Abstract
Endoscopic ultrasound (EUS)-guided interventions have evolved rapidly in recent years, with dedicated metal stents playing a crucial role in this process. Specifically, the invention of biflanged short metal-covered stents, including lumen-apposing metal stents (LAMS), and modifications in a variety of tubular self-expandable metal stents (SEMS), have led to innovations in EUS-guided interventions. LAMS or non-LAMS stents are commonly used in the EUS-guided drainage of pancreatic fluid collections, especially in cases of walled-off necrosis. Additionally, LAMS is commonly considered for drainage of the EUS-guided gallbladder or dilated common bile duct and EUS-guided gastroenterostomy. Fully or partially covered tubular SEMS with several new designs are being considered for EUS-guided biliary drainage. This review focuses on advances in SEMS for EUS-guided interventions and discusses related research results.
Endoscopic ultrasound (EUS)-guided interventions have rapidly evolved in recent years for the management of various pancreatobiliary diseases, with dedicated stents playing a crucial role in this process. In particular, lumen-apposing metal stents (LAMS) have revolutionized EUS-guided interventions. More recently, with the development of electrocautery-enhanced (EC)-LAMS, the procedure has become safer and more efficient with a shorter procedure time. Novel dedicated tubular self-expandable metal stents (SEMS), either fully or partially covered with innovative antimigratory designs, are being considered for EUS-guided biliary drainage (EUS-BD) procedures. This review focuses on advances in SEMS for EUS-guided interventions and discusses related research results.
LAMS is a short, fully covered dumbbell-shaped metal stent with a large diameter and antimigratory properties and was first reported by Binmoeller and Shah1 in 2011 for transluminal drainage. LAMS offers several advantages over traditionally used double-pigtail plastic stents for EUS-guided transmural drainage (EUS-TD) of pancreatic fluid collections, including a large diameter that provides improved drainage of thick or viscous contents, a wide lumen as a conduit for subsequent endoscopic interventions in the target structure, and reduced risk of migration or leakage due to their apposing property.2 Conventionally delivered LAMS also have these advantages; however, they require multiple steps for avoiding adverse events and failure of accurate deployment.3 To overcome these limitations, the novel EC-LAMS has been developed for EUS-TD that allows the endoscopist alone to deploy the SEMS in quick sequential steps. Currently, there are two popularly used types of EC-LAMS in EUS-TD: the Hot AXIOS (Boston Scientific Corporation) and the Niti-S Hot SPAXUS (Taewoong Medical), both of which were modified from an earlier version of the non-cautery-based LAMS (Fig. 1).1,4-7 The EC system eliminates the need for additional accessories such as EUS puncture needles, guidewires, or dilator devices, thereby reducing procedural complexity and the risk of adverse events.5,7 LAMS are commonly used for draining intra-abdominal fluid collections (especially peri-pancreatic fluid collections [PFC]), decompressing obstructed common bile duct (CBD), establishing gastrointestinal luminal anastomosis, and creating fistulous tracts between organs for potential future endoscopic interventions.2 In contrast to the two aforementioned LAMS, the biflanged SEMS with reduced lumen apposing capability including–NAGI stent (Taewoong Medical), Aixstent (Leufen Medical), and Hanarostent Plumber (M.I Tech.) are being used mainly for the drainage of PFC (Table 1). Therefore, the choice of LAMS type can be determined based on the clinical situation and local availability.
PFC can develop as a local complication of severe acute pancreatitis, progressing to pseudocyst or walled-off necrosis (WON) after a variable period. The PFC is the most common indication for EUS-guided transmural drainage.8 Traditionally, double double-pigtail plastic stents have been mainly used for the transmural drainage of pancreatic pseudocysts or WON. However, currently available dedicated short biflanged SEMS, with or without lumen-apposing capability, which effectively drain owing to their wider diameter and reduce the risk of stent occlusion, are increasingly being used (Fig. 2). Wide flares at both ends prevent stent migration.
In a recent randomized controlled trial, the duration of the drainage procedure was the only notable difference between LAMS and plastic stents in the treatment of WON, as treatment outcomes showed no significant difference.9 The authors suggested that patients should undergo follow-up imaging and have the stent removed after 3 weeks if the WON has resolved. This approach helps reduce the adverse events associated with LAMS.9 In contrast, a recent meta-analysis suggests that SEMS are more effective in resolving WON, with fewer instances of bleeding, a slight tendency towards lower occlusion and perforation rates, but a higher migration rate compared to plastic stents.10 However, in a more recent randomized trial, LAMS were not found to be superior to double-pigtail plastic stents for the treatment of a large WON (>15 cm).11 The result of this study may have been influenced by the protocol applied to the plastic stents group, which involved weekly dilatation to 20 mm. The insertion of coaxial short double-pigtail plastic stents through LAMS can reduce the rate of adverse events and stent occlusion in EUS-guided drainage of WON.12 The European Society of Gastrointestinal Endoscopy recommends that LAMS should be retrieved within 4 weeks to prevent stent-related adverse effects.13
A recent multicenter study demonstrated that a 15-mm-long EC-LAMS was feasible and safe for the drainage of the PFC located 10 to 14 mm from the luminal wall. In the present study, the technical and clinical success rates were high (97%). Only one patient experienced recurrence during a median follow-up of 123 days, with minimal complications.14
EUS-BD is performed frequently when endoscopic retrograde cholangiopancreatography (ERCP) fails or is infeasible. Depending on the access routes and clinical practice methods, EUS-BD procedures include EUS-guided antegrade stenting (EUS-AGS), choledochoduodenostomy (EUS-CDS), and hepaticogastrostomy (EUS-HGS).
EUS-AGS involves the placement of a stent through the stricture or papilla to the duodenum using an intrahepatic duct access. The benefit of EUS-AGS is the re-establishment of the physiological drainage of bile into the duodenum; however, there is a risk of pancreatitis due to antegrade guidewire and stent manipulation outside the papilla.15 Stent dysfunction can occur due to the reflux of duodenal contents (similar to SEMS placed using a duodenoscope) such as food material, leading to sludge formation or ascending infection.16 A recently available covered SEMS with a long duodenal extension has been used in EUS-AGS to prevent reflux cholangitis and tumor in-growth.17 This approach has demonstrated favorable clinical outcomes, with technical and clinical success rates reaching 96% and 84%, respectively. The reintervention rate was low at 8.3%; however, but there was an increased incidence of post-procedural pancreatitis (24%) in patients with non-pancreatic cancers.17
In EUS-CDS, an anastomosis is established between the duodenum and CBD using either a tubular-covered biliary SEMS or LAMS. According to a recent literature review spanning from 2015 to 2020, the overall technical success rate was 95.0%, and the overall clinical success rate was 97.0%.18 Sufficient sealing of the anastomosis can be challenging when using a plastic stent or an uncovered SEMS, that can increase the risk of bile leakage. As a result, either a fully covered SEMS or LAMS is preferred for EUS-CDS. According to a recent meta-analysis comparing the efficacy of LAMS and SEMS for EUS-CDS, the rates of clinical and technical success, post-procedure adverse events, and reintervention were similar between the two types of stents.19
In a recent guideline, it is recommended to utilize partially or fully covered SEMS for EUS-HGS because of the increased risk of bile leakage with uncovered SEMS and challenges associated with placing pure double-pigtail stents.15 However, there is a risk of migration of fully covered SEMS, which can lead to bile leak, persistent biliary obstruction, and subsequent cholangitis. To address these limitations, the use of hybrid SEMS has been proposed.20,21 Hybrid SEMS are partially covered SEMS equipped with anchoring flaps specifically designed to prevent stent migration (Fig. 3). In a long-term follow-up study evaluating the effectiveness of newly developed hybrid SEMS, no instances of stent migration were observed during the median follow-up period of 148.5 days, and the mean duration of stent patency was recorded as 166.3 days.20 Based on a recent study, it was found that covered SEMS could be a more suitable choice for patients with malignant biliary obstruction undergoing EUS-HGS. This preference is justified by the longer time until recurrent biliary obstruction.22
For patients with cholecystitis and high-risk surgical candidates, EUS-guided gallbladder drainage (EUS-GBD) has demonstrated technical and clinical success rates comparable to those of percutaneous techniques.23,24 In the transmural approach to EUS-GBD, a stent is inserted under EUS guidance from the duodenum or stomach into the gallbladder. The use of pigtail plastic stents and biliary SEMS is not suitable due to their risk of leakage, potential for contralateral wall injury or occlusions caused by their longer length, and the risk of migration due to the absence of flanges.25 LAMS have successfully addressed these limitations by featuring a short length with large flanges and offering a wide range of diameters (6–20 mm, Fig. 4). This allows for the passage of gallstones or the endoscope as required during therapeutic cholecystoscopy.26,27 The commonly employed LAMS sizes for EUS-GBD encompass saddle lengths of 10 and 15 mm, along with inner diameters of 10, 15, and 20 mm.25 It is customary to routinely place a double-pigtail plastic biliary stent across the LAMS as a potential measure to mitigate complications such as bleeding, stent obstruction, and contralateral wall injury resulting from the LAMS. However, this practice has not been universally adopted.28
Advancements in EUS have facilitated the emergence of EUS-guided gastroenterostomy (EUS-GE) as a potentially minimally invasive approach that utilizes LAMS. It has emerged as a safe and effective palliative procedure for gastric outlet obstruction (GOO) in patients unfit for surgery.29-31 A recent large multicenter study including 310 patients with GOO showed that EUS-GE can be performed in patients with nutritional deficiencies without compromising the technical (97.9% vs. 100%) and clinical success rates (94.1% vs. 94.3%) when compared to surgical gastrojejunostomy. Additionally, EUS-GE showed a lower occurrence of adverse events (13.4% vs. 33.3%, p<0.001) while enabling earlier resumption of diet and chemotherapy. A 15 mm or 20 mm diameter, 10 mm long EC-LAMS was used in this study.32 Another comparative study demonstrated that the technical success rate of EUS-GE was comparable to that of surgical gastrojejunostomy, while exhibiting significantly fewer adverse events (8% vs. 41%, p=0.01).33
Endoscopic ultrasound-directed transgastric ERCP (EDGE) provides a nonsurgical biliary intervention in patients with Roux-en-Y gastric bypass for bariatric treatment. EUS-guided gastrogastrostomy is first performed using LAMS to establish a conduit between the gastric pouch and the excluded remnant stomach. Once the LAMS stent is sufficiently dilated, a duodenoscope is passed to perform ERCP. EDGE appears to have successfully addressed the technical difficulties associated with enteroscopy-assisted ERCP.34 While there is growing guidance on the technical execution of EDGE, with limited data regarding the removal of LAMS and the long-term closure of fistulas. While immediate removal of the LAMS during the index procedure might provide practical and economic benefits, it could also result in free perforation. Therefore, it is recommended to remove the stent gradually over time as long as no further interventions are deemed necessary after tract maturation.15
Recently, several new types of stents have been developed. Tornado stent (S&G Biotech Inc.) is a fully covered, braided, twisted SEMS with spirally coiled ends. It was constructed using a nitinol wire coated with silicone (Fig. 5A). An animal study showed that EUS-GBD using the Tornado stent was technically feasible without any adverse events.35 The authors highlighted that the Tornado stent’s relatively flexible spiral coiled ends may help to reduce the occurrence of serious adverse events such as bleeding or stent burying. The risk of mechanical complications may be lower because the spiral-coiled ends on both sides have a fixation effect without a flange. Furthermore, the presence of spiral-coiled ends is expected to decrease the risk of stent migration and ascending infection compared with tubular SEMS. Spring Stopper Stent (Taewoong Medical) is a recently developed, partially covered SEMS designed for EUS-HGS. It features a spring-line anchoring function on the gastric side (Fig. 5B). This pilot retrospective study demonstrated the feasibility and safety of a Spring Stopper Stent for EUS-HGS. Spring-like anchoring on the gastric side appears to be an effective mechanism for preventing migration.36
With the remarkable advancements in SEMS, the scope of EUS-guided interventions is expanding and becoming more efficient. Recent developments in dedicated stents have made this procedure simpler and safer. However, the development of LAMS requires further technical refinements to enhance its safety profile and broaden its applications to other indications. In clinical practice, LAMS is recommended for EUS-guided WON/pseudocyst drainage, EUS-GBD, and EUS-GE, with different diameters based on the distance between the lumens. Covered or hybrid SEMS are useful in EUS-CDS and EUS-HGS. Additionally, it is crucial to conduct well-designed randomized trials and prospective studies to critically evaluate the impact of this technology on EUS-guided interventions.
REFERENCES
1. Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy. 2011; 43:337–342.
2. Bang JY, Varadarajulu S. Lumen-apposing metal stents for endoscopic ultrasonography-guided interventions. Dig Endosc. 2019; 31:619–626.
3. Walter D, Teoh AY, Itoi T, et al. EUS-guided gall bladder drainage with a lumen-apposing metal stent: a prospective long-term evaluation. Gut. 2016; 65:6–8.
4. Song TJ, Lee SS, Moon JH, et al. Efficacy of a novel lumen-apposing metal stent for the treatment of symptomatic pancreatic pseudocysts (with video). Gastrointest Endosc. 2019; 90:507–513.
5. Rinninella E, Kunda R, Dollhopf M, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video). Gastrointest Endosc. 2015; 82:1039–1046.
6. Yoo HW, Moon JH, Jo SJ, et al. A novel electrocautery-enhanced delivery system for one-step endoscopic ultrasound-guided drainage of the gallbladder and bile duct using a lumen-apposing metal stent: a feasibility study. Endoscopy. 2021; 53:922–926.
7. Mangiavillano B, Moon JH, Crinò SF, et al. Safety and efficacy of a novel electrocautery-enhanced lumen-apposing metal stent in interventional EUS procedures (with video). Gastrointest Endosc. 2022; 95:115–122.
8. Oh CH, Lee JK, Song TJ, et al. Clinical practice guidelines for the endoscopic management of peripancreatic fluid collections. Clin Endosc. 2021; 54:505–521.
9. Bang JY, Navaneethan U, Hasan MK, et al. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut. 2019; 68:1200–1209.
10. Bazerbachi F, Sawas T, Vargas EJ, et al. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: a systematic review and meta-analysis. Gastrointest Endosc. 2018; 87:30–42.
11. Karstensen JG, Novovic S, Hansen EF, et al. EUS-guided drainage of large walled-off pancreatic necroses using plastic versus lumen-apposing metal stents: a single-centre randomised controlled trial. Gut. 2023; 72:1167–1173.
12. Vanek P, Falt P, Vitek P, et al. EUS-guided transluminal drainage using lumen-apposing metal stents with or without coaxial plastic stents for treatment of walled-off necrotizing pancreatitis: a prospective bicentric randomized controlled trial. Gastrointest Endosc. 2023; 97:1070–1080.
13. Arvanitakis M, Dumonceau JM, Albert J, et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018; 50:524–546.
14. Zhang LY, Kunda R, Aerts M, et al. Novel 15-mm-long lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections located ≥10 mm from the luminal wall. Endoscopy. 2022; 54:706–711.
15. van Wanrooij RL, Bronswijk M, Kunda R, et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2022; 54:310–332.
16. Misra SP, Dwivedi M. Reflux of duodenal contents and cholangitis in patients undergoing self-expanding metal stent placement. Gastrointest Endosc. 2009; 70:317–321.
17. So H, Oh D, Takenaka M, et al. Initial experience of endoscopic ultrasound-guided antegrade covered stent placement with long duodenal extension for malignant distal biliary obstruction (with video). J Hepatobiliary Pancreat Sci. 2021; 28:1130–1137.
18. Ogura T, Itoi T. Technical tips and recent development of endoscopic ultrasound-guided choledochoduodenostomy. DEN Open. 2021; 1:e8.
19. Amato A, Sinagra E, Celsa C, et al. Efficacy of lumen-apposing metal stents or self-expandable metal stents for endoscopic ultrasound-guided choledochoduodenostomy: a systematic review and meta-analysis. Endoscopy. 2021; 53:1037–1047.
20. Cho DH, Lee SS, Oh D, et al. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest Endosc. 2017; 85:1067–1075.
21. De Cassan C, Bories E, Pesenti C, et al. Use of partially covered and uncovered metallic prosthesis for endoscopic ultrasound-guided hepaticogastrostomy: results of a retrospective monocentric study. Endosc Ultrasound. 2017; 6:329–335.
22. Shibuki T, Okumura K, Sekine M, et al. Covered self-expandable metallic stents versus plastic stents for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Clin Endosc. 2023; 56:802–811.
23. Jang JW, Lee SS, Song TJ, et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012; 142:805–811.
24. Small AJ, Irani S. EUS-guided gallbladder drainage vs. percutaneous gallbladder drainage. Endosc Ultrasound. 2018; 7:89–92.
25. Irani SS, Sharzehi K, Siddiqui UD. AGA clinical practice update on role of EUS-guided gallbladder drainage in acute cholecystitis: commentary. Clin Gastroenterol Hepatol. 2023; 21:1141–1147.
26. Chan SM, Teoh AY, Yip HC, et al. Feasibility of per-oral cholecystoscopy and advanced gallbladder interventions after EUS-guided gallbladder stenting (with video). Gastrointest Endosc. 2017; 85:1225–1232.
27. Yoo HW, Moon JH, Lee YN, et al. Peroral cholecystoscopy using a multibending ultraslim endoscope through a lumen-apposing metal stent for endoscopic ultrasound-guided gallbladder drainage: a feasibility study. Endoscopy. 2022; 54:384–388.
28. Higa JT, Irani SS. Endoscopic methods for gallbladder drainage. Curr Treat Options Gastroenterol. 2019; 17:357–366.
29. Ribas PH, De Moura DT, Proença IM, et al. Endoscopic ultrasound-guided gastroenterostomy for the palliation of gastric outlet obstruction (GOO): a systematic review and meta-analysis of the different techniques. Cureus. 2022; 14:e31526.
30. Şentürk H, Köker İH, Koçhan K, Kiremitçi S, et al. Endoscopic ultrasound-guided gastrojejunostomy with a direct technique without previous intestinal filling using a tubular fully covered self-expandable metallic stent. Clin Endosc. 2024; 57:209–216.
31. Lim SG, Kim CG. No difference in outcomes with 15 mm vs. 20 mm lumen-apposing metal stents for endoscopic ultrasound-guided gastroenterostomy for gastric outlet obstruction: a meta-analysis. Clin Endosc. 2023; 56:298–307.
32. Canakis A, Bomman S, Lee DU, et al. Benefits of EUS-guided gastroenterostomy over surgical gastrojejunostomy in the palliation of malignant gastric outlet obstruction: a large multicenter experience. Gastrointest Endosc. 2023; 98:348–359.
33. Abbas A, Dolan RD, Bazarbashi AN, et al. Endoscopic ultrasound-guided gastroenterostomy versus surgical gastrojejunostomy for the palliation of gastric outlet obstruction in patients with peritoneal carcinomatosis. Endoscopy. 2022; 54:671–679.
34. Kedia P, Kumta NA, Widmer J, et al. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: a novel technique. Endoscopy. 2015; 47:159–163.
35. Huh G, Choi JH, Lee SH, et al. Innovation of EUS-guided transmural gallbladder drainage using a novel self-expanding metal stent. Sci Rep. 2020; 10:11159.
36. Ishii S, Isayama H, Sasahira N, et al. A pilot study of Spring Stopper Stents: novel partially covered self-expandable metallic stents with anti-migration properties for EUS-guided hepaticogastrostomy. Endosc Ultrasound. 2023; 12:266–272.
Fig. 1.
Commercially available electrocautery-enhanced lumen-apposing metal stents. (A) Hot AXIOS (Boston Scientific Corporation). (B) Niti-S Hot SPAXUS (Taewoong Medical).
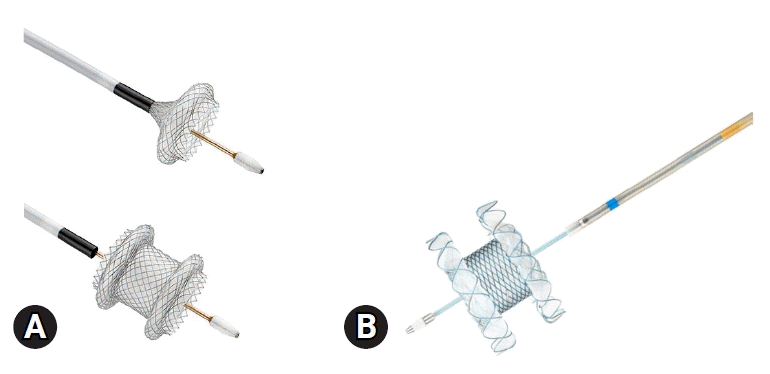
Fig. 2.
A case of EUS-guided walled-off necrosis treated using a lumen-apposing metal stent (15 mm, 1 cm, Hot AXIOS; Boston Scientific Corporation). (A) A large walled-off necrosis is noted around the pancreas. (B). A sonographic view of stent deployment. (C) An endoscopic view of the lumen-apposing metal stent in the stomach. (D) An X-ray view of the stent.
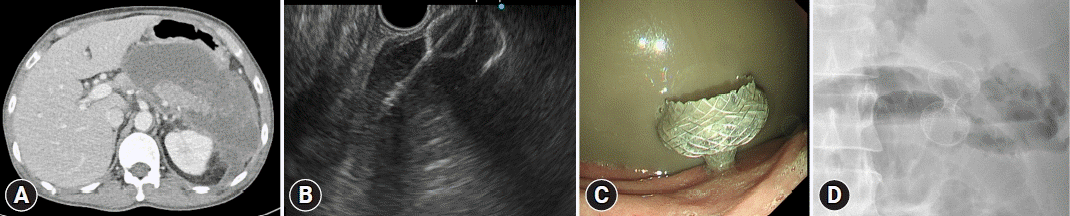
Fig. 3.
A case of EUS-guided hepatico-gastrostomy treated using a hybrid self-expandable metal stent (10 mm, 8 cm, Giobor; Taewoong Niti-S Biliary Covered Stent). (A) Dilated bile ducts are noted in a patient with cholangiocarcinoma. (B, C) A process of stent deployment. (D) An X-ray view of the stent (arrow).
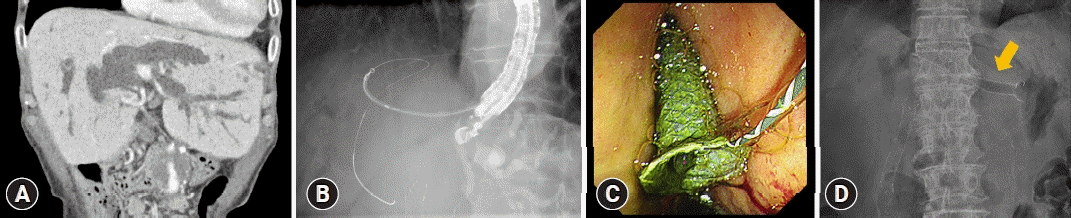
Fig. 4.
A case of EUS-guided gallbladder drainage treated using a lumen-apposing metal stent (10 mm, 2 cm, Hot AXIOS; Boston Scientific Corporation). (A) Acute cholecystitis is noted on computed tomography. (B) A sonographic view of the stent deployment. (C) An endoscopic view of the lumen-apposing metal stent in the duodenum. (D) An X-ray view of the stent (circle).
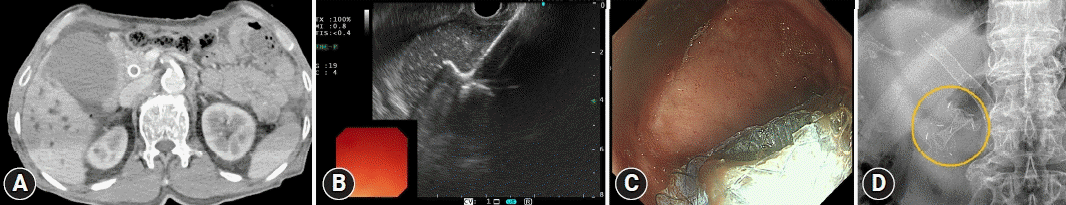
Fig. 5.
Newly developed self-expandable metal stents for endoscopic ultrasound-guided interventions. (A) Tornado stent (S&G Biotech Inc.). (B) Spring Stopper Stent (Taewoong Medical).
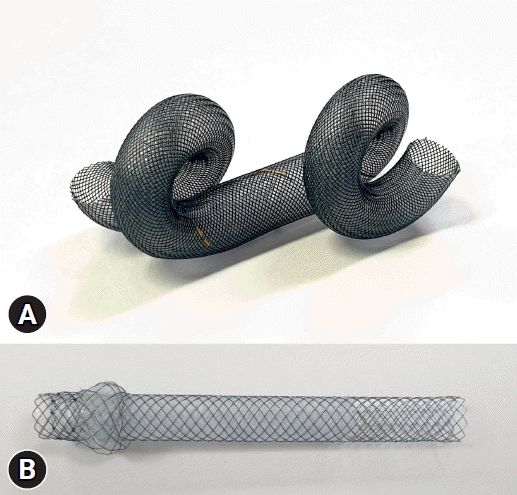
Table 1.
Types of lumen-apposing metal stents and biflanged self-expandable metal stents




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download