1. Baron TH, DiMaio CJ, Wang AY, et al. American Gastroenterological Association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020; 158:67–75.
2. van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010; 362:1491–1502.
3. van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018; 391:51–58.
4. Hollemans RA, Bakker OJ, Boermeester MA, et al. Superiority of step-up approach vs open necrosectomy in long-term follow-up of patients with necrotizing pancreatitis. Gastroenterology. 2019; 156:1016–1026.
5. ASGE Standards of Practice Committee, Muthusamy VR, Chandrasekhara V, et al. The role of endoscopy in the diagnosis and treatment of inflammatory pancreatic fluid collections. Gastrointest Endosc. 2016; 83:481–488.
6. Keane MG, Sze SF, Cieplik N, et al. Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: a 14-year experience from a tertiary hepatobiliary centre. Surg Endosc. 2016; 30:3730–3740.
7. Khan MA, Hammad T, Khan Z, et al. Endoscopic versus percutaneous management for symptomatic pancreatic fluid collections: a systematic review and meta-analysis. Endosc Int Open. 2018; 6:E474–E483.
8. Hookey LC, Debroux S, Delhaye M, et al. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006; 63:635–643.
9. Chandrasekhara V, Elhanafi S, Storm AC, et al. Predicting the need for step-up therapy after EUS-guided drainage of pancreatic fluid collections with lumen-apposing metal stents. Clin Gastroenterol Hepatol. 2021; 19:2192–2198.
10. Park DH, Lee SS, Moon SH, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009; 41:842–848.
11. Siddiqui AA, Adler DG, Nieto J, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc. 2016; 83:699–707.
13. Shah RJ, Shah JN, Waxman I, et al. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015; 13:747–752.
14. Rinninella E, Kunda R, Dollhopf M, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video). Gastrointest Endosc. 2015; 82:1039–1046.
15. Bang JY, Hasan M, Navaneethan U, et al. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut. 2017; 66:2054–2056.
16. Chandrasekhara V, Barthet M, Devière J, et al. Safety and efficacy of lumen-apposing metal stents versus plastic stents to treat walled-off pancreatic necrosis: systematic review and meta-analysis. Endosc Int Open. 2020; 8:E1639–E1653.
17. Goff D, Lloyd-Jones D; Risk Assessment Working Group. Assessing cardiovascular risk: systematic evidence review from the Risk Assessment Work Group. United States Department of Health and Human Services/National Institutes of Health/National Heart, Lung, and Blood Institute;2013. [cited 2023 Oct 1]. Available from:
https://www.nhlbi.nih.gov/health-topics/assessing-cardiovascular-risk.
18. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
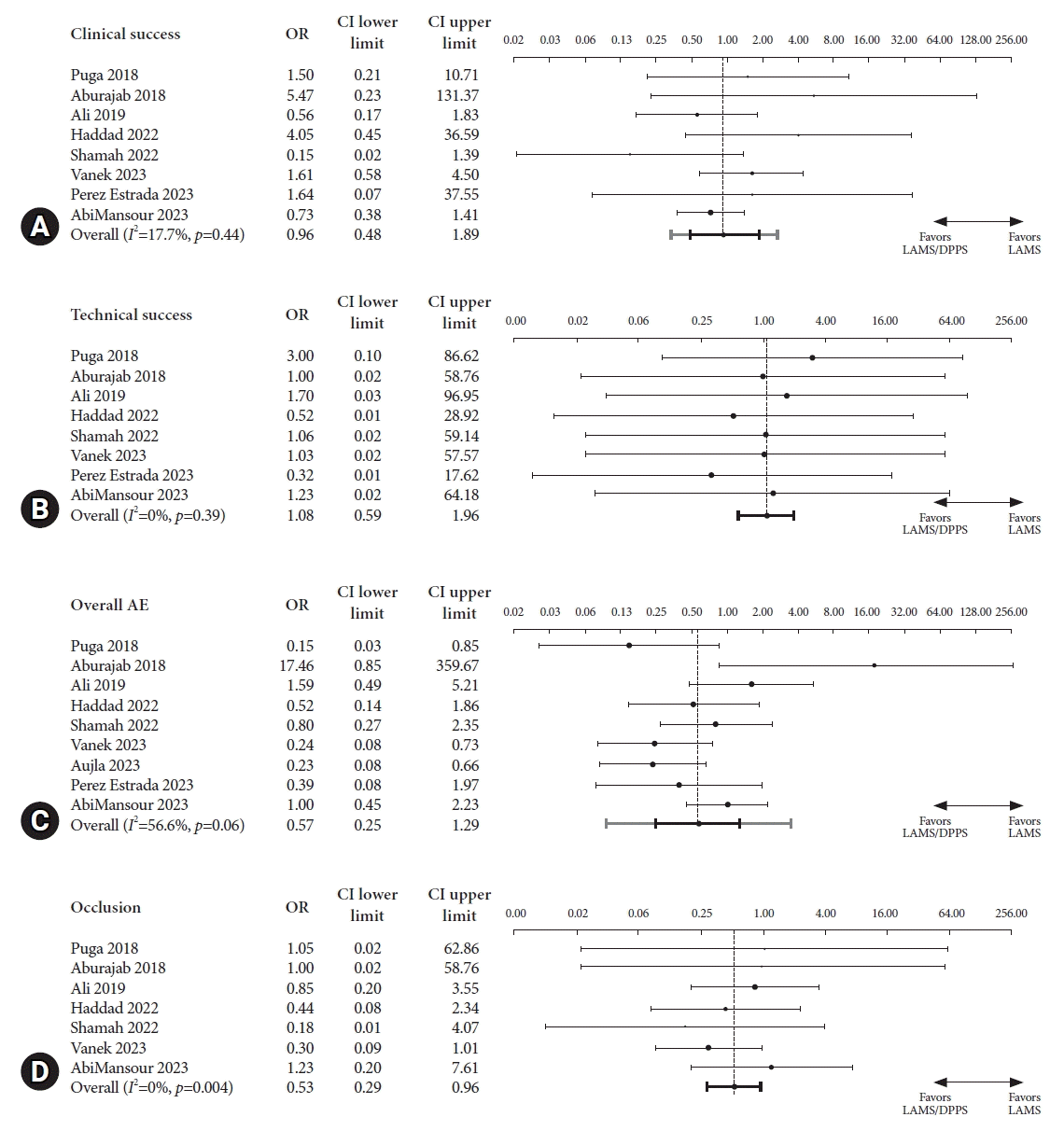
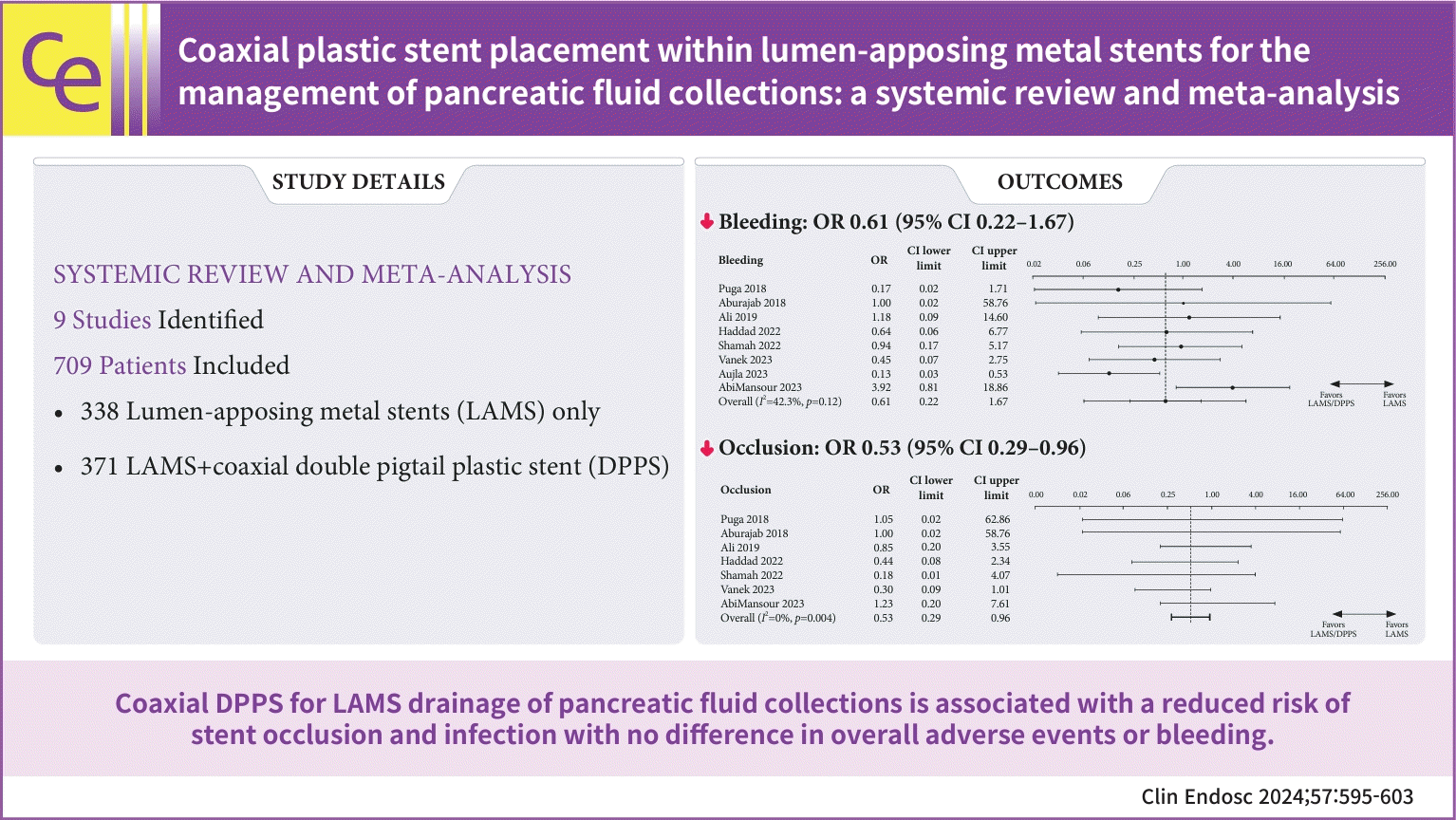
19. AbiMansour JP, Jaruvongvanich V, Velaga S, et al. Lumen-apposing metal stents with or without coaxial plastic stent placement for the management of pancreatic fluid collections. Gastrointest Endosc. 2024; 99:104–107.
20. Puga M, Consiglieri CF, Busquets J, et al. Safety of lumen-apposing stent with or without coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a retrospective study. Endoscopy. 2018; 50:1022–1026.
21. Aburajab M, Smith Z, Khan A, et al. Safety and efficacy of lumen-apposing metal stents with and without simultaneous double-pigtail plastic stents for draining pancreatic pseudocyst. Gastrointest Endosc. 2018; 87:1248–1255.
22. Ali SE, Benrajab K, Mardini H, et al. Anchoring lumen-apposing metal stent with coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: any benefit? Ann Gastroenterol. 2019; 32:620–625.
23. Haddad JD, Tielleman T, Fuller A, et al. Safety and efficacy of lumen-apposing metal stents with and without coaxial plastic stents for pancreatic fluid collections. Tech Innov Gastrointest Endosc. 2023; 25:113–118.
24. Shamah SP, Sahakian AB, Chapman CG, et al. Double pigtail stent placement as an adjunct to lumen-apposing metal stentsfor drainage of pancreatic fluid collections may not affect outcomes: a multicenter experience. Endosc Ultrasound. 2022; 11:53–58.
25. Vanek P, Falt P, Vitek P, et al. EUS-guided transluminal drainage using lumen-apposing metal stents with or without coaxial plastic stents for treatment of walled-off necrotizing pancreatitis: a prospective bicentric randomized controlled trial. Gastrointest Endosc. 2023; 97:1070–1080.
26. Aujla P, Aleem A, Subramanian SK, et al. Stent within a stent: when lumen apposing metal stent meets its match in coaxial double pigtail stent. Gastrointest Endosc. 2023; 97:AB920.
27. Perez Estrada C, Adalberto R. Efficacy and safety of lumen-apposing metal stents in endoscopic ultrasound-guided drainage of abdominal fluid collections and the influence of inserting a coaxial double pigtail plastic stent. United Eur Gastroenterol J. 2022; 10(Suppl 8):984–985.
28. Gluck M, Ross A, Irani S, et al. Dual modality drainage for symptomatic walled-off pancreatic necrosis reduces length of hospitalization, radiological procedures, and number of endoscopies compared to standard percutaneous drainage. J Gastrointest Surg. 2012; 16:248–257.
29. Varadarajulu S, Phadnis MA, Christein JD, et al. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011; 74:74–80.
30. Bakker OJ, van Santvoort HC, van Brunschot S, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012; 307:1053–1061.
31. Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013; 145:583–590.
32. Penn DE, Draganov PV, Wagh MS, et al. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 2012; 76:679–684.
33. Bang JY, Mel Wilcox C, Arnoletti JP, et al. Importance of disconnected pancreatic duct syndrome in recurrence of pancreatic fluid collections initially drained using lumen-apposing metal stents. Clin Gastroenterol Hepatol. 2021; 19:1275–1281.
34. van Dijk SM, Timmerhuis HC, Verdonk RC, et al. Treatment of disrupted and disconnected pancreatic duct in necrotizing pancreatitis: a systematic review and meta-analysis. Pancreatology. 2019; 19:905–915.
35. Wang L, Elhanafi S, Storm AC, et al. Impact of disconnected pancreatic duct syndrome on endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endoscopy. 2021; 53:603–610.
36. Giri S, Harindranath S, Afzalpurkar S, et al. Does a coaxial double pigtail stent reduce adverse events after lumen apposing metal stent placement for pancreatic fluid collections? A systematic review and meta-analysis. Ther Adv Gastrointest Endosc. 2023; 16:26317745231199364.
37. Lang GD, Fritz C, Bhat T, et al. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: comparison of efficacy and adverse event rates. Gastrointest Endosc. 2018; 87:150–157.
38. Dayyeh BK, Chandrasekhara V, Shah RJ, et al. Combined drainage and protocolized necrosectomy through a coaxial lumen-apposing metal stent for pancreatic walled-off necrosis: a prospective multicenter trial. Ann Surg. 2023; 277:e1072–e1080.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download