Abstract
Background/Aims
Endoscopic biliary drainage using self-expandable metallic stents (SEMSs) for malignant biliary strictures occasionally induces acute cholecystitis (AC). This study evaluated the efficacy of prophylactic gallbladder stents (GBS) during SEMS placement.
Methods
Among 158 patients who underwent SEMS placement for malignant biliary strictures between January 2018 and March 2023, 30 patients who attempted to undergo prophylactic GBS placement before SEMS placement were included.
Results
Technical success was achieved in 21 cases (70.0%). The mean diameter of the cystic duct was more significant in the successful cases (6.5 mm vs. 3.7 mm, p<0.05). Adverse events occurred for 7 patients (23.3%: acute pancreatitis in 7; non-obstructive cholangitis in 1; perforation of the cystic duct in 1 with an overlap), all of which improved with conservative treatment. No patients developed AC when the GBS placement was successful, whereas 25 of the 128 patients (19.5%) without a prophylactic GBS developed AC during the median follow-up period of 357 days (p=0.043). In the multivariable analysis, GBS placement was a significant factor in preventing AC (hazard ratio, 0.61; 95% confidence interval, 0.37–0.99; p=0.045).
Endoscopic biliary drainage has become the standard treatment for malignant biliary strictures.1,2 Although self-expandable metallic stents (SEMSs) have been increasingly used because of their long patency, acute cholecystitis (AC) after SEMS placement is a reported adverse event.3-5 The incidence rate of AC, reported to be 5.3% to 12.1%, is not negligible. In addition, it is often challenging to manage AC after SEMS placement due to debilitation related to advanced malignancy. Although surgical cholecystectomy is recommended for common calculous AC, it is often not an option for complicated cases.
Tumor involvement of the cystic duct (CD), tumor invasion into an artery that feeds the gallbladder, and coverage of the CD orifice by an SEMS have been reported as risk factors for AC after SEMS placement.3-5 However, even when these factors are absent, if the CD orifice is adjacent to the malignant stricture, the orifice will become occluded by the SEMS with a sufficient length to cover the stricture, possibly leading to AC in the future. Therefore, prophylactic gallbladder stent (GBS) placement during the SEMS placement session may prevent future AC. Although there have been a few reports on the efficacy of prophylactic GBSs,6,7 evidence is limited. Recently, a 5-Fr plastic stent designed for the gallbladder was developed, and there have been no reports on its prophylactic use. In this study, we retrospectively investigated the incidence of AC after simultaneous placement of a SEMS and a prophylactic GBS and their safety.
This was a single-center retrospective observational study. Patients for whom prophylactic GBS placement was attempted in the initial SEMS placement session from January 2018 to March 2023 for malignant biliary strictures were included in this study. The following patients were excluded: (1) patients with perihilar biliary obstructions (≥Bismuth level II), (2) those who were followed up for <30 days, (3) those who previously underwent surgical cholecystectomy, (4) those in whom a SEMS would not cover the CD orifice, and (5) those with concomitant AC. Patient data were extracted from a prospectively maintained endoscopic retrograde cholangiopancreatography (ERCP) database.
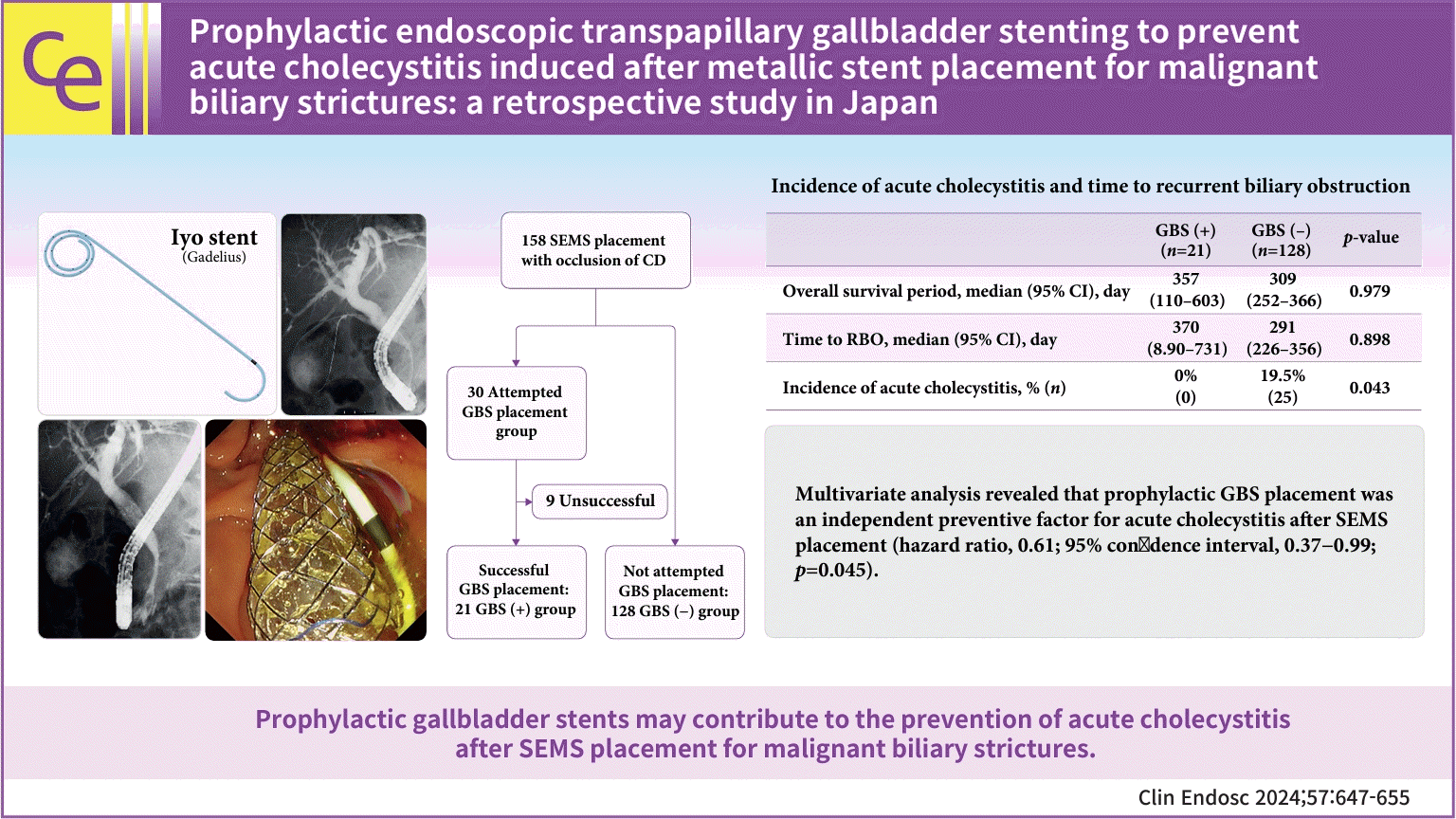
Endoscopic procedures were performed using a duodenoscope (JF-260V, TJF-260V, or TJF-Q290V; Olympus Co.). The endoscopists judged whether GB stenting should be attempted, considering the risk factors of AC after SEMS placement and if the orifice of the CD was covered. After confirming the presence of a biliary stricture by using cholangiography, a contrast agent was injected to visualize the CD orifice. A 0.025-inch guidewire (Radifocus, Terumo; NaviPro, Boston Scientific; VisiGlide2, Olympus) was then inserted into the CD. After successful guidewire insertion, a tapered catheter (5 or 7 Fr, MultiFunction Catheter; Gadelius Medical K.K.) was inserted to confirm whether a GBS could be inserted. Then, a 5-Fr, 30-cm plastic stent (IYO stent; Gadelius Medical K.K.) was placed into the gallbladder (Fig. 1). After the GBS was placed, a SEMS was deployed over the biliary stricture (Fig. 2).
The primary outcomes were the technical success, adverse events, and the incidence of AC. Technical success was defined as the successful placement of a GBS. Adverse events were defined as clinically significant unfavorable events after the procedure (for example, post-ERCP pancreatitis [PEP], acute cholangitis, peritonitis). The diagnosis and severity were determined according to the consensus guidelines by Cotton et al.8 Regarding the incidence of AC in patients with GBS, the diagnostic criteria for AC were based on the Tokyo Guidelines 2018.9
The following factors related to the primary outcomes were evaluated: the diameter of the CD at the time of cholangiography, the bifurcation direction of the CD, the total procedure time, the procedure time purely related to GBS placement (time for GBS), time to recurrent biliary obstruction (RBO), overall survival (OS), and risk factor for AC. The diameter of the CD was determined from the findings of the cholecystography. Regarding the CD diameter, the width of the CD orifice was measured when the orifice was visualized using cholecystography. When the orifice was not clear and the CD was visible, the width of the CD nearest to the orifice was measured. The bifurcation of the CD was morphologically classified into cranially and caudally directed bifurcations in reference to previous reports.10 The total procedure time was defined as the time from scope insertion to withdrawal. The time for GBS was defined as the time from the start of the attempt of cholecystography to complete deployment of the GBS or procedural secession due to abandoning.
Continuous variables were compared using a Mann-Whitney U test, and categorical variables were compared using the chi-square test or the Fisher’s exact test. The incidence of AC and time to RBO and OS were analyzed using the Kaplan-Meier method and a log-rank test. The risk factors for AC were evaluated using univariate and multivariate analyses. The multivariate analyses were performed using a Cox hazard model. Statistical analyses were performed using the IBM SPSS software ver. 24.0 (IBM Japan Ltd.) and StatMate III for Macintosh (Atms). A p-value of <0.05 was considered statistically significant.
SEMSs (uncovered or covered, excluding partially covered) were initially placed into malignant biliary strictures in 300 patients. One hundred fifty-eight patients were recruited for this study after the exclusion criteria. Of these, 30 patients for whom prophylactic GBS placement was attempted during the SEMS placement session were included in this study. GBS placement was successful for 21 patients (GBS [+] group), and was not attempted in the SEMS placement session for 128 patients (GBS [−] group) (Fig. 3). The patients’ characteristics are described in Table 1.
Treatment outcomes are summarized in Table 2. The technical success rate was 70.0% (21/30). The total procedure time was significantly longer for the attempted GBS placement group. GBS placement was unsuccessful because the CD could not be visualized in two cases, and the guidewire could not be inserted into the gallbladder through the CD in seven cases. Intervention-related adverse events were not significantly different between the two groups. All adverse events improved with conservative treatment.
Details of cholecystography findings for the attempted GBS placement group are shown in Table 3. The detection rate of the CD in cholecystography was significantly higher for the successful cases. The mean diameter of the CD tended to be larger in the successful cases than that in the unsuccessful cases.
AC was observed in no cases with successful GBS placement (GBS [+] group) during the median follow-up period of 357 days (Table 4). There was no dislocation of the GBS during the follow-up. On the other hand, two patients developed AC after failed GBS placement. They underwent additional procedures (percutaneous transhepatic gallbladder aspiration and endoscopic ultrasound-guided gallbladder drainage [EUS-GBD]). Among the 128 patients for whom prophylactic GBS placement was not attempted during the study period (GBS [-] group), 25 patients (19.5%) developed AC and required additional treatment. The median interval from SEMS insertion to the incidence of AC was 18 days. There was a statistically significant difference in the incidence of AC between the GBS (+) and GBS (−) groups (p=0.043). There was no difference in the time to RBO and OS between the two groups (370 days vs. 291 days, p=0.898; 357 days vs. 309 days, p=0.979) (Table 4, Fig. 4).
The results of univariate and multivariate analyses for risk factors for AC after SEMS placement are provided in Table 5. Multivariate analysis revealed that prophylactic GBS placement was an independent preventive factor for AC (hazard ratio [HR], 0.61; 95% confidence interval, 0.37−0.99; p=0.045). Although PEP frequently occurred for the attempted GBS placement group, multivariate analysis showed that the attempt to prophylactically place a GBS was not associated with an increased risk of PEP (odds ratio, 2.18; 95% confidence interval, 0.76−6.26; p=0.146) (Table 6).
Endoscopic GBS placement is a valuable alternative to emergency cholecystectomy and percutaneous transhepatic gallbladder drainage (PTGBD) for AC in patients at a high risk. However, it is technically challenging, as shown in a recent meta-analysis reporting a technical success rate of 83%.11,12 In a retrospective study with a setting similar to ours, the technical success rate of prophylactic GBS placement was reported to be 58% to 75%, indicating the technical difficulty of prophylactic GBS placement for malignant biliary strictures.6,7 Several possible factors can make this technique more difficult. First, the CD often cannot be visualized due to tumor involvement. Second, the maneuverability of a guidewire is highly restricted due to interference from the biliary stricture. Appropriate devices and improved techniques are needed to improve the success rate of prophylactic GBS placement.
In the present study, the rate of CD detection in cholecystography was higher, and the mean diameter of the CD was larger for successful GBS placement cases. Likewise, another study has reported that a large CD diameter is associated with technical success.10 This procedure is well suited for patients with a large CD.
In our study, a GBS could be deployed for all patients with successful guidewire insertion, similar to Nakahara’s study wherein the technical success rate of 7-Fr GBS placement was reported to be 100% after successful insertion of a guidewire and tapered catheter.13 Guidewire insertion appears essential for successful GBS placement. The mean time for GBS placement in successful cases was only 16 min, indicating that the intervention is relatively easy once the guidewire is inserted. The thinness of the 5-Fr IYO stent used in this study could be advantageous for GBS placement. In addition, the thinness might help the SEMS to function. There were no statistical differences in the times to RBO and OS between groups with and without a GBS. Moreover, GBS dislocation was not observed in the study period. Furthermore, the GBS did not interfere with re-intervention for the biliary SEMS. If the functions of the 5 and 7 Fr GBS are similar, then the former, which may have less impact on the biliary SEMS afterward, might be more desirable.
The rate of adverse events in prophylactic GBS placement was 23%, which was slightly higher than that reported in previous studies.7,11,12 In this study, PEP was the most frequent adverse event in the attempted GBS placement group. Prolongation of the total procedure time due to attempted GBS placement might be related to an increased rate of PEP. However, from multivariate analyses, prophylactic GBS placement was not a risk factor for PEP. Perforation of the CD has been reported to be a significant adverse event in endoscopic GBS placement (2.9%–15.8%).6,11,12,14 Fortunately, in this study, one patient for whom the contrast leaked outside the bile duct due to guidewire perforation did not develop clinically significant events. A cholecystography image could not be obtained due to tumor invasion. Since the success rate of GBS placement was significantly lower in the case without cholecystography images, one should not search for a CD in such a case.
No patients developed AC during the follow-up periods in the GBS (+) group, whereas 19.5% of the GBS (–) group suffered from AC, requiring additional treatments, including surgery, PTGBD, and EUS-GBD (p=0.043). The reason for the absence of AC in the GBS (+) group could be due to a few factors. Regarding the GBS patency, multiple side holes spirally opening at 5-mm intervals might be related to the long patency. In addition, the thinness of the 5-Fr GB stent might be associated because it creates gaps between the stent and CD wall as well as the stent and SEMS. Bile flow would be maintained through these gaps even after the stent lumen was obstructed.
Prophylactic GBS placement was found to be a preventative factor for AC after SEMS placement (HR, 0.61; 95% confidence interval, 0.37–0.99; p=0.045). Likewise, in a retrospective study by Ishii et al.,7 the cumulative incidence of AC was reported to be significantly lower for patients with a prophylactic GBS than in those without one (4% vs. 21%, p=0.045). Other single-arm trials have reported that AC does not occur when a GBS is successfully placed in biliary strictures.6,15 Therefore, prophylactic GBS placement helps prevent AC after SEMS placement. Since invasive or time-consuming treatment should be avoided for patients with unresectable malignancies, prophylactic GBS placement appears valuable.
In recent years, there have been several studies about palliation rather than the prevention of AC after SEMS placement. EUS-GBD, an alternative to traditional percutaneous techniques, has favorable outcomes.16–19 Since the method has not been established yet, prevention of AC is desirable if possible. In addition, a transpapillary GBS placement after removing the previously covered SEMS or through a mesh hole of the previously uncovered SEMS has recently been reported as a palliation.20,21 Although it has a surprisingly high procedural success rate (83.3%, 10/12), this intervention is extremely challenging for average endoscopists. Preferable strategies, including prophylactic GBS placement, endoscopic GBS placement after AC occurrence, EUS-GBD, PTGBD, and surgery, must be established with research.
This study had several limitations derived from the single-center setting with a small population. First, regarding patient selection, there might have been selection bias. The tumor invasion of the CD, which is a known risk factor of AC after SEMS placement for malignant biliary strictures, was low (17%) for the attempted GBS group. In contrast, it was 33% for the not attempted GBS group. Although there was no significant difference in the characteristics of patients in this study, there was a selection bias due to the nature of retrospective studies as the GBS was placed at the operator’s discretion. In addition, there might have been an observer bias since a single gastroenterologist evaluated the image findings. Although our data suggested that prophylactic GBS placement helped prevent AC after SEMS placement for malignant biliary strictures, prospective comparative studies are needed to confirm its usefulness.
In conclusion, no AC developed after biliary SEMS placement when a prophylactic GBS was placed in the same session. Further accumulation of cases and prospective evaluations are necessary.
Notes
Author Contributions
Conceptualization: FK, YK; Data curation: FK, YK, SK, TO, HK, TS, KY, KM, HO, KH, HS; Formal analysis: FK; Investigation: FK, YK, SK, TO, HK, TS, KY, KM, HO, YM, KH, HS; Methodology: FK, YK; Project administration: FK, YK; Supervision: YK, KI; Validation: YK, KI; Writing–original draft: FK, YK; Writing–review & editing: all authors.
REFERENCES
1. Isayama H, Yasuda I, Ryozawa S, et al. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): covered Wallstent versus double-layer stent. Dig Endosc. 2011; 23:310–315.
2. Almadi MA, Barkun A, Martel M. Plastic vs. self-expandable metal stents for palliation in malignant biliary obstruction: a series of meta-analyses. Am J Gastroenterol. 2017; 112:260–273.
3. Isayama H, Kawabe T, Nakai Y, et al. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2006; 4:1148–1153.
4. Suk KT, Kim HS, Kim JW, et al. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest Endosc. 2006; 64:522–529.
5. Sogabe Y, Kodama Y, Honjo H, et al. Tumor invasion to the arteries feeding the gallbladder as a novel risk factor for cholecystitis after metallic stent placement in distal malignant biliary obstruction. Dig Endosc. 2018; 30:380–387.
6. Gosain S, Bonatti H, Smith L, et al. Gallbladder stent placement for prevention of cholecystitis in patients receiving covered metal stent for malignant obstructive jaundice: a feasibility study. Dig Dis Sci. 2010; 55:2406–2411.
7. Ishii T, Kin T, Yamazaki H, et al. Prophylactic endoscopic gallbladder stent placement for cholecystitis after covered metal stent placement for distal biliary obstruction (with video). Gastrointest Endosc. 2023; 98:36–42.
8. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991; 37:383–393.
9. Yokoe M, Hata J, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:41–54.
10. Maruta A, Iwata K, Iwashita T, et al. Factors affecting technical success of endoscopic transpapillary gallbladder drainage for acute cholecystitis. J Hepatobiliary Pancreat Sci. 2020; 27:429–436.
11. Khan MA, Atiq O, Kubiliun N, et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017; 85:76–87.
12. Mohan BP, Khan SR, Trakroo S, et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020; 52:96–106.
13. Nakahara K, Michikawa Y, Morita R, et al. Endoscopic transpapillary gallbladder stenting using a newly designed plastic stent for acute cholecystitis. Endosc Int Open. 2019; 7:E1105–E1114.
14. Nakahara K, Sato J, Morita R, et al. Incidence and management of cystic duct perforation during endoscopic transpapillary gallbladder drainage for acute cholecystitis. Dig Endosc. 2022; 34:207–214.
15. Wong M, Sánchez-Luna SA, Rustagi T. Endoscopic transpapillary gallbladder stenting to prevent acute cholecystitis in patients receiving FCEMS for benign biliary stricture. Endosc Int Open. 2021; 9:E1386–E1390.
16. Kozakai F, Kanno Y, Ito K, et al. Endoscopic ultrasonography-guided gallbladder drainage as a treatment option for acute cholecystitis after metal stent placement in malignant biliary strictures. Clin Endosc. 2019; 52:262–268.
17. Kanno Y, Kozakai F, Koshita S, et al. Technical issues stemming from endoscopic-ultrasound-guided gallbladder drainage: a single center experience. Turk J Gastroenterol. 2019; 30:1055–1061.
18. Podboy A, Yuan J, Stave CD, et al. Comparison of EUS-guided endoscopic transpapillary and percutaneous gallbladder drainage for acute cholecystitis: a systematic review with network meta-analysis. Gastrointest Endosc. 2021; 93:797–804.
19. McCarty TR, Hathorn KE, Bazarbashi AN, et al. Endoscopic gallbladder drainage for symptomatic gallbladder disease: a cumulative systematic review meta-analysis. Surg Endosc. 2021; 35:4964–4985.
20. Nakahara K, Michikawa Y, Morita R, et al. Endoscopic transpapillary gallbladder stent placement in the presence of uncovered biliary metal stents using a through-the-mesh technique. VideoGIE. 2020; 5:296–299.
21. Nakahara K, Morita R, Michikawa Y, et al. Endoscopic transpapillary gallbladder drainage for acute cholecystitis after biliary self-expandable metal stent placement. Surg Laparosc Endosc Percutan Tech. 2020; 30:416–423.
Fig. 1.
Plastic stent for the gallbladder, IYO stent (Gadelius Medical K.K.). The stent is 5 Fr with a 10-cm long straight section. The distal end is coiled in a multi-layered pigtail configuration, allowing it to be placed and secured in gallbladders of various shapes and sizes. In addition, side holes are spirally opened at 5-mm intervals, which are expected to prevent stent occlusion.
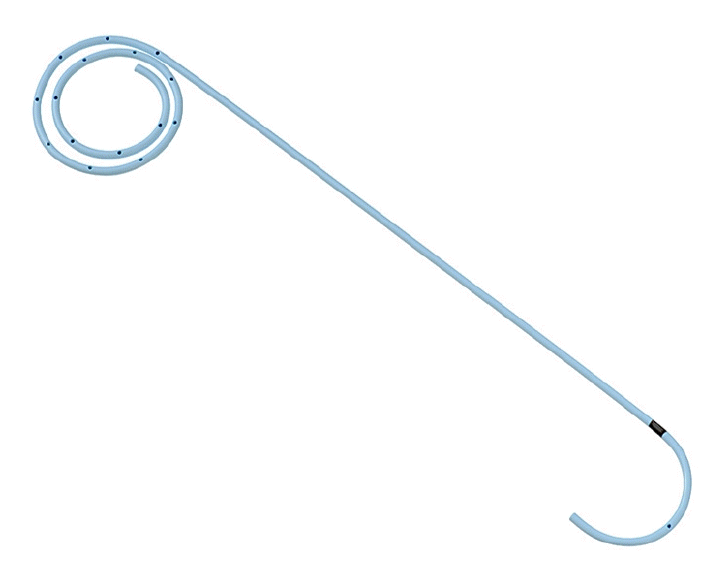
Fig. 2.
Gallbladder stent placement in combination with a biliary metallic stent for malignant biliary strictures. After confirming the presence of a biliary stricture and cystic duct by cholangiography (A), a guidewire was placed in the gallbladder (B), and the IYO stent was inserted along the wire (C). A covered self-expandable metallic stent was placed into the biliary stricture afterward (D, E).
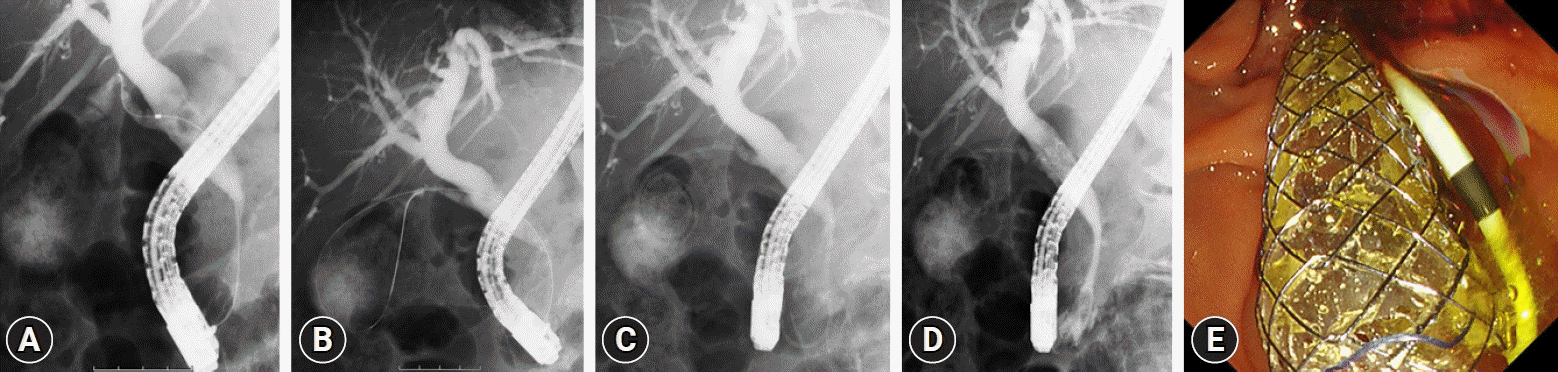
Fig. 3.
Flowchart of this study. SEMS, self-expandable metallic stent; CD, cystic duct; GBS, gallbladder stent.
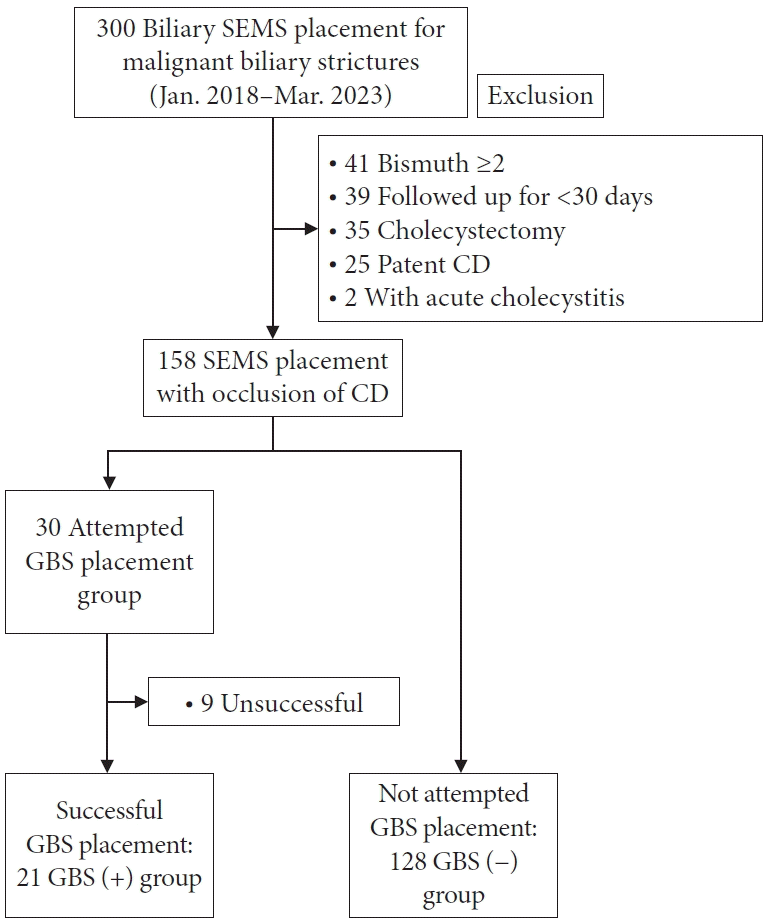
Fig. 4.
Kaplan-Meier analysis of the cumulative rate of recurrent obstruction (RBO) (A) and overall survival (OS) (B). There was no statistical difference in time to RBO and OS between the GBS (+) and GBS (–) groups (370 days vs. 291 days; p=0.898; 357 days vs. 309 days, p=0.979). GBS, gallbladder stent.
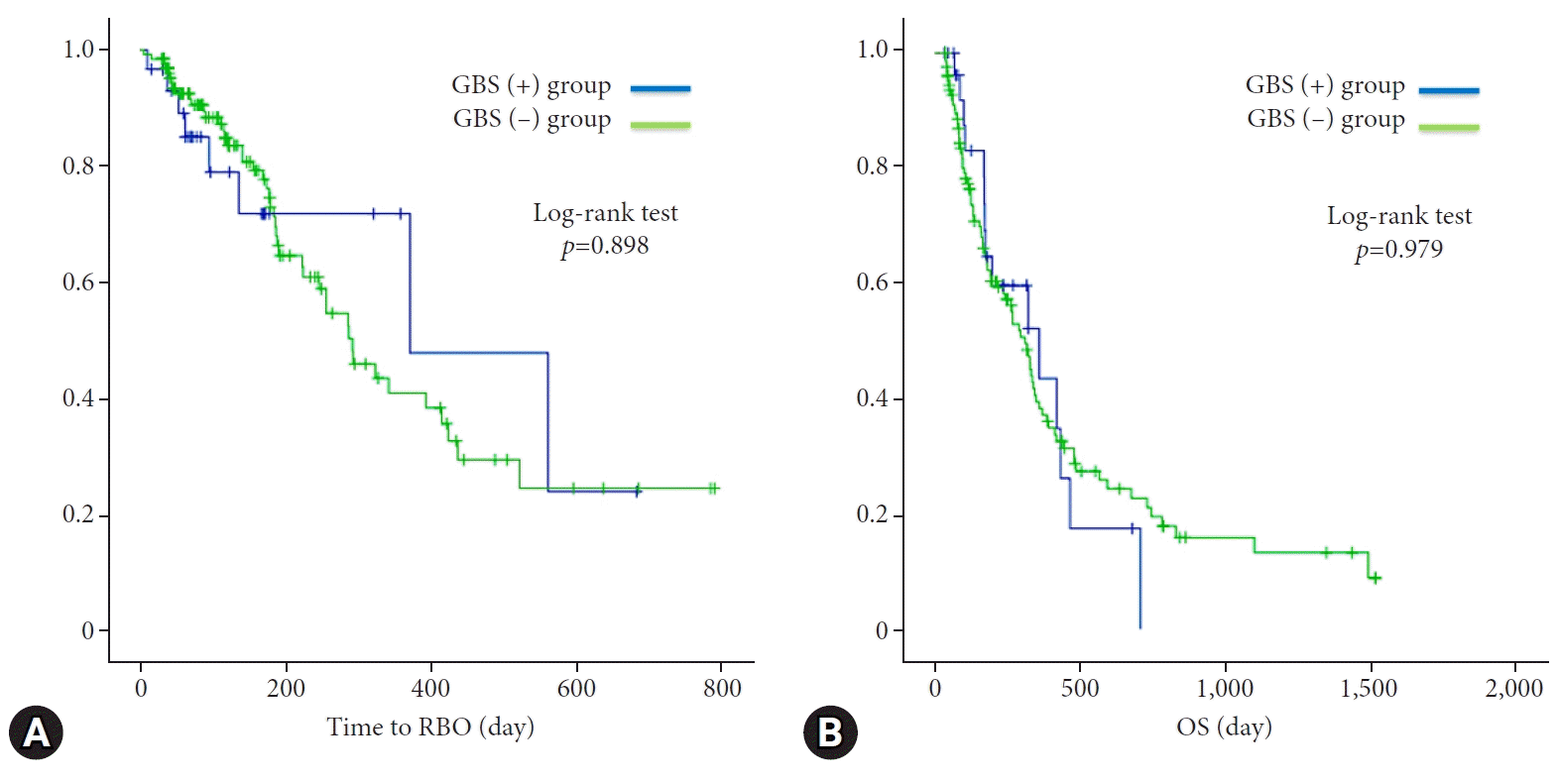
Table 1.
Characteristics of patients in this study
| Characteristic | All patient (n=158) | Attempted GBS placement group (n=30) | Not attempted GBS placement group (n=128) | p-value |
|---|---|---|---|---|
| Age (yr) | 77±10 | 78±9 | 77±10 | 0.616 |
| Sex (male/female) | 79/79 | 17/13 | 62/66 | 0.543 |
| Etiology | 0.836 | |||
| Pancreatic cancer | 101 (63.9) | 20 (66.7) | 81 (63.3) | |
| Bile duct cancer | 50 (31.6) | 8 (26.7) | 42 (32.8) | |
| Others | 7 (4.4) | 2 (6.7) | 5 (3.9) | |
| Unresectable cancer (including poor condition) | 137 (86.7) | 26 (86.7) | 111 (86.7) | 1.000 |
| Tumor invasion of the cystic duct | 47 (29.7) | 5 (16.7) | 42 (32.8) | 0.119 |
| Tumor invasion of an artery to gallbladder | 41 (25.9) | 7 (23.3) | 34 (26.6) | 0.820 |
| Gallbladder stone | 22 (13.9) | 4 (13.3) | 18 (14.1) | 0.850 |
| Enlarged gallbladder | 146 (92.4) | 30 (100.0) | 116 (90.6) | 0.125 |
| History of endoscopic biliary drainage | 49 (31.0) | 9 (30.0) | 40 (31.3) | 0.894 |
| Pancreatography | 60 (38.0) | 15 (50.0) | 45 (35.2) | 0.147 |
| Stent type (covered/uncovered) | 139/19 | 27/3 | 112/16 | 0.947 |
| Length of stent (>60 mm/≤60 mm) | 87/71 | 20/10 | 67/61 | 0.221 |
| Cholecystography findings | ||||
| Cholecystography detected (yes/no) | 89/69 | 26/4 | 63/65 | <0.001 |
| Diameter of cystic duct (mm)a) | 5.9±2.3 | 5.9±2.3 | 5.6±2.3 | 0.577 |
| Bifurcation direction (cranially directed/caudally directed)a) | 80/9 | 21/5 | 59/4 | 0.115 |
Table 2.
Treatment outcomes
Table 3.
Details of cholecystography findings at the time of prophylactic GBS placement
| Detail | Successful GBS placement (n=21) | Unsuccessful GBS placement (n=9) | p-value |
|---|---|---|---|
| Cholecystography findings | |||
| Cholecystography detected (yes/no) | 20/1 | 6/3 | 0.035 |
| Diameter of cystic duct (mm) | 6.5±2.2 | 3.7±1.3 | 0.007 |
| Bifurcation direction (cranially directed/caudally directed)a) | 17/3 | 4/2 | 0.318 |
| Time for GBS (min)a) | 16±8 | 19±20 | 0.090 |
Table 4.
Incidence of acute cholecystitis and time to recurrent biliary obstruction
Table 5.
Risk factors for acute cholecystitis after SEMS placement
Table 6.
Risk factors for post-ERCP pancreatitis after SEMS placement




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download