk for metastasis. In cases where there is a significant risk of metastasis, additional treatments, such as esophagectomy or chemoradiotherapy, are administered.5,6
This organized treatment strategy has significantly improved the outcome of esophageal cancer after ER.3,7,8 Notable progress in the prognosis of esophageal cancer highlights the importance of surveillance for subsequent disease. This review aims to summarize the existing literature on surveillance after treatment for esophageal SCC, offering insights into its current status and future perspectives.
The PubMed database was systematically searched for relevant articles published between January 2000 and August 2023. Any articles not captured through the systematic search were identified by a manual search. The following search terms were used: (esophageal cancer) AND (endoscopic resection) AND (outcome); (esophageal cancer) AND (ESD) AND (outcome); (esophageal cancer) AND (surveillance) AND (endoscopy); (esophageal cancer) AND (metachronous) AND (endoscopy); (esophageal cancer) AND (second primary); (esophageal cancer) AND (other organ); (esophageal cancer) AND (endoscopy) AND (detection) AND (prospective); (head and neck cancer) AND (endoscopy) AND (detection) AND (prospective); (pharyngeal cancer) AND (endoscopy) AND (detection) AND (prospective); and (artificial intelligence) AND (esophagus) AND (endoscopy). The search was restricted to studies published in English.
Several studies have investigated the long-term survival outcomes following ER of esophageal SCC. Inclusion criteria for this analysis comprised studies with a sample size exceeding 100 subjects and providing detailed insights into the causes of death.3,7,8 Yamashina et al.3 collected detailed data from 402 patients who underwent ER for esophageal SCC, with a high follow-up rate of 98.2%. Notably patients with SCC involving the epithelium/lamina propria exhibited a favorable prognosis, with a 5-year survival rate of 90.5%. Conversely, those with SCC involving the muscularis mucosa and submucosa experienced lower 5-year survival rates (71.1% and 70.8%, respectively), with increased mortality from both esophageal SCC and other diseases. In the study, malignant tumors contributed to 25 out of 49 total deaths, originating from the esophagus (n=7), lung (n=6), pharynx (n=4), liver (n=2), gallbladder (n=2), and other sites (n=4). Non-cancer-related causes of death included pneumonia (n=6), heart disease (n=6), liver cirrhosis (n=3), and other miscellaneous causes (n=9). Similarly, Iwai et al.7 conducted an analysis of outcomes in 566 patients treated with ER, identifying 64 deaths during the follow-up period. In their study, malignant tumors were the predominant cause of death in 44 out of 64 cases. The origin of the malignant tumors included the esophagus (n=11), pharynx (n=7), lung (n=6), liver (n=4), pancreas (n=3), hematologic neoplasm (n=3), stomach (n=2), kidney (n=2), larynx (n=2), and other sites (n=4). Causes of death unrelated to cancer encompassed pneumonia (n=8), liver cirrhosis (n=5), heart failure (n=2), miscellaneous reasons (n=9), and an unknown cause of death (n=1).
Mortality rates are frequently compared between a study population and the general population to assess the excess mortality risk in the study subjects. The standardized mortality ratio (SMR) represents the ratio of the actual observed mortality in the study group and the expected mortality calculated from the general population. We have previously assessed the SMR in patients with esophageal SCC.8 In that population, 16 out of 22 deaths were attributed to malignant tumors. Notably, mortality from malignant tumors (SMR, 3.14; 95% confidence interval [CI], 1.79–5.09), particularly head/neck cancer (SMR, 68.40; 95% CI, 24.98–148.88), exhibited a significantly higher SMR compared to the general population. While the SMR in the population with esophageal cancer was higher (SMR, 4.82; 95% CI: 0.06–26.81), it was not a significant difference from that in the general population.
The findings from these reports indicate that over half of the patients with esophageal mucosal SCC experience fatal events from malignant tumors of the esophagus or another primary cancer. Furthermore, the mortality attributed to malignancies in these patients exceeds that observed in the general population. This underscores the importance of care, not only addressing esophageal cancers but also vigilance in monitoring other primary cancers.
The risk of metachronous primary cancer is usually evaluated using the standardized incidence ratio (SIR), representing the relative incidence of cancer with reference to the general population. Numerous strudies have reported a high SIR for metachronous primary cancer in patients with esophageal SCC. The high SIR for SCC in the upper aerodigestive tract among patients with esophageal SCC is explained by the field cancerization theory.9
The reported high SIR for pharyngeal cancer in patients with esophageal SCC can be attributed to the shared risk factors between these cancers, including alcohol consumption, smoking, and aldehyde dehydrogenase-2 (ALDH2) enzyme deficiency.10 Similarly, a high SIR for lung cancer is observed in these patients because of the shared risk factor of smoking.11 Other studies have also demonstrated high SIRs for other cancer types, including stomach, colorectal, pancreatic, thyroid, and renal cancers.12-14
However, SIR values can be susceptible to ascertainment bias15 or surveillance bias, potentially influencing the reported incidence of metachronous cancers. For instance, routine examinations, such as endoscopy, computed tomography, and abdominal ultrasonography, commonly performed during esophageal cancer staging, may increase the chances of detecting metachronous primary cancers. A change in the SIR during the surveillance period may provide important insights into interpreting and reducing this bias. Another approach to address this bias is to evaluate the SMR, which gauges mortality relative to that of the general population.16 Unlike the SIR, the SMR remains unaffected by variation in examination frequency. Accordingly, this review section exclusively incorporates articles that evaluated the periodic change in the SIR (Table 1)12,13 or the SMR of metachronous primary cancer.17
Chuang et al.12 investigated 13 population-based cancer registries and assessed the excess risk of metachronous primary cancer following esophageal cancer. In their study, metachronous cancer was defined as cancer that developed subsequent to esophageal cancer. During the first six months of follow-up, increased SIRs were observed for metachronous primary cancers in the oral cavity and pharynx, stomach, liver, and kidney. Beyond the 6-month mark, increased SIR values were associated with cancers of the oral cavity and pharynx, larynx, and lung. Similarly, Chen et al.13 also assessed the SIR in 18,026 patients with esophageal cancer, defining metachronous cancer as cancer developing after one year. The study consistently reported high SIRs for cancers of the head/neck, stomach, and lung/mediastinum. In a study by Ohmori et al.,17 assessing the SIR and SMR values in patients with superficial or localized esophageal cancer without lymph node metastasis (using data from the integrated database of hospital-based cancer registry and Vital Statistics of Japan), metachronous cancer was defined as cancer developing after two months. Significantly higher SMR and SIR values were confirmed for cancers in the mouth/pharynx, larynx, pancreas, and leukemia, with respective values of 10.78/16.16, 8.56/6.44, 2.33/2.31, and 3.96/4.42. SIRs were significantly higher for stomach, lung, and skin cancers, with respective values of 2.84, 2.36, and 3.38, while SMRs were not significantly elevated in these cancers. The three studies demonstrated ingenuity in eliminating surveillance bias and revealed the heightened risk of metachronous primary cancer, particularly in the oral cavity, pharynx, larynx, and lung, compared to those in the general population, with more than one of the studies showing a high SMR or a consistently high SIR for these cancers. Therefore, strict surveillance of these organs is required post-ER for esophageal SCC. However, further investigations are needed to determine the risks associated with gastric and pancreatic cancer and leukemia. This review of metachronous primary cancer post-ER for esophageal cancer identified the elevated risk of head/neck cancers (oral cavity, pharynx, and larynx) and lung cancer in patients with esophageal cancer. The subsequent section outlines the recommended surveillance strategy for these cancers, excluding specific comments on lung cancer, as this aspect is best addressed by experts in the field.
Patients with esophageal SCC exhibit a notable predisposition to the development of metachronous esophageal SCC and head/neck SCC.3,18,19 Yamashina et al.3 reported cumulative 3-year and 5-year incidence rates for metachronous esophageal SCC at 15.5% and 20.6%, respectively. Similarly, Kato et al.18 reported cumulative 3-year and 5-year incidence rates for metachronous head/neck SCC at 5.3% and 9.7%, respectively. Risk stratification is needed for effective surveillance of these metachronous cancers.
Various predictors of metachronous esophageal or head/neck SCC have been reported, with Lugol-voiding lesions (LVL) identified as the most well-established predictor (Fig. 1). LVL is defined as the number of lesions per endoscopic view (A, no lesions; B, 1–9 lesions; C, ≥10 lesions) after spraying with iodine solution. Katada et al.19 demonstrated a significant association between LVL grade and the 2-year cumulative incidence of metachronous multiple SCC of the esophagus (A, 4.0%; B, 9.4%; and C, 24.7%; p<0.0001) and of the head/neck region (A, 0.0%; B, 1.7%; and C, 8.6%; p=0.016 for A vs. C and p=0.008 for B vs. C). This method is widely accepted as a reliable means of stratifying the risk of metachronous esophageal and head/neck SCC globally. Despite its effectiveness, the use of iodine for LVL assessments can sometimes be associated with adverse events, such as chest pain and discomfort.20 Therefore, less invasive methods for stratification of the risk of metachronous esophageal and head/neck SCC have been investigated.
Recently, the use of equipment-based image-enhanced endoscopy, specifically narrow band imaging (NBI) and blue laser imaging (BLI), has gained global recognition in the diagnosis of esophageal SCC. Previous studies have explored endoscopic findings in the noncancerous background esophageal mucosa, suggesting a high risk for metachronous esophageal or head/neck SCC using these modalities.21-23 In retrospective investigations, we investigated the NBI/BLI findings corresponding to individual small LVLs and identified that a focus of dilated vascular areas corresponds primarily to individual small LVLs.21 Moreover, we investigated the association between the grade of multiple LVLs and various NBI/BLI findings and revealed that the presence of multiple foci of dilated vascular areas (MDV) (Fig. 2) in the noncancerous background esophageal mucosa significantly correlates with multiple LVLs.21 In a more recent prospective study, we explored the association between number of MDVs in the entire esophagus and metachronous esophageal SCC. The study demonstrated that the 2-year incidence of metachronous esophageal SCC was 7.1% in patients with an MDV of ≤4 and 13.9% in those with an MDV of ≥5 (p<0.01). In multivariate analysis, MDV emerged as an independent predictor of metachronous esophageal SCC, with an odds ratio of 2.37 (95% CI, 1.06–5.31).22 Additionally, Azuma et al.23 retrospectively investigated seven endoscopic findings (1, a brownish area with an unclear margin; 2, white flat deposits; 3, multiple foci of dilated vessels; 4, low capillary permeability; 5, multiple glycogenic acanthosis; 6, horizontal lines; and 7, a nonuniform color tone) and found that metachronous esophageal SCC was significantly associated with multiple foci of dilated vessels, low capillary permeability, and a nonuniform color tone. While these endoscopic findings may allow for stratification of a patient’s risk of esophageal and head/neck SCC without iodine staining, they are more subtle than LVL and are more challenging to assess. Further innovation, such as computer-assisted diagnosis of MDV, is needed before this method can be widely adopted.
Multiple LVL or MDV requires endoscopic assessment of the background esophageal mucosa; however, risk stratification of metachronous esophageal and head/neck SCC can be achieved without endoscopic examination. For instance, one study demonstrated that the cumulative incidence of metachronous head/neck SCC was significantly higher in younger patients (aged <60 years) compared to older patients (aged ≥ 60 years, p=0.001).24 Conversely, the cumulative incidence of cancer in other organs was found to be significantly higher in older patients than in younger patients (p=0.03). Another study revealed that macrocytosis, defined by a high mean corpuscular volume (MCV) ≥106 fL, was associated with a higher 2-year cumulative incidence of metachronous esophageal SCC (11.4% without high MCV vs. 38.1% with high MCV, p=0.002).25 This study suggested that macrocytosis, a traditional marker of alcohol abuse or alcoholism, could serve as a marker to identify individuals at high risk for esophageal SCC. Additionally, Kagemoto et al.26 assessed the relationship between ADH1B and LADH2 risk alleles and metachronous SCC post-ER, revealing that ADH1B rs1229984 GG, ALDH2 rs671 GA, and smoking status were independently associated with the risk of developing metachronous esophageal SCC. However, the discriminability of these factors in predicting the risk of metachronous SCC may not be as precise as that achieved using multiple LVL. Therefore, the current standard practice involves stratifying the risk of metachronous esophageal and head/neck SCC using LVL.
Metachronous esophageal SCC adversely affects post-ER outcomes of esophageal SCC. However, there is currently no consensus on the optimal approach for surveillance of metachronous esophageal and head/neck SCCs following ER. Additionally, there are limited studies examining the impact of different surveillance methods and intervals on early detection and mortality after ER for esophageal SCC. In a recent investigation, we analyzed the impact of long-term surveillance endoscopy on mortality from metachronous esophageal and head/neck SCC after ER.27 This study included patients who underwent ER for esophageal SCC, excluding those with other primary invasive cancers diagnosed or treated within one year before ER. Patients who were lost to periodic surveillance (i.e., no surveillance endoscopy for more than two years) were also excluded. Patients generally underwent surveillance endoscopy with observation of the pharynx every 6 to 12 months. Data on development of metachronous cancers and causes of death were collected from the integrated Osaka Cancer Registry and Vital Statistics of Japan database. During a median follow-up of 67.4 months, 230 patients (36.7%) developed 500 metachronous esophageal SCCs, and 126 (20.1%) developed 239 metachronous head/neck SCCs. The 3-, 5-, and 7-year cumulative incidences were 25.8%, 36.0%, and 43.6%, respectively, for metachronous esophageal SCCs, and 10.9%, 16.0%, and 26.9% for metachronous head/neck SCCs. By merging the Vital Statistics survey death in Japan, the cause of death was confirmed in all 104 patients who died during follow-up. No patient died of metachronous esophageal cancer, and only seven patients (1.1%) died of metachronous head/neck cancer. The 5- and 7-year disease-specific survival rates were 99.6% and 98.6%, respectively. The findings of this study indicate that regular and continuous endoscopic surveillance every 6 to 12 months may reduce the mortality from metachronous esophageal and head/neck SCCs. However, it is important to recognize that 1.1% of patients died from metachronous head/neck SCC even with regular surveillance.
Based on the results of previous studies,3,18,19,27-32 the annual incidence rates of esophageal SCC is expected to be 4% to 6% for esophageal SCC and 1.5% to 3% for head/neck SCC. Despite the majority of metachronous esophageal cancers being described as superficial cancers and curable with local treatment, the high incidence of metachronous cancers underscores the importance of surveillance for these patients.
The primary goal of cancer surveillance is to reduce mortality associated with the targeted cancer. However, a more practical objective is to achieve a cure for metachronous cancers through minimally invasive treatments, such as ER, by detecting them at an early stage. Only studies of early detection of metachronous cancers during surveillance that included more than 100 subjects were selected for inclusion in this section. All the studies selected were analyzed in terms of surveillance interval, application of invasive treatment, and early detection (Table 2).3,28-31 The literature search identified five studies,3,28-31 including three from Japan, one from Taiwan, and one from China. The surveillance interval varied among the studies, with three studies recommending 6 to 12 months interval,3,28,31 while two studies recommended a shorter interval.29,30 One study from China28 found that an exceptionally high proportion of patients underwent invasive treatment, such as esophagectomy or chemoradiotherapy. In the other four studies, although the stage of metachronous esophageal SCC was not clearly reported, more than 90% of cases were amenable to minimally invasive treatment such as ER. Based on these reports, endoscopic examinations may be best performed at 6 to 12-month intervals as surveillance for metachronous esophageal SCC after ER. Particularly close endoscopic surveillance is required for patients with multiple LVLs in the esophagus.
Shinozaki et al.32 investigated the effectiveness of planned surveillance by gastrointestinal endoscopists and otolaryngologists for head/neck SCCs. In their study, gastrointestinal endoscopists examined the head/neck regions 3 to 6 months after ER and at 6-monthly intervals thereafter. Otolaryngologists also examined the head/neck regions at the time of ER and at 12-month intervals thereafter. During a median follow-up of 49.4 months, 33 new SCCs were detected in 20 patients using this surveillance schedule. Thirty-two of the 33 lesions were detected on endoscopic examination by gastrointestinal endoscopists. Twenty-nine lesions in 17 patients were treated by transoral surgery. One of these patients had SCC in the uvula and developed cervical lymph node metastasis after transoral surgery, which was treated successfully by neck dissection followed by radiotherapy. One patient with two lesions in the pyriform sinus and glottis was treated by radiotherapy. Two lesions in the pyriform sinus and posterior wall of the hypopharynx disappeared after biopsy. Using this strict surveillance program, most of the head/neck SCCs were detected at an early stage and could be treated by minimally invasive transoral surgery.
Considering the available evidence, I would suggest regular surveillance of the esophagus and the head/neck region by gastrointestinal endoscopists every 6 to 12 months. Additionally, incorporating annual examinations by otolaryngologists is advisable and may reduce the risk of missing lesions, especially in the post-cricoid area or posterior wall the hypopharynx (Fig. 3).
Superficial esophageal SCCs, especially intra-mucosal SCCs, often present as flat lesions with minimal color change on the mucosal surface. These changes can be challenging to identify using standard white light imaging (WLI) endoscopy; therefore, WLI is of limited value for the surveillance of metachronous esophageal SCC. Iodine chromoendoscopy is used to highlight areas of abnormality, thereby significantly increasing the ability to detect esophageal SCC. While iodine staining is considered useful because of its high sensitivity for esophageal SCC, it has limitations, including low specificity and potential side effects such as iodine hypersensitivity, laryngitis, chest discomfort, and nausea.20 Equipment-based image-enhanced endoscopy is an optical technology that allows for better visualization of the microsurface and microvascular patterns on the mucosal surface. This section explores the usefulness of these imaging modalities in prospective comparative studies that included more than 100 subjects (Table 3).33-42
Three studies comparing the WLI with equipment-based image-enhanced endoscopy were identified.33-35 Muto et al.33 conducted a multicenter randomized controlled trial (RCT) comparing the diagnostic yield of esophageal and head/neck SCC between WLI and NBI. The sensitivity of NBI was significantly higher than that of WLI for the esophagus and head/neck. Only one case (7.7%) of head/neck SCC was detected by primary WLI, whereas all 15 head/neck SCCs were detected by primary NBI. For esophageal SCCs, 58 cases (55%) were detected by primary WLI, while 104 cases (97%) were detected by primary NBI (p<0.001). Kawai et al.34 confirmed that the sensitivity of NBI was significantly higher than that of WLI using ultrathin transnasal endoscopy. Ono et al.35 conducted a multicenter RCT comparing linked color imaging (LCI) and WLI for detecting neoplastic lesions in the upper gastrointestinal tract, including the pharynx, esophagus, and stomach. In that trial, the percentage of patients diagnosed with one or more neoplastic lesions was higher with primary LCI than with primary WLI (60/750 patients [8.0%] vs. 36/752 patients [4.8%]; p=0.011). Ten cases of esophageal SCC were detected by primary WLI, while 16 esophageal SCCs were detected by primary LCI. Additionally, two head/neck SCCs were detected by primary WLI, and seven head/neck SCCs were detected by primary LCI. The findings of these studies indicate that NBI has higher sensitivity for the detection of esophageal and head/neck SCCs. Furthermore, LCI may have higher sensitivity than WLI for the detection of these SCCs. Representative cases of head/neck cancer detected by gastrointestinal endoscopy are shown in Figures 4–7.
Two studies compared the various types of equipment-based image-enhanced endoscopy.36,37 Kawada et al.36 performed a non-randomized study comparing WLI followed by NBI with WLI followed by BLI for the detection of esophageal SCCs. The percentage of patients detected to have one or more malignant lesions was similar between the WLI followed by NBI group and the WLI followed by BLI group (5.8% [15/258] vs. 5.5% [14/254]). Ogata et al.37 compared BLI and LCI for the detection of esophageal SCC in a multicenter RCT and found no significant difference in the rate of detection of esophageal SCC between primary BLI and primary LCI (4.0% [14/351] vs. 4.9% [17/348]; p=0.565). However, the rate of esophageal SCC missed by the primary mode was lower in the BLI primary group than in the LCI primary group (26.3% [5/19] vs. 63.3% [19/30]; p=0.012). These two studies suggest that BLI may have similar sensitivity to NBI. Furthermore, the detectability of NBI/BLI may be better than that of LCI, although further investigation is needed.
Five studies were identified that compared iodine staining with other modalities.38-42 All studies38-40 comparing WLI and iodine staining demonstrated that iodine staining had higher sensitivity than WLI, indicating that WLI has limited value for detection of esophageal SCC. In all four studies39-42 comparing NBI and iodine staining, NBI showed high sensitivity equivalent to that of iodine staining. Moreover, two studies41,42 found that the specificity of NBI was higher than that of iodine staining.
Based on these comparative studies of various imaging modalities, NBI appears to be the optimal surveillance modality for esophageal and head/neck SCC. BLI is expected to have similar performance considering that BLI employs a similar system to NBI with a laser light source developed for the observation of narrow band light. However, in Japan, some facilities still use iodine staining for the surveillance of patients at high risk for SCC, including those who have undergone ER of esophageal SCC. This is likely because iodine staining allows clearer visualization of esophageal or head/neck SCC than NBI and has a better ability to predict the risk of metachronous SCC in the esophagus and head/neck. Both NBI and iodine staining have advantages and disadvantages. Iodine staining has high sensitivity for SCC and clear visualization of SCC compared to areas not stained with iodine, whereas NBI has high specificity and better acceptability by patients. There is no consensus regarding the choice between these two modalities. However, NBI is considerably preferred for the surveillance of the esophagus and head/neck post-ER. Careful observation by an experienced endoscopist in an environment with less-air condition43 is considered sufficiently sensitive and specific for detecting esophageal and head/neck SCC.
I would like to introduce the method used to observe the head/neck region (Fig. 8). To facilitate pharyngeal observation, a dose of pethidine hydrochloride (35 mg) is injected.44 Prior to biting on a mouthpiece, the examination begins with the wide opening of the mouth for the observation of the oral cavity and oropharynx. To observe the ventral surface and the lateral sides of the tongue, the patient is instructed to move their tongue. For a detailed observation of the oropharynx, the patient is instructed to say ‘aaah’ with the tongue extended as far forward as possible. To observe the hypopharynx, a Valsalva maneuver is performed using a specific small mouthpiece (MC Medical) that can be completely contained in the mouth.45 This method enhances the observation of the hypopharynx, especially the post-cricoid area and posterior wall.
The advancement of imaging modalities has facilitated the detection of esophageal and head/neck SCCs. Despite these improvements, some metachronous cancers are still identified in advanced stages during surveillance.3,28-32 A previous study revealed that the diagnosis of SCCs using NBI was susceptible to interobserver variability.46 Another report demonstrated that inexperienced endoscopists had lower sensitivity in detecting esophageal SCC compared to experts.47 A potential solution to mitigate both the variability and complexity of endoscopic diagnosis is to apply an artificial intelligence system. Previous studies have shown that artificial intelligence has high sensitivity and specificity for detection of esophageal SCC that is comparable with or even better than that reported for expert endoscopists.46,48,49 Therefore, artificial intelligence-assisted detection systems have potential for real-time assistance to endoscopists in diagnosing metachronous esophageal and head/neck SCCs in the near future.
Strict surveillance measures may enable early detection of metachronous SCCs. However, achieving a more favorable outcome involves prevention. Radiofrequency ablation for esophageal neoplasia involves delivering 465-kHz energy waveform through a bipolar electrode array, mounted on the outside of a balloon or on an articulated platform at the distal end of an endoscope (a HALO 360 or 90 system, Barrx Medical Inc.). Chen et al.50 reported that combination of ER and radiofrequency ablation effectively eliminated multiple LVLs in the background mucosa, leading to a reduced incidence of metachronous esophageal squamous neoplasms and local recurrence after ER. These innovative approaches hold promise in improving outcomes for patients post-ER by enhancing early detection of metachronous SCCs or potentially eliminating the need for surveillance in the future through treatment of high-risk background mucosa.
More than half of death in patients with esophageal SCC is due to malignancies. Furthermore, the mortality from malignancy in these patients surpass that in the general population, underscoring the importance of care, not only for esophageal cancers but also for other primary cancers. The review of metachronous primary cancer post-ER for esophageal cancer identified an increased risk of cancer in the head/neck region (oral cavity, pharynx, and larynx) and the lung. The expected annual incidence of metachronous esophageal and head/neck SCC is estimated at 4% to 6% and 1.5% to 3%, respectively. Previous studies have suggested the potential benefits of regular and continuous endoscopic surveillance in reducing mortality from metachronous esophageal and head/neck SCCs. While there may be a lack of evidence, regular surveillance of the esophagus and head/neck region every 6 to 12 months, particularly by gastrointestinal endoscopists is recommended. Additionally, the inclusion of annual examinations by otolaryngologists may further reduce the risk of missing lesions.
Notes
Conflicts of Interest
The author has received honoraria for lectures from Olympus, FUJIFILM Medical, Daiichi-Sankyo, Miyarisan Pharmaceutical, AI Medical Service, and Astra Zeneca.
Acknowledgments
The author would like to thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
2. Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet. 2017; 390:2383–2396.
3. Yamashina T, Ishihara R, Nagai K, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol. 2013; 108:544–551.
4. Kato K, Ito Y, Nozaki I, et al. Parallel-group controlled trial of surgery versus chemoradiotherapy in patients with stage I esophageal squamous cell carcinoma. Gastroenterology. 2021; 161:1878–1886.
5. Ishihara R, Arima M, Iizuka T, et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. 2020; 32:452–493.
6. Kitagawa Y, Ishihara R, Ishikawa H, et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 1. Esophagus. 2023; 20:343–372.
7. Iwai N, Dohi O, Yamada S, et al. Prognostic risk factors associated with esophageal squamous cell carcinoma patients undergoing endoscopic submucosal dissection: a multi-center cohort study. Surg Endosc. 2022; 36:2279–2289.
8. Ishihara R, Tanaka H, Iishi H, et al. Long-term outcome of esophageal mucosal squamous cell carcinoma without lymphovascular involvement after endoscopic resection. Cancer. 2008; 112:2166–2172.
9. Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer. 1953; 6:963–968.
10. Yokoyama A, Ohmori T, Muramatsu T, et al. Cancer screening of upper aerodigestive tract in Japanese alcoholics with reference to drinking and smoking habits and aldehyde dehydrogenase-2 genotype. Int J Cancer. 1996; 68:313–316.
11. Dresler CM, León ME, Straif K, et al. Reversal of risk upon quitting smoking. Lancet. 2006; 368:348–349.
12. Chuang SC, Hashibe M, Scelo G, et al. Risk of second primary cancer among esophageal cancer patients: a pooled analysis of 13 cancer registries. Cancer Epidemiol Biomarkers Prev. 2008; 17:1543–1549.
13. Chen SC, Teng CJ, Hu YW, et al. Secondary primary malignancy risk among patients with esophageal cancer in Taiwan: a nationwide population-based study. PLoS One. 2015; 10:e0116384.
14. Matsubara T, Yamada K, Nakagawa A. Risk of second primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003; 21:4336–4341.
15. Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004; 58:635–641.
16. dos Santos SI. Cancer epidemiology: principles and methods. International Agency for Research on Cancer;1999.
17. Ohmori M, Ishihara R, Morishima T, et al. Excessive risk of second-cancer incidence and cancer mortality in patients with esophageal cancer. J Gastroenterol. 2021; 56:434–441.
18. Kato M, Ishihara R, Hamada K, et al. Endoscopic surveillance of head and neck cancer in patients with esophageal squamous cell carcinoma. Endosc Int Open. 2016; 4:E752–E755.
19. Katada C, Yokoyama T, Yano T, et al. Alcohol consumption and multiple dysplastic lesions increase risk of squamous cell carcinoma in the esophagus, head, and neck. Gastroenterology. 2016; 151:860–869.
20. Kondo H, Fukuda H, Ono H, et al. Sodium thiosulfate solution spray for relief of irritation caused by Lugol's stain in chromoendoscopy. Gastrointest Endosc. 2001; 53:199–202.
21. Matsuno K, Ishihara R, Nakagawa K, et al. Endoscopic findings corresponding to multiple Lugol-voiding lesions in the esophageal background mucosa. J Gastroenterol Hepatol. 2019; 34:390–396.
22. Kawakami Y, Ishihara R, Matsuno K, et al. Multiple foci of dilated vessels as a new predictor of metachronous esophageal cancer. Dig Endosc. 2024; 36:421–427.
23. Azuma Y, Dohi O, Naito Y, et al. Blue laser imaging identifies endoscopic findings corresponding to metachronous esophageal squamous cell carcinoma. Esophagus. 2022; 19:278–286.
24. Maekawa A, Ishihara R, Iwatsubo T, et al. High incidence of head and neck cancers after endoscopic resection for esophageal cancer in younger patients. J Gastroenterol. 2020; 55:401–407.
25. Katada C, Yokoyama T, Yano T, et al. Association between macrocytosis and metachronous squamous cell carcinoma of the esophagus after endoscopic resection in men with early esophageal squamous cell carcinoma. Esophagus. 2020; 17:149–158.
26. Kagemoto K, Urabe Y, Miwata T, et al. ADH1B and ALDH2 are associated with metachronous SCC after endoscopic submucosal dissection of esophageal squamous cell carcinoma. Cancer Med. 2016; 5:1397–1404.
27. Matsueda K, Ishihara R, Morishima T, et al. Impact of endoscopic surveillance on mortality of metachronous esophageal and head and neck cancer after esophageal endoscopic resection. J Gastroenterol Hepatol. 2022; 37:2098–2104.
28. Jiang D, Li X, Wang H, et al. A retrospective study of endoscopic resection for 368 patients with early esophageal squamous cell carcinoma or precancerous lesions. Surg Endosc. 2017; 31:2122–2130.
29. Oda I, Shimizu Y, Yoshio T, et al. Long-term outcome of endoscopic resection for intramucosal esophageal squamous cell cancer: a secondary analysis of the Japan Esophageal Cohort study. Endoscopy. 2020; 52:967–975.
30. Suzuki Y, Kikuchi D, Hoteya S, et al. Effectiveness of chemoradiotherapy for metachronous esophageal squamous cell carcinoma. Digestion. 2021; 102:622–629.
31. Hsu MH, Wang WL, Chen TH, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma in Taiwan. BMC Gastroenterol. 2021; 21:308.
32. Shinozaki T, Katada C, Shiga K, et al. Effectiveness of planned surveillance for detecting second primary head and neck cancers after endoscopic resection of esophageal squamous cell carcinoma. Jpn J Clin Oncol. 2020; 50:1162–1167.
33. Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010; 28:1566–1572.
34. Kawai T, Takagi Y, Yamamoto K, et al. Narrow-band imaging on screening of esophageal lesions using an ultrathin transnasal endoscopy. J Gastroenterol Hepatol. 2012; 27 Suppl 3:34–39.
35. Ono S, Kawada K, Dohi O, et al. Linked color imaging focused on neoplasm detection in the upper gastrointestinal tract: a randomized trial. Ann Intern Med. 2021; 174:18–24.
36. Kawada K, Arima M, Miyahara R, et al. Effect of adding magnifying BLI, magnifying NBI, and iodine staining to white light imaging in diagnosis of early esophageal cancer. Endosc Int Open. 2021; 9:E1877–E1885.
37. Ogata Y, Hatta W, Koike T, et al. Blue light imaging and linked color imaging as a screening mode for esophageal squamous cell carcinoma in high-risk patients: multicenter randomized trial. Dig Endosc. 2023; 35:835–844.
38. Dubuc J, Legoux JL, Winnock M, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006; 38:690–695.
39. Ide E, Maluf-Filho F, Chaves DM, et al. Narrow-band imaging without magnification for detecting early esophageal squamous cell carcinoma. World J Gastroenterol. 2011; 17:4408–4413.
40. Yokoyama A, Ichimasa K, Ishiguro T, et al. Is it proper to use non-magnified narrow-band imaging for esophageal neoplasia screening? Japanese single-center, prospective study. Dig Endosc. 2012; 24:412–418.
41. Gruner M, Denis A, Masliah C, et al. Narrow-band imaging versus Lugol chromoendoscopy for esophageal squamous cell cancer screening in normal endoscopic practice: randomized controlled trial. Endoscopy. 2021; 53:674–682.
42. Nagami Y, Tominaga K, Machida H, et al. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol. 2014; 109:845–854.
43. Iwatsubo T, Ishihara R, Yamasaki Y, et al. Narrow band imaging under less-air condition improves the visibility of superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2020; 20:389.
44. Yamasaki Y, Ishihara R, Hanaoka N, et al. Pethidine hydrochloride is a better sedation method for pharyngeal observation by transoral endoscopy compared with no sedation and midazolam. Dig Endosc. 2017; 29:39–48.
45. Iwatsubo T, Ishihara R, Nakagawa K, et al. Pharyngeal observation via transoral endoscopy using a lip cover-type mouthpiece. J Gastroenterol Hepatol. 2019; 34:1384–1389.
46. Ohmori M, Ishihara R, Aoyama K, et al. Endoscopic detection and differentiation of esophageal lesions using a deep neural network. Gastrointest Endosc. 2020; 91:301–309.
47. Ishihara R, Takeuchi Y, Chatani R, et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010; 23:480–486.
48. Luo H, Xu G, Li C, et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019; 20:1645–1654.
49. Waki K, Ishihara R, Kato Y, et al. Usefulness of an artificial intelligence system for the detection of esophageal squamous cell carcinoma evaluated with videos simulating overlooking situation. Dig Endosc. 2021; 33:1101–1109.
50. Chen Z, Dou L, Liu Y, et al. Combination of endoscopic resection and radiofrequency ablation for the treatment of esophageal squamous cell neoplasia with multiple lugol-voiding lesions. Front Oncol. 2021; 11:786015.
Fig. 3.
Surveillance protocol for the head/neck region and the esophagus. GI, gastrointestinal; NBI, narrow-band imaging; BLI, blue laser imaging.
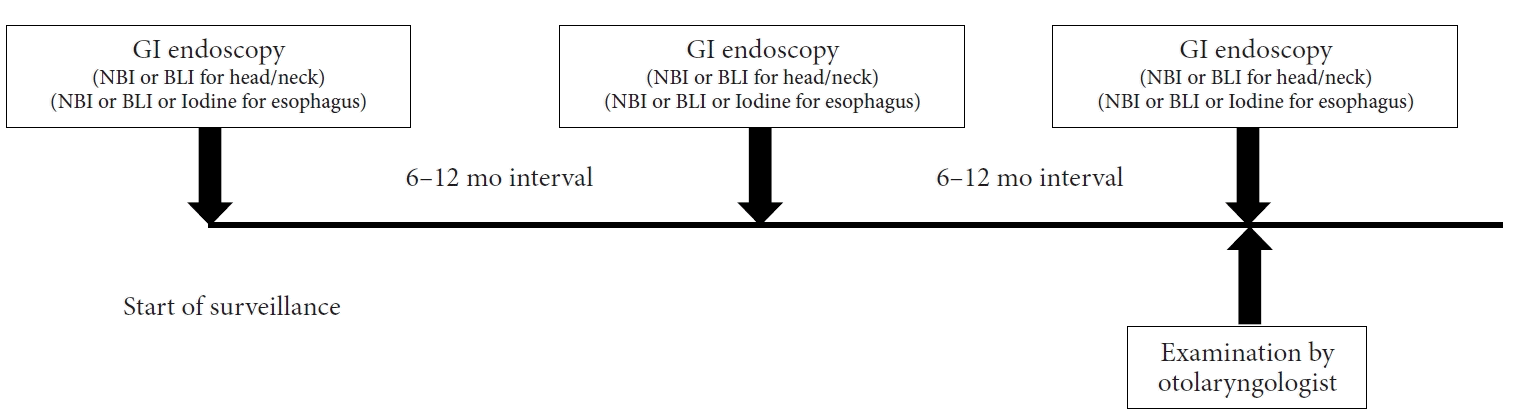
Fig. 4.
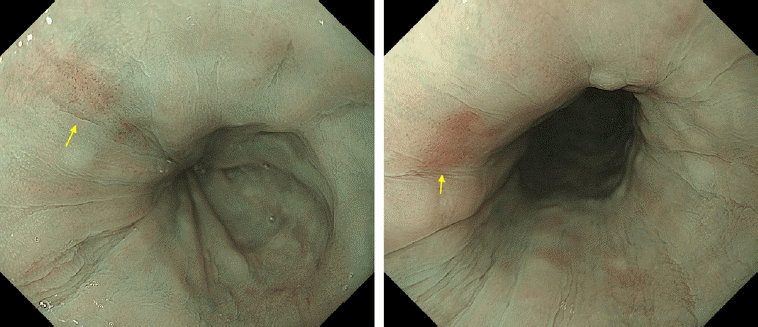
Metachronous cancer of the tongue (arrows) detected by gastrointestinal endoscopic examination.
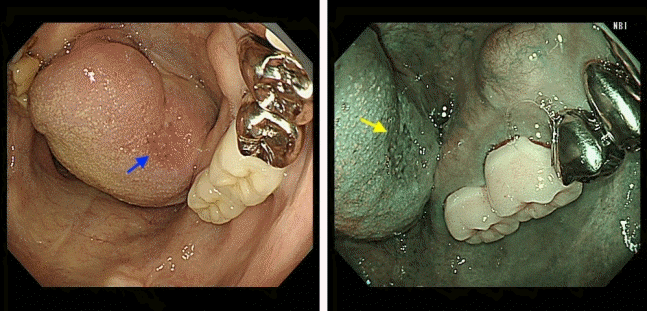
Fig. 5.
Metachronous cancer of the buccal mucosa (arrow) detected by gastrointestinal endoscopic examination.
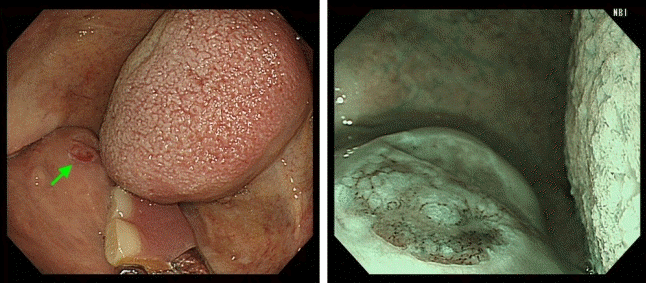
Fig. 6.
Metachronous cancer of the soft palate (arrow) detected by gastrointestinal endoscopic examination.
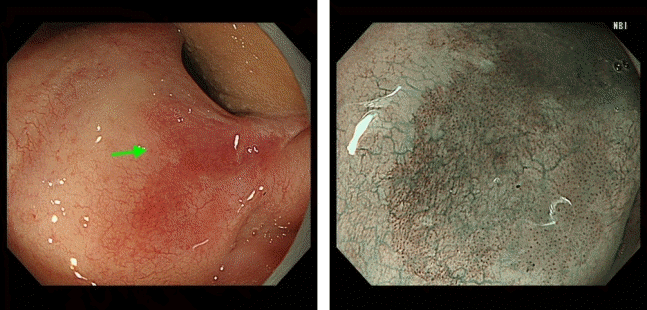
Fig. 7.
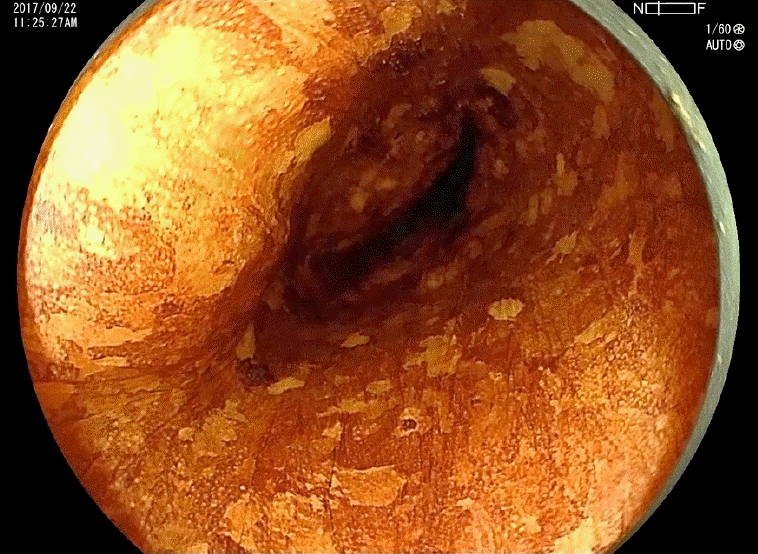
Metachronous cancer of the hypopharynx (arrows, post-cricoid area) detected by gastrointestinal endoscopic examination using the Valsalva maneuver. This maneuver entails a forceful attempt to exhale against a closed airway and can be used to observe the post-cricoid area.
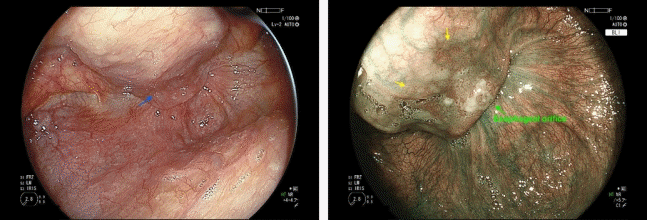
Fig. 8.
Observation of the tongue (A), the oropharynx (B), and the post-cricoid area and posterior wall (C).
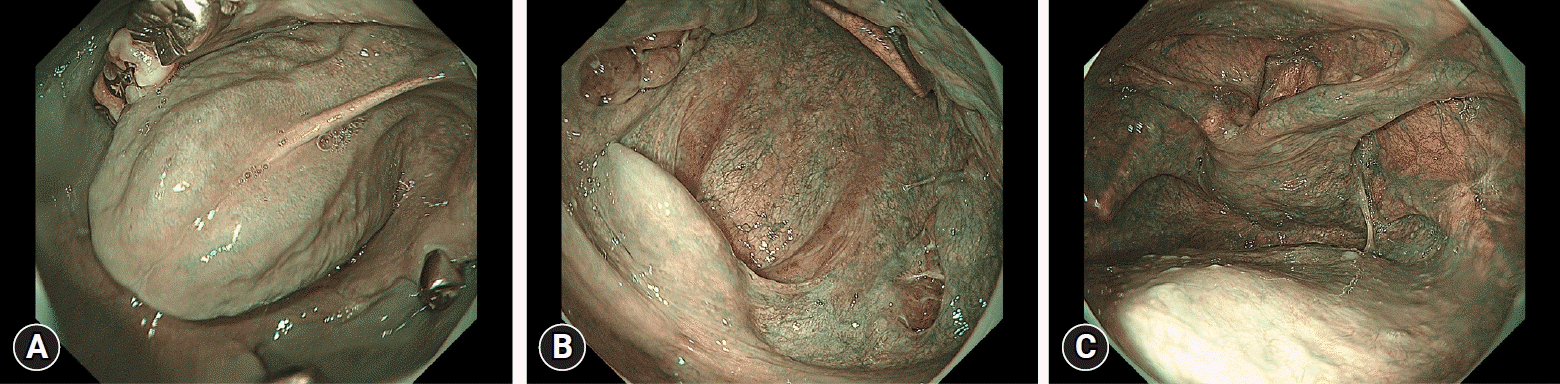
Table 1.
Risk of second primary cancer in patients with esophageal cancer
| Study | Organ | Follow SIR | |||
|---|---|---|---|---|---|
| Chuang et al.12 | Time since first cancer diagnosis | <6 mo | 6–11 mo | 1–4 yr | ≥5 yr |
| Oral cavity | 6.35 (4.02–9.52) | 5.02 (2.30–9.53) | 10.1 (7.17–13.8) | 4.06 (2.22–6.82) | |
| Larynx | 1.34 (0.16–4.85) | 1.36 (0.03–7.56) | 5.02 (2.17–9.88) | 4.19 (1.54–9.11) | |
| Lung | 1.47 (0.99–2.10) | 0.60 (0.22–1.31) | 1.98 (1.43–2.67) | 1.64 (1.13–2.31) | |
| Stomach | 2.82 (1.87–4.07) | 0.62 (0.13–1.81) | 1.35 (0.72–2.31) | 0.79 (0.32–1.62) | |
| Chen et al.13 | Follow-up time after esophageal cancer (yr) | 1–5 | 5–10 | ≥10 | |
| Head and neck | 15.40 (13.28–17.78) | 17.25 (13.10–22.29) | 16.61 (7.17–32.73) | ||
| Lung and mediastinum | 1.87 (1.27–2.65) | 1.96 (1.01–3.42) | 6.37 (2.56–13.12) | ||
| Stomach | 2.69 (1.60–4.26) | 4.76 (2.38–8.51) | 5.01 (0.61–18.10) | ||
Table 2.
Surveillance and treatment for metachronous esophageal cancer
| Study | Surveillance interval | Treatment for metachronous cancer |
|---|---|---|
| Yamashina et al.3 | Every 6–12 mo | 61 ER, 1 CRT, 1 esophagectomy |
| Jiang et al.28 | Every 6–12 mo | 38 ER, 13 esophagectomy, 3 RT, 2 symptomatic therapy |
| Oda et al.29 | Every 3 mo for 6 mo | 70 ER |
| Every 6 mo after 6 mo | 2 CRT, 2 ER+CRT | |
| Suzuki et al.30 | Every 6 mo | 17 ESD |
| Hsu et al.31 | Every 6 mo for 1 yr | 38 Endoscopic treatment |
| Annually after 1 yr | 3 Surgery or CRT |
Table 3.
Detection of cancer in the esophagus and the head and neck region
| Study | Site | Study design | Comparison | No. of patients | Results |
|---|---|---|---|---|---|
| Muto et al.33 | Esophagus, head and neck | RCT | WLI vs. NBI | 320 | Significantly higher sensitivity and accuracy by NBI |
| Kawai et al.34 | Esophagus | Tandem | WLI vs. NBI (Ultrathin) | 105 | Significantly higher sensitivity by NBI |
| Ono et al.35 | Upper gastrointestinal tract | RCT | WLI vs. LCI | 752 | Significantly more patients with neoplastic lesions by LCI |
| Kawada et al.36 | Mainly esophagus | Non-randomized | NBI vs. BLI | 512 | No significant difference in the detection |
| Ogata et al.37> | Esophagus | RCT | BLI vs. LCI | 699 | No difference in detection |
| Significantly lower miss rate by BLI | |||||
| Dubuc et al.38 | Esophagus | Tandem | WLI vs. Lugol | 1,095 | Significantly higher sensitivity by Lugol |
| Ide et al.39 | Esophagus | Tandem | WLI vs. NBI vs. Lugol | 129 | All lesions were detected by NBI or Lugol chromoendoscopy |
| Only 4 of 9 lesions were detected by WLI | |||||
| Yokoyama et al.40 | Esophagus | Tandem | WLI vs. NBI vs. Iodine | 117 | Significantly higher sensitivity by NBI |
| Equivalent sensitivity by NBI to iodine | |||||
| Gruner et al.41 | Esophagus | RCT | NBI vs. Lugol | 334 | Same sensitivity |
| Significantly higher specificity by NBI | |||||
| Nagami et al.42 | Esophagus | Non-randomized | NBI vs. Iodine | 202 | Significantly higher specificity and accuracy by NBI |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download