1. Lee TH, Choi JH, Park do H, et al. Similar efficacies of endoscopic ultrasound-guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2016; 14:1011–1019.
2. Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc. 2017; 85:904–914.
3. Artifon EL, Marson FP, Gaidhane M, et al. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: is there any difference? Gastrointest Endosc. 2015; 81:950–959.
4. Uemura RS, Khan MA, Otoch JP, et al. EUS-guided choledochoduodenostomy versus hepaticogastrostomy: a systematic review and meta-analysis. J Clin Gastroenterol. 2018; 52:123–130.
5. Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: a systematic review. Gastrointest Endosc. 2016; 83:1218–1227.
6. Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: a systematic review and meta-analysis. Dig Dis Sci. 2016; 61:684–703.
7. Moole H, Bechtold ML, Forcione D, et al. A meta-analysis and systematic review: success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine (Baltimore). 2017; 96:e5154.
8. Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012; 76:1133–1141.
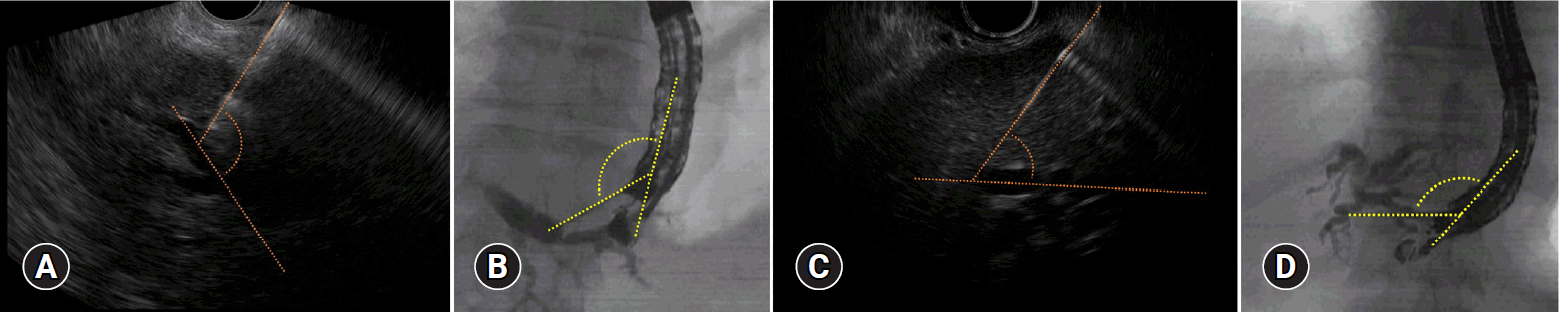
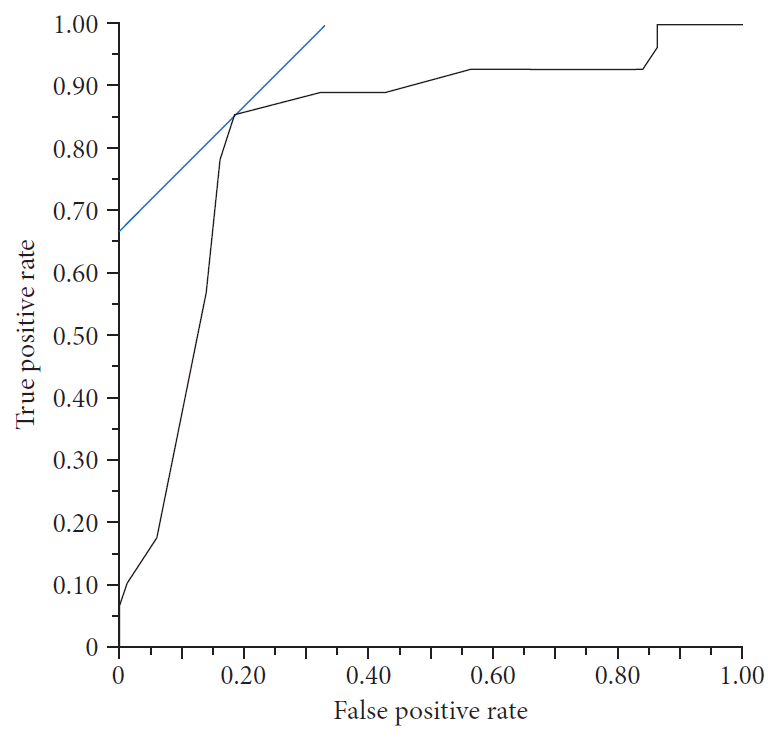
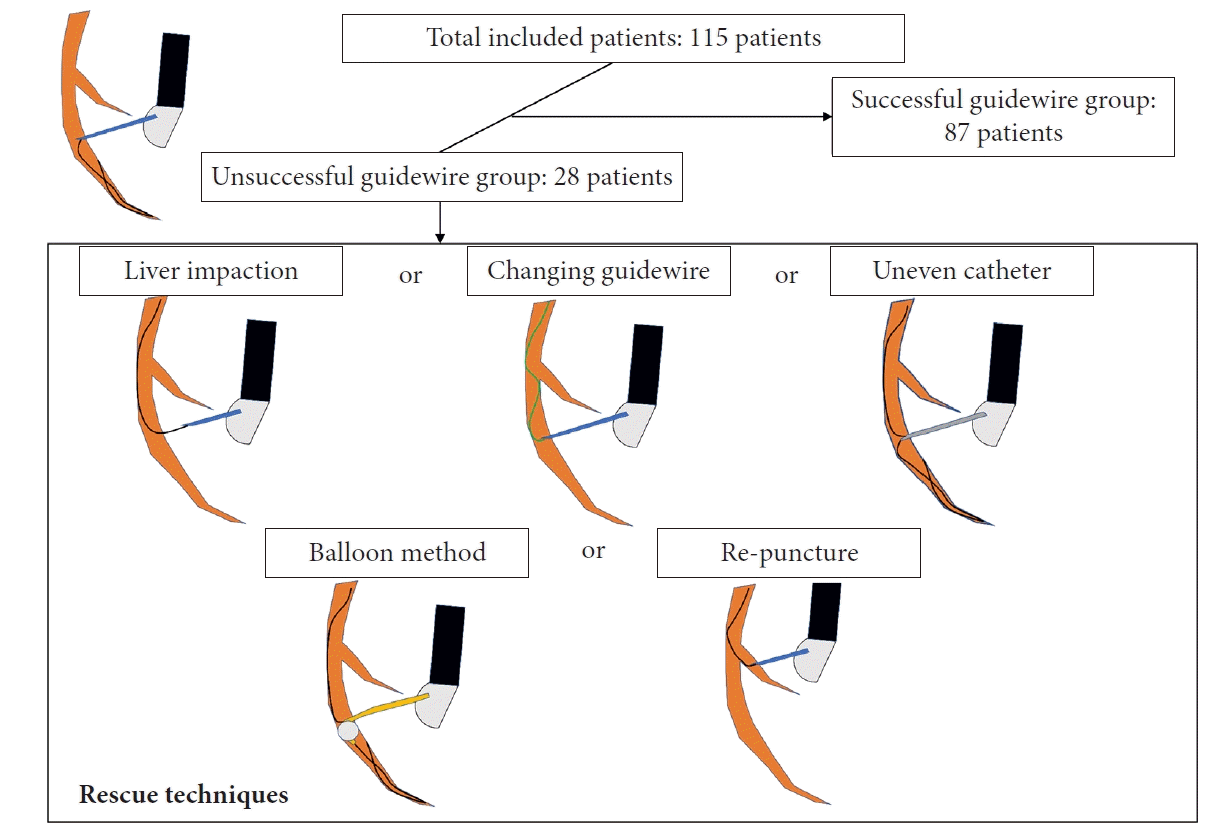
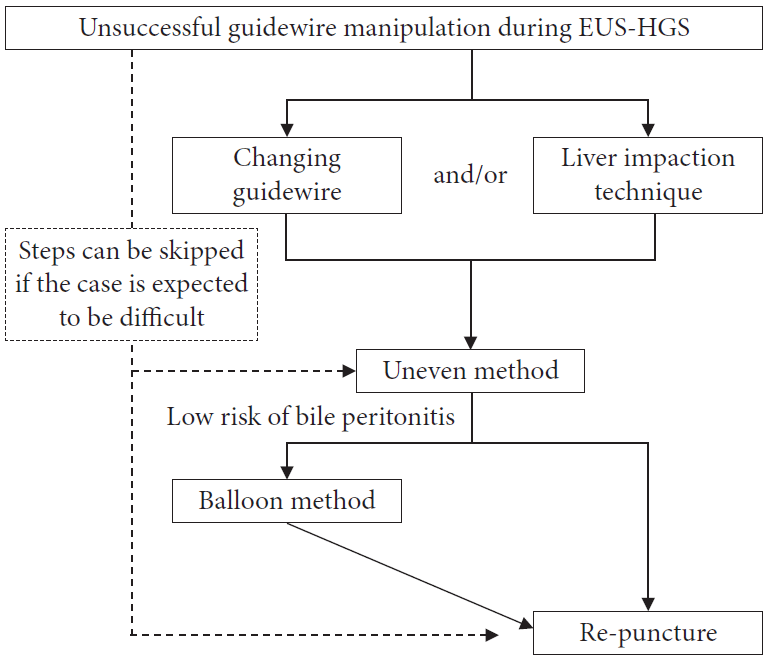
9. Ogura T, Nishioka N, Ueno S, et al. Effect of echoendoscope angle on success of guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2021; 53:369–375.
10. Ogura T, Masuda D, Takeuchi T, et al. Liver impaction technique to prevent shearing of the guidewire during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2015; 47(S 01):E583–E584.
11. Kawakami H, Kubota Y, Makiyama H, et al. Uneven double-lumen cannula for rescue guidewire technique in endoscopic ultrasonography-guided hepaticogastrostomy. Endoscopy. 2017; 49:E264–E265.
12. Ohno A, Kaku T, Fujimori N. Balloon guidewire technique during EUS-guided hepaticogastrostomy. Endosc Ultrasound. 2022; 11:330–331.
13. Ohno A, Fujimori N, Kaku T, et al. Feasibility and efficacy of endoscopic ultrasound-guided hepaticogastrostomy without dilation: a propensity score matching analysis. Dig Dis Sci. 2022; 67:5676–5684.
14. Ogura T, Okuda A, Ueno S, et al. Prospective comparison study between 19-gauge needle with .025-inch guidewire and 22-gauge needle with novel .018-inch guidewire during EUS-guided transhepatic biliary drainage (with video). Gastrointest Endosc. 2022; 96:262–268.
15. Hara K, Okuno N, Haba S, et al. How to perform EUS-guided hepaticogastrostomy easier and safer. J Hepatobiliary Pancreat Sci. 2020; 27:563–564.
16. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
17. Isayama H, Hamada T, Yasuda I, et al. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc. 2015; 27:259–264.
18. Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017; 10:42–53.
19. Fugazza A, Colombo M, Spadaccini M, et al. Relief of jaundice in malignant biliary obstruction: when should we consider endoscopic ultrasonography-guided hepaticogastrostomy as an option? Hepatobiliary Pancreat Dis Int. 2022; 21:234–240.
20. Matsubara S, Nakagawa K, Suda K, et al. Practical tips for safe and successful endoscopic ultrasound-guided hepaticogastrostomy: a state-of-the-art technical review. J Clin Med. 2022; 11:1591.
21. Ueno S, Ogura T, Higuchi K. Moving scope technique for guidewire insertion during endoscopic ultrasound-guided hepaticogastrostomy. Dig Endosc. 2021; 33:e109–e110.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download