Abstract
Malignant gastric outlet obstruction (GOO) is a condition characterized by blockage or narrowing where the stomach empties its contents into the small intestine due to primary malignant tumors or metastatic diseases. This condition leads to various symptoms such as nausea, vomiting, abdominal pain, and weight loss. To manage malignant GOO, different treatment options have been employed, including surgical gastrojejunostomy (SGJ), gastroduodenal stenting (GDS) using self-expandable metallic stent (SEMS), and endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ). This review focuses on comparing the clinical outcomes of endoscopic stenting (GDS and EUS-GJ) with SGJ for malignant GOO. Studies have shown that GDS with SEMS provides comparable clinical outcomes and safety for the palliation of obstructive symptoms. The choice between covered and uncovered SEMS remains controversial, as different studies have reported varying results. EUS-GJ, performed via endoscopic ultrasound guidance, has shown promising efficacy and safety in managing malignant GOO, but further studies are needed to establish it as the primary treatment option. Comparative analyses suggest that GDS has higher recurrence and reintervention rates compared to EUS-GJ and SGJ, with similar overall procedural complications. However, bleeding rates were lower with GDS than with SGJ. Randomized controlled trials are required to determine the optimal treatment approach for malignant GOO.
Malignant gastric outlet obstruction (GOO) refers to a condition in which there is a blockage or narrowing at the point where the stomach empties its contents into the small intestine by primary malignant tumors or metastatic diseases. Symptoms caused by malignant GOO include nausea, vomiting, early satiety, abdominal pain or discomfort, loss of appetite, and weight loss. Dehydration, electrolyte imbalances, malnutrition, or gastroparesis can also be complicated.1,2 To resolve and manage clinical problems complicated by malignant GOO, several treatment options have been applied clinically. Surgical gastrojejunostomy (SGJ) was initially applied for the resolution of malignant GOO. Then, gastroduodenal stenting (GDS) with self-expandable metallic stents (SEMS) was popular as an alternative option for malignant GOO. Recently, endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ) was introduced with the rapid development of interventional endoscopic ultrasound.3-5 Herein, we reviewed clinical outcomes of endoscopic stenting (ES) such as GDS and EUS-GJ and compared them with SGJ for the treatment of malignant GOO.
The treatment modalities for the resolution of symptomatic malignant GOO are shown in Figure 1. Palliative approaches for malignant GOO are divided into ES and SGJ. ES includes GDS and endoscopic gastrojejunostomy. GDS is the conventional endoscopic placement of SEMS at the obstruction site in malignant GOO. Endoscopic gastrojejunostomy has been performed in the following three ways: endoscopic ultrasound (EUS-GJ), forward-viewing endoscopy (endoscopic magnetic gastrojejunostomy), and the natural orifice transluminal endoscopic surgery (NOTES) approach. In the following sections, we will focus on GDS and SGJ, which are currently being actively implemented clinically; and EUS-GJ, which has been started relatively recently but is being tried by several groups.
The SGJ, which involved creating a connection between the stomach and the jejunum, had been selected for a conventional treatment of malignant GOO. However, this procedure is invasive and carries a higher risk of complications.6-10 Gastroduodenal stent placement using a SEMS (Fig. 2) has shown comparable clinical outcomes and safety for palliation of obstructive symptoms complicated by malignant GOO.11-14 Initially, SEMS in the early developmental stages of the procedure were uncovered. The uncovered SEMS inevitably developed tumor ingrowth over time, which covered SEMS were developed to overcome. However, because the SEMS in the early developmental stages had high axial force with poor conformability, covered SEMS resulted in frequent stent migration.15 The covered stents used in esophageal obstruction are full-covered types, but covered stents for malignant GOO are mostly partially covered and uncovered at both ends to reduce migration. The clinical studies about the efficacy of SEMS on malignant GOO are summarized in Table 1.15-27 Previous studies reported stent malfunction due to stent migration in 0–8.3% of patients with malignant gastroduodenal obstruction with uncovered stents16,19,24 and 8.8% to 28% of patients with malignant GOO with covered stents.28-30 A meta-analysis of 61 articles analyzing the clinical results of GDS for malignant GOO published from January 2015 to February 2021 showed that technical and clinical successes were 99.4% (95% confidence interval [CI], 98.9%–99.8 %) and 88.9% (95% CI, 86.7%–90.9%). The recurrence rates were 28.7% (95% CI, 19.7%–38.6%), and the reintervention rate was 20.3% (95% CI, 16.9%–23.9%).6
According to the results of a meta-analysis, which analyzed studies on the clinical performance and safety of covered and uncovered SEMS in malignant GOO, technical and clinical success were not statistically different between the two SEMS (odds ratio [OR], 0.69; 95% CI, 0.21–2.3 and OR ,1.1; 95% CI, 0.76–1.61, respectively).31 Stent patency, defined as the time between stent deployment and stent dysfunction was higher in covered than in uncovered SEMS (hazard ratio, 0.68; 95% CI, 0.48–0.96). Covered SEMS were associated with higher stent migration (OR, 4.28; 95% CI, 2.79–6.57). Uncovered SEMS were associated with a higher rate of stent occlusion (OR, 0.34; 95% CI, 2.79–6.57). However, there were no differences in terms of overall adverse events, reintervention, and dysfunction rates. In addition, patient survival was similar in covered and uncovered stent groups (hazard ratio, 0.96; 95% CI, 0.75–1.23).31
Thus far, four randomized, controlled studies in which clinical efficacy and safety of endoscopic placement of covered vs. uncovered SEMS were sufficiently demonstrated, have been conducted worldwide, three in Korea and one in Japan.15-18 In terms of clinical efficacy, the two randomized trials showed similar results in stent malfunction caused by stent migration and restenosis in covered and uncovered SEMS.15,16 However, in the two other studies, the clinical outcomes of covered SEMS were better than those of uncovered SEMS.17,18
When looking at the results of these meta-analyses and comparative studies, it seems difficult to draw any conclusions about which type of stent should be chosen as a first option between covered and uncovered SEMS. However, we must consider the following several points. Firstly, it is highly likely that the performance of the stents used in previous studies was not the same. The manufacturing methods and stent designs are different in the various companies that produce SEMS and they could cause differences in the clinical performance of SEMS. Secondly, the causative diseases of malignant GOO were different in the prior studies. The patient cohorts of many studies consisted of various cancers, such as pancreatic, gastric, duodenal, bile duct, gallbladder, and metastatic cancers, and some conducted studies on patients with a single cancer type, such as gastric cancer.16,32,33 Thirdly, the cancer progression and the clinical disease severity during the follow-up period were different among studies. These factors could affect the clinical outcome and prognosis of SEMS placement in malignant GOO. In addition, whether chemotherapy is administered can also affect the clinical outcomes of SEMS placement. Several retrospective studies have shown that chemotherapy is associated with prolonged uncovered stent patency in patients with malignant pyloric obstruction.34-36 A long time-to-progression and first-line chemotherapy were substantial protective factors against re-stenosis.37 Chemotherapy has been associated with stent migration.15,35,36 In a study conducted by Lim et al.,15 the rate of stent migration in the covered SEMS group was higher in the patients who underwent chemotherapy than in those who did not undergo chemotherapy. However, in most studies, it is difficult to find statistically significant results analyzing the influence of chemotherapy on SEMS placed endoscopically.
Innovations in the design of SEMS should be made to improve clinical efficacy in malignant GOO. Two of the above-mentioned randomized, controlled trials indicated a triple-layered design for covered SEMS; clinical outcomes in the covered SEMS group were superior to the uncovered SEMS group. This warrants attention. It is speculated that this innovative design substantially influenced the decrease in the rate of stent migration. The superiority of triple-layered, covered SEMS must be validated in more clinical prospective studies.
Since endoscopic gastrojejunostomy has advantages of both SGJ and endoscopic metal stent placement, such as a short length of anastomosis and less invasiveness, endoscopic gastrojejunostomy may be an ideal treatment for malignant GOO. Endoscopic approaches can be divided into two: (1) using flexible forward-view endoscopy, and (2) using endoscopic ultrasound (Fig. 3).
Studies with animal models on endoscopic gastrojejunostomy began to be reported in the early 1990s and early 2000s.38-40 Later, endoscopic magnetic gastrojejunostomy41-43 and the NOTES approach44,45 were suggested. However, all of these were small-scale studies with insufficient progress to merit successful implementation in a clinical situation, for the following reasons. Firstly, flexible forward-view endoscopy is very difficult to perform with only a conventional endoscope. Secondly, in the case of the magnetic method, it takes approximately 10 days after magnet installation until a gastroenteric fistula is formed. Thirdly, the safety of the procedure has not been sufficiently proven. Therefore, the concept of endoscopic gastrojejunostomy has evolved into an approach using endoscopic ultrasound.
Endoscopic ultrasound-guided drainage therapy has been consistently published since the early 2000s.41 EUS-GJ is usually proceeded with the following steps. (1) The patient is placed under conscious sedation or general anesthesia to ensure comfort during the procedure. (2) An endoscope with an integrated ultrasound probe is inserted into the patient's mouth and guided down the esophagus into the stomach. (3) Under ultrasound guidance, a needle is advanced through the stomach wall and into the jejunum, creating a tract. (4) A guidewire is then threaded through the needle and into the jejunum. (5) Over the guidewire, a stent or a balloon catheter is placed to create a connection (anastomosis) between the stomach and the jejunum. (6) The stent is deployed, expanding and securing the connection. (7) The endoscope is withdrawn, and the procedure is completed.
Recent studies and case reports have shown comparable efficacy and safety of EUS-GJ in managing malignant GOO, contributing to its increasing adoption as a minimally invasive therapeutic option.3,46-55 There have also been studies on ES in cases of both malignant biliary obstruction and GOO. The extent of GOO can be divided into three types: (1) invasion of only the duodenal bulb; (2) invasion to the second part of the duodenum, to the main papilla; and (3) invasion of the third part of the duodenum, without the involvement of the papilla. According to a study analyzing the results of 11 studies examining the treatment results of endoscopic treatment in patients with both malignant biliary and GOO, stenting was performed on the GOO site in 10 studies, and EUS-GJ was used to treat malignant GOO in one study.56 Endoscopic ultrasound-guided biliary drainage (EUS-BD) therapies therapies were performed at the same time or within seven days. The clinical outcomes of EUS-BD were very good (a mean technical success of 96.4%; 95% CI, 92.2%–99.0%) and a mean clinical success of 85.0% (95% CI, 68.0%–96.3%). Clinical success of duodenal stenting and EUS-GJ for malignant GOO was 90% and 100%, respectively.56 Therefore, when malignant biliary obstruction and malignant GOO occur at the same time, GOO is not a problem at all even if it is solved by conventional ES or relatively recently introduced EUS-GJ. However, it is still too early to say that EUS-GJ can be applied as a primary treatment of choice for malignant GOO. A randomized trial comparing the method with conventional SEMS placement should be conducted to verify the efficacy and safety of EUS-GJ.
In a meta-analysis comparing the clinical results of GDS, EUS-GJ, and SGJ in the studies published between January 2015 and February 2021, the technical success was lowest in EUS-GJ (95.3% [95% CI, 89.3%–98.9%] in EUS-GJ, 99.4 % [95% CI, 98.9%–99.8%] in GDS, and 99.9% [95% CI, 99.5%–100%] in SGJ, p=0.0048).6 In addition, the recurrence and reintervention rates of GDS were higher than those of EUS-GJ and SGJ; the recurrence rates were 28.7% (95% CI, 19.7%–38.6%) in GDS, 4.0% (95% CI, 0%–15.0%) in EUS-GJ, and 16.9% (95% CI, 11.6%–23.0%) in SGJ, respectively (p=0.0036), and the reintervention rates were 20.3% (95% CI, 16.9%–23.9%) in GDS, 11.2% (95% CI, 4.9%–19.6%) in EUS-GJ, and 12.6% (95% CI, 6.6%–20.1%) in SGJ, respectively (p=0.041).
In terms of safety, overall procedural complications were similar (GDS, 18.7% vs. EUS-GE, 21.9% vs. surgical GJ, 23.8%; p=0.32). Although estimated bleeding rates were similar between GDS and EUS-GJ (1.7% [95% CI, 0.9%–2.7%] vs. 2.9% [95% CI, 0.2%–8.6%], p=0.999), the bleeding rate for GDS was lower than that for SGJ (5.2% [95% CI, 3.2%–7.5%], p=0.0033 for pairwise comparison).6
There have only been small reports on the comparison between SGJ and GDS.8,9,11,57-59 In a multicenter-based, randomized, controlled trial performed by Jeurnink et al.,11 SGJ was associated with better long-term outcomes. In the study, the majority of enrolled patients had pancreatic cancer and GDS was compared with bypass surgery. Approximately half of the 77 initially enrolled patients refused to participate in the randomization, which was described as a limitation of the study.
A multidisciplinary, team-based decision-making process including gastroenterologists, surgeons, and oncologists is important to select the most appropriate and personalized treatment option for each patient with malignant GOO. The team has to assess the individual patient's overall health status, tumor characteristics, and potential risks and benefits of each treatment option.
GDS is advantageous in several aspects. It is a minimally invasive procedure, avoiding the need for open surgery. It is associated with shorter procedure time, reduced hospital stay, and faster recovery compared to SGJ. SEMS can be easily removed or exchanged if needed. GDS carries a lower risk of complications compared to SGJ.60,61 GDS can preclude the potential risks associated with open surgery, such as general anesthesia, surgical wound infections, postoperative pain, and longer hospital stay.
However, GDS is not always feasible and is preferred to SGJ. If the sites of GOO show extensive or complex obstruction or an attempt to place a SEMS is unsuccessful, in these cases, SGJ may be preferred. Tumor-related factors should also be considered. If the tumor is bulky, invasive, or associated with a high risk of tumor ingrowth, SGJ could provide more durable relief of obstruction compared to ES. Finally, consideration of clinical factors such as patient preferences for SGJ, life expectancy, anticipated treatment course, and quality of life could inform the decision of SGJ over GDS.
According to a recent guideline released by the American Society for Gastrointestinal Endoscopy, GDS is preferentially recommended in patients who are poor surgical candidates with short life expectancy (<6 months) and want early resumption of oral diet and discharge from the facility.62 Conversely, SGJ is preferentially recommended in patients with a life expectancy of >6 months and a good performance status.62
More clinical data are required on the circumstances in which EUS-GJ is preferentially selected for patients with malignant GOO. If the procedure is successful, the rates of restenosis and reintervention are lower than those of GDS. However, there is still doubt about the feasibility and safety of the procedure. More randomized clinical trials are needed to validate the feasibility and safety of EUS-GJ.
Since the early 2000s, ES has shown excellent clinical outcomes and safety, affirming that it can replace surgical bypass. In terms of the difficulty of the endoscopic procedure, ES can be performed if the gastrointestinal endoscopist is familiar with the operation of conventional endoscopic devices, such that clinical implementation of the procedure has succeeded worldwide. More recently, EUS-GJ has shown remarkable clinical results, although only a few studies have been reported.63,64 However, there are still procedural difficulties to be solved and safety issues to determine. Ultimately, non-inferiority compared to conventional endoscopic stent treatment should be demonstrated in a randomized, prospective clinical study, such that the endoscopic ultrasound-based approach can be applied more often in clinical situations. Although ES has optimistic clinical outcomes, there are some potential future directions for ES. Innovation in stent designs has to be attempted to enhance clinical efficacy and reduce complications. Research on anti-migration mechanisms, drug elution to the surface of SEMS in techniques to prevent tumor ingrowth, and modifications of stent design to promote better luminal patency should be done. Advanced endoscopic technology may lead to the development of more minimally invasive approaches for ES. Robotic-assisted endoscopic platforms or endoscopic suturing devices may have a crucial role in improving clinical outcomes and widening the indications of endoscopy while reducing invasiveness. To prove the possibility of these innovative approaches, prospective and randomized clinical trials are warranted.
REFERENCES
1. Chekan EG, Clark L, Wu J, et al. Laparoscopic biliary and enteric bypass. Semin Surg Oncol. 1999; 16:313–320.
2. Mintziras I, Miligkos M, Wächter S, et al. Palliative surgical bypass is superior to palliative endoscopic stenting in patients with malignant gastric outlet obstruction: systematic review and meta-analysis. Surg Endosc. 2019; 33:3153–3164.
3. Itoi T, Ishii K, Ikeuchi N, et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016; 65:193–195.
4. Tyberg A, Perez-Miranda M, Sanchez-Ocaña R, et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016; 4:E276–E281.
5. Cheung SL, Teoh AY. Optimal management of gastric outlet obstruction in unresectable malignancies. Gut Liver. 2022; 16:190–197.
6. Krishnamoorthi R, Bomman S, Benias P, et al. Efficacy and safety of endoscopic duodenal stent versus endoscopic or surgical gastrojejunostomy to treat malignant gastric outlet obstruction: systematic review and meta-analysis. Endosc Int Open. 2022; 10:E874–E897.
7. Medina-Franco H, Abarca-Pérez L, España-Gómez N, et al. Morbidity-associated factors after gastrojejunostomy for malignant gastric outlet obstruction. Am Surg. 2007; 73:871–875.
8. Wong YT, Brams DM, Munson L, et al. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surg Endosc. 2002; 16:310–312.
9. Yim HB, Jacobson BC, Saltzman JR, et al. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc. 2001; 53:329–332.
10. Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc. 2005; 61:421–426.
11. Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010; 71:490–499.
12. Zheng B, Wang X, Ma B, et al. Endoscopic stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Dig Endosc. 2012; 24:71–78.
13. Jeurnink SM, van Eijck CH, Steyerberg EW, et al. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007; 7:18.
14. Kang HW, Kim SG. Upper gastrointestinal stent insertion in malignant and benign disorders. Clin Endosc. 2015; 48:187–193.
15. Lim SG, Kim JH, Lee KM, et al. Conformable covered versus uncovered self-expandable metallic stents for palliation of malignant gastroduodenal obstruction: a randomized prospective study. Dig Liver Dis. 2014; 46:603–608.
16. Kim CG, Choi IJ, Lee JY, et al. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc. 2010; 72:25–32.
17. Maetani I, Mizumoto Y, Shigoka H, et al. Placement of a triple-layered covered versus uncovered metallic stent for palliation of malignant gastric outlet obstruction: a multicenter randomized trial. Dig Endosc. 2014; 26:192–199.
18. Lee H, Min BH, Lee JH, et al. Covered metallic stents with an anti-migration design vs. uncovered stents for the palliation of malignant gastric outlet obstruction: a multicenter, randomized trial. Am J Gastroenterol. 2015; 110:1440–1449.
19. van Hooft J, Mutignani M, Repici A, et al. First data on the palliative treatment of patients with malignant gastric outlet obstruction using the WallFlex enteral stent: a retrospective multicenter study. Endoscopy. 2007; 39:434–439.
20. van Hooft JE, Uitdehaag MJ, Bruno MJ, et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009; 69:1059–1066.
21. Havemann MC, Adamsen S, Wøjdemann M. Malignant gastric outlet obstruction managed by endoscopic stenting: a prospective single-centre study. Scand J Gastroenterol. 2009; 44:248–251.
22. Kim YW, Choi CW, Kang DH, et al. A double-layered (comvi) self-expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig Dis Sci. 2011; 56:2030–2036.
23. Isayama H, Sasaki T, Nakai Y, et al. Management of malignant gastric outlet obstruction with a modified triple-layer covered metal stent. Gastrointest Endosc. 2012; 75:757–763.
24. Sasaki T, Isayama H, Maetani I, et al. Japanese multicenter estimation of WallFlex duodenal stent for unresectable malignant gastric outlet obstruction. Dig Endosc. 2013; 25:1–6.
25. van den Berg MW, Haijtink S, Fockens P, et al. First data on the evolution duodenal stent for palliation of malignant gastric outlet obstruction (DUOLUTION study): a prospective multicenter study. Endoscopy. 2013; 45:174–181.
26. Jung K, Ahn JY, Jung HY, et al. Outcomes of endoscopically inserted self-expandable metal stents in malignancy according to the type of stent and the site of obstruction. Surg Endosc. 2016; 30:4001–4010.
27. Hori Y, Naitoh I, Hayashi K, et al. Predictors of outcomes in patients undergoing covered and uncovered self-expandable metal stent placement for malignant gastric outlet obstruction: a multicenter study. Gastrointest Endosc. 2017; 85:340–348.
28. Lee KM, Choi SJ, Shin SJ, et al. Palliative treatment of malignant gastroduodenal obstruction with metallic stent: prospective comparison of covered and uncovered stents. Scand J Gastroenterol. 2009; 44:846–852.
29. Park KB, Do YS, Kang WK, et al. Malignant obstruction of gastric outlet and duodenum: palliation with flexible covered metallic stents. Radiology. 2001; 219:679–683.
30. Seo EH, Jung MK, Park MJ, et al. Covered expandable nitinol stents for malignant gastroduodenal obstructions. J Gastroenterol Hepatol. 2008; 23(7 Pt 1):1056–1062.
31. Tringali A, Costa D, Anderloni A, et al. Covered versus uncovered metal stents for malignant gastric outlet obstruction: a systematic review and meta-analysis. Gastrointest Endosc. 2020; 92:1153–1163.
32. Shi D, Ji F, Bao YS, et al. A multicenter randomized controlled trial of malignant gastric outlet obstruction: tailored partially covered stents (placed fluoroscopically) versus standard uncovered stents (placed endoscopically). Gastroenterol Res Pract. 2014; 2014:309797.
33. Park CI, Kim JH, Lee YC, et al. What is the ideal stent as initial intervention for malignant gastric outlet obstruction? Dig Liver Dis. 2013; 45:33–37.
34. Telford JJ, Carr-Locke DL, Baron TH, et al. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc. 2004; 60:916–920.
35. Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007; 66:256–264.
36. Cho YK, Kim SW, Hur WH, et al. Clinical outcomes of self-expandable metal stent and prognostic factors for stent patency in gastric outlet obstruction caused by gastric cancer. Dig Dis Sci. 2010; 55:668–674.
37. Kim CG, Park SR, Choi IJ, et al. Effect of chemotherapy on the outcome of self-expandable metallic stents in gastric cancer patients with malignant outlet obstruction. Endoscopy. 2012; 44:807–812.
38. Swain CP, Mills TN. Anastomosis at flexible endoscopy: an experimental study of compression button gastrojejunostomy. Gastrointest Endosc. 1991; 37:628–631.
39. Kantsevoy SV, Jagannath SB, Niiyama H, et al. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc. 2005; 62:287–292.
40. Bergström M, Ikeda K, Swain P, et al. Transgastric anastomosis by using flexible endoscopy in a porcine model (with video). Gastrointest Endosc. 2006; 63:307–312.
41. Chopita N, Vaillaverde A, Cope C, et al. Endoscopic gastroenteric anastomosis using magnets. Endoscopy. 2005; 37:313–317.
42. van Hooft JE, Vleggaar FP, Le Moine O, et al. Endoscopic magnetic gastroenteric anastomosis for palliation of malignant gastric outlet obstruction: a prospective multicenter study. Gastrointest Endosc. 2010; 72:530–535.
43. Ryou M, Cantillon-Murphy P, Azagury D, et al. Smart Self-Assembling MagnetS for ENdoscopy (SAMSEN) for transoral endoscopic creation of immediate gastrojejunostomy (with video). Gastrointest Endosc. 2011; 73:353–359.
44. Song TJ, Seo DW, Kim SH, et al. Endoscopic gastrojejunostomy with a natural orifice transluminal endoscopic surgery technique. World J Gastroenterol. 2013; 19:3447–3452.
45. Yi SW, Chung MJ, Jo JH, et al. Gastrojejunostomy by pure natural orifice transluminal endoscopic surgery using a newly designed anastomosing metal stent in a porcine model. Surg Endosc. 2014; 28:1439–1446.
46. Chen YI, Itoi T, Baron TH, et al. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017; 31:2946–2952.
47. Ge PS, Young JY, Dong W, et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019; 33:3404–3411.
48. Kerdsirichairat T, Irani S, Yang J, et al. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endosc Int Open. 2019; 7:E144–E150.
49. Kastelijn JB, Moons LM, Garcia-Alonso FJ, et al. Patency of endoscopic ultrasound-guided gastroenterostomy in the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2020; 8:E1194–E1201.
50. Xu G, Shen Y, Lv Y, et al. Safety and efficacy of endoscopic ultrasound-guided gastroenterostomy using double balloon occlusion methods: a clinical retrospective study in 36 patients with malignant gastric outlet obstruction. Endosc Int Open. 2020; 8:E1690–E1697.
51. Hindryckx P, Degroote H. Lumen-apposing metal stents for approved and off-label indications: a single-centre experience. Surg Endosc. 2021; 35:6013–6020.
52. Kouanda A, Binmoeller K, Hamerski C, et al. Endoscopic ultrasound-guided gastroenterostomy versus open surgical gastrojejunostomy: clinical outcomes and cost effectiveness analysis. Surg Endosc. 2021; 35:7058–7067.
53. Khashab MA, Bukhari M, Baron TH, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017; 5:E275–E281.
54. Perez-Miranda M, Tyberg A, Poletto D, et al. EUS-guided gastrojejunostomy versus laparoscopic gastrojejunostomy: an international collaborative study. J Clin Gastroenterol. 2017; 51:896–899.
55. Tonozuka R, Tsuchiya T, Mukai S, et al. Endoscopic ultrasonography-guided gastroenterostomy techniques for treatment of malignant gastric outlet obstruction. Clin Endosc. 2020; 53:510–518.
56. Rizzo GE, Carrozza L, Quintini D, et al. A systematic review of endoscopic treatments for concomitant malignant biliary obstruction and malignant gastric outlet obstruction and the outstanding role of endoscopic ultrasound-guided therapies. Cancers (Basel). 2023; 15:2585.
57. Fiori E, Lamazza A, Volpino P, et al. Palliative management of malignant antro-pyloric strictures: gastroenterostomy vs. endoscopic stenting: a randomized prospective trial. Anticancer Res. 2004; 24:269–271.
58. Maetani I, Akatsuka S, Ikeda M, et al. Self-expandable metallic stent placement for palliation in gastric outlet obstructions caused by gastric cancer: a comparison with surgical gastrojejunostomy. J Gastroenterol. 2005; 40:932–937.
59. Espinel J, Sanz O, Vivas S, et al. Malignant gastrointestinal obstruction: endoscopic stenting versus surgical palliation. Surg Endosc. 2006; 20:1083–1087.
60. Keymling M, Wagner HJ, Vakil N, et al. Relief of malignant duodenal obstruction by percutaneous insertion of a metal stent. Gastrointest Endosc. 1993; 39:439–441.
61. Mosler P, Mergener KD, Brandabur JJ, et al. Palliation of gastric outlet obstruction and proximal small bowel obstruction with self-expandable metal stents: a single center series. J Clin Gastroenterol. 2005; 39:124–128.
62. ASGE Standards of Practice Committee, Jue TL, Storm AC, et al. ASGE guideline on the role of endoscopy in the management of benign and malignant gastroduodenal obstruction. Gastrointest Endosc. 2021; 93:309–322.
63. Şentürk H, Köker İH, Koçhan K, et al. Endoscopic ultrasound-guided gastrojejunostomy with a direct technique without previous intestinal filling using a tubular fully covered self-expandable metallic stent. Clin Endosc. 2024; 57:209–216.
64. Vedantam S, Shah R, Bhalla S, et al. No difference in outcomes with 15 mm vs. 20 mm lumen-apposing metal stents for endoscopic ultrasound-guided gastroenterostomy for gastric outlet obstruction: a meta-analysis. Clin Endosc. 2023; 56:298–307.
Fig. 1.
The treatment modalities for palliation of malignant gastric outlet obstruction (GOO). NOTES, natural orifice transluminal endoscopic surgery.
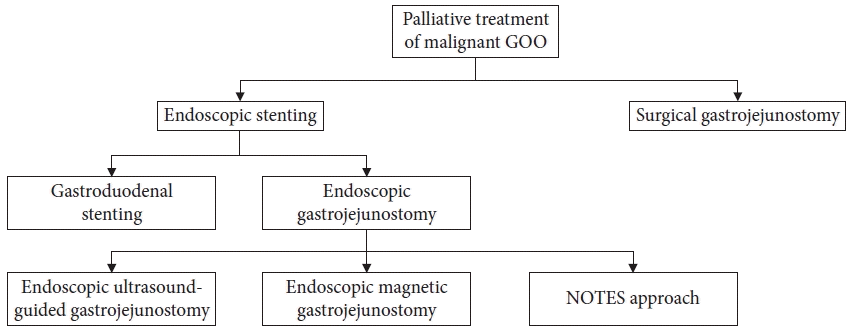
Fig. 2.
Photographs of self-expanding metallic stents. Covered (A), uncovered (B), and triple-layer, covered (C) self-expanding metallic stents.
Fig. 3.
An example of endoscopic ultrasound (EUS)-guided gastrojejunostomy in a patient with recurrent intraductal papillary neoplasm of the bile duct and long afferent loop stricture undergoing right anterior partial sectionectomy, S1 and radical bile duct resection, and subtotal gastrectomy with Billroth-II reconstruction. (A) A coronal image of the dilated afferent loop caused by mid afferent loop stricture (yellow arrow) on computed tomography scan. (B, C) A needle puncture (black arrowhead) on fluoroscopic and EUS view. (D) A guidewire insertion through the punctured needle into dilated afferent loop. (E) Stent (yellow arrowheads) deployment along the inserted guidewire. (F, G) Completed stent (yellow arrowheads) deployment on fluoroscopic and endoscopic view.
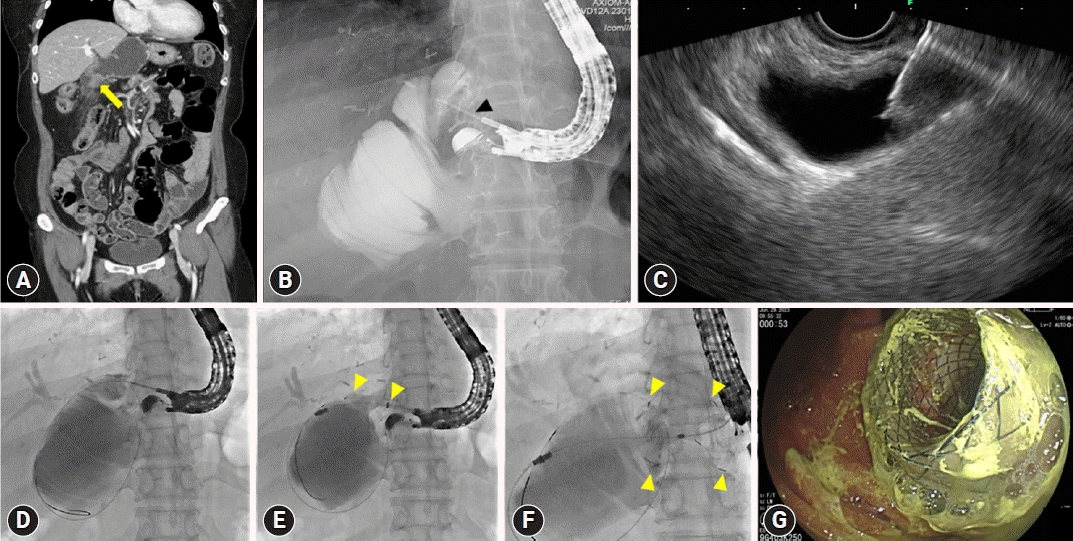
Table 1.
Characteristics of studies about clinical efficacy of self-expandable metal stents on malignant gastric outlet obstruction
| Study | Design/centers | Country | No. of patients finally analyzed | Type of malignancy | C-SEMS | U-SEMS | Technical success (%)a) | Clinical success (%)a) | 8-wk patency (%)a) | Stent patency (wk) | Stent migration during follow-up (%)a) | Restenosis during follow-up (ingrowth,overgrowth, stent compression) (%)a) | Reintervention during follow-up (%)a) | Major complications (%)b) | Follow-up (wk)a) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al. (2010)16 | RCT/single center | Korea | 80 | GC | Niti-S pyloric or Niti-S ComVi pyloric stent (double layered) | Enteral Wallstent or Enteral WallFlex | 100/100 | 95/90 | 61.3/61.1 | NA | 32.3/8.3 | 3.2/44.4 | NA | 2/0 | 14.5 (1–117)/14 (1–114) |
| Maetani et al. (2014)17 | RCT/multicenter | Japan | 62 | GC, PC, BDC | Niti-S ComVi stent (triple layered) | Niti-S stent | 100/100 | 87.1/93.5 | NA | NA | 6.5/3.3 | 0/19.4 | 3.3/19.4 | 3.2/3.2 | 10.4/13.3 |
| Lim et al. (2014)15 | RCT/multicenter | Korea | 120 | GC, PC, DC, AC, BDC, GBC | Niti-S ComVi pyloric stent (double layered) | Niti-S pyloric/duodenal D-type stent | 100/100 | 100/98.4 | NA | 13.6 (8–19)/13.1 (8.9–17.3) | 13.6/0 | 6.8/21.3 | 22.0/21.3 | 0/0 | 14.0 (8–26)/14.0 (7–22) |
| Lee et al. (2015)18 | RCT/multicenter | Korea | 102 | GC | WAVE SEMS with an anti-migration design (Bona stents) | U-SEMS (Bona stents) | 98.0/96.1 | 94.1/86.2 | 72.5/62.7 | NA | 9.5/5.4 | 7.1/37.8 | 23.5/39.2 | 0/0 | 21.3 (±10)/20.1 (±9.5) |
| van Hooft et al. (2007)19 | Retrospective/multicenter | Netherlands, Italy, Germany | 62 | PC, GC, MD, BDC, GBC, AC, DC | WallFlex duodenal stent | 97 | 85 | NA | NA | 1 | 1 | NA | 8 | 4.3 | |
| van Hooft et al. (2009)20 | Prospective/multicenter | Netherlands | 51 | PC, BDC, GBC, GC, DC, AC, MD | WallFlex duodenal stent | 98 | 84 | NA | 43.8 | 2 | 12 | 14 | 10 | 8.9 | |
| Havemann et al. (2009)21 | Prospective/single center | Denmark | 45 | PC, GC, GyC, UC, HC, MD | Hanaro stents | 91 | 63 | NA | NA | 7 | 14 | 14 | 4 | 17.3 (9–25.9) | |
| Kim et al. (2011)22 | Prospective/multicenter | Korea | 50 | GC, PC, BDC, GBC | Niti-S Comvi pyloric stent | 100 | 88 | NA | 13.1±8.0 | 10 | 18 | 28 | 0 | 15.7 (±9) | |
| Isayama et al. (2012)23 | Prospective/multicenter | Japan | 50 | PC, GC, BDC, MD | Niti-S M-ComVi stent (triple layered) | 100 | 90 | NA | 21.4±1.3 | 6 | 10 | NA | 4 | 20 (±2.8) | |
| Sasaki et al. (2013)24 | Retrospective/multicenter | Japan | 42 | PC, GC, BDC, DC | WallFlex duodenal stent | 100 | 83.3 | NA | 16.0 (3–18) | 0 | 23.8 | 26.2 | 2.4 | 3.3 (1.8–6) | |
| van den Berg et al. (2013)25 | Prospective/multicenter | Netherland | 46 | PC, BDC, GC, MD, DC, GBC | The Evolution enteral stents | 89 | 72 | NA | 9.6 | 4 | 26 | NA | 6 | 12.4 (5–33.9) | |
| Jung et al. (2016)26 | Retrospective/single center | Korea | 220 | PC, GC, BDC, GBC, DC, MD | Fully C-SEMSs, partially C-SEMSsc) | Variablec) | 96.8 | 86.4 | NA | NA | 37.5/9.1/5.8d) | 12.5/22.1/26.7d) | NA | 0/0.5/0d) | 17.7 (8–32) |
| Hori et al. (2017)27 | Retrospective/multicenter | Japan | 252 | PC, GC, BDC, GBC | Ultraflex stent, Niti-S ComVi stent | WallFlex duodenal stent, Niti-S pyloric stent | 99.2/100 | 82.5/88.1 | NA | 12.3/9 | 4.8/0.8 | 9.5/11.9 | NA | 7.9/3.2 | 12.4/9 |
Values are presented as median (range) or mean±standard deviation unless otherwise indicated.
C-SEMS, covered self-expanding metal stent; U-SEMS, uncovered self-expanding metal stent; RCT, randomized controlled trial; NA, not available; GC, gastric cancer; PC, pancreatic cancer; BDC, bile duct cancer; DC, duodenal cancer; AC, ampullary cancer; GBC, gallbladder cancer; MD, metastatic disease; GyC, gynecological cancer; UC, urological cancer; HC, hematological cancer.
b) Major complication is defined as adverse events caused by SEMS placement requiring additional interventions or hospitalization.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download