Gastric variceal hemorrhages (GVH) represent a significant source of bleeding in patients with portal hypertension (PH) and are associated with considerable morbidity and mortality. Gastric varices (GV) are found in up to 30% of patients with gastrointestinal hemorrhage and PH, regardless of a cirrhotic or non-cirrhotic etiology.
1,
2 Although less frequently symptomatic compared to esophageal varices, GVH is associated with treatment failure and a mortality rate of up to 50%.
2,
3 However, mortality in these patients is largely caused by subsequent decompensation of the underlying liver disease.
4 While most studies have focused on GVH in cirrhotic patients, insufficient data exists regarding the long-term outcome of GVH due to non-cirrhotic portal hypertension (NCPH).
Recent European Society of Gastrointestinal Endoscopy Guidelines conclude that the recurrence of GVH after initial treatment remains a significant clinical problem, occurring in up to 45% of cases during long-term follow-up.
3,
5 Therefore, after initial bleeding control, repeated endoscopies with cyanoacrylate (CYA) injection every two to four weeks are recommended until complete eradication of residual GV is accomplished.
3 However, whether ongoing endoscopic or endosonographic surveillance results in better clinical outcomes has not been shown conclusively, and prospective controlled trials evaluating this crucial clinical problem are scarce.
To address this issue, the authors conducted a retrospective single-center analysis of all patients with GVH treated with CYA injection and NCPH as the underlying condition. The project was approved by a competent ethics committee (Kantonale Ethikkommission Zürich, ID 2022-01490). All patients treated with CYA injection due to GVH at the University Hospital Zürich between 2003 and 2021 were evaluated for eligibility. Patients with documented refusal of further use of medical data for research were excluded from the study, as was one patient who had undergone a splenectomy and another patient with insufficient follow-up data. Data describing patient characteristics, disease course, and treatment, as well as endoscopic reports and laboratory values, were retrieved from the electronic clinical information system.
In total, 34 patients with GVH treated with CYA were found, of which 25 were patients with liver cirrhosis. The remaining nine patients had NCPH as the underlying condition.
Table 1 delineates the main results. Two patients had initially undergone transjugular intrahepatic portosystemic shunt (TIPS) placement. However, in both instances, the intervention was futile because TIPS thrombosis mirrored clinically recurrent GVH. Among 25 CYA procedures, 19 (76.0%) were endoscopic ultrasound (EUS)-guided, whereas the remainder were under direct visualization. GV treatment was subdivided into an eradication period, where GV was treated until considered sufficiently eradicated, and a surveillance period until the last EUS. During follow-up, an adequate therapeutic result was defined as “no remaining intramural vessels larger than 2 mm detectable on EUS,” which was achieved in all nine patients. On average, patients received 12 EUS examinations during the surveillance period.
Table 1.
Patient characteristics, procedure, and follow-up data
|
Characteristic |
Value |
|
Total |
9 (100) |
|
Age (yr) |
48 (17–73) |
|
Underlying etiology |
|
|
EHPVOa)
|
7 (77.8) |
|
NRH |
1 (11.1) |
|
CHF |
1 (11.1) |
|
Sarin classification |
|
|
GOV1 |
1 (11.1) |
|
GOV2a)
|
7 (77.8) |
|
IGV1 |
1 (11.1) |
|
Prior NSBB |
4 (44.4) |
|
Indication for GV treatment |
|
|
Active bleeding |
3 (33.3) |
|
History of GV bleeding |
6 (66.7) |
|
Initial treatment |
|
|
Type |
|
|
CYA or EUS-CYA |
8 (88.9) |
|
EUS-CYA+coil |
1 (11.1) |
|
No. of interventions per patient |
2 (1–3) |
|
NSBB secondary prophylaxis |
7 (77.8) |
|
Surveillance |
|
|
GV obliterated on EUS follow-up |
9 (100) |
|
Follow-up time (mo) |
140 (89–258) |
|
Time under EUS surveillance (mo) |
107 (22–159) |
|
Repeat EUS per patient |
12 (5–24) |
|
Patients with repeat GV treatment |
4 (44.4) |
|
Time to repeat treatment (mo) |
3, 13, 25, 46 |
|
No. of repeat treatments per patient (prophylactic) |
1, 1, 2, 2 |
|
Patients with recurrent GVH (n, %) |
2/9 (22.2) |
|
Time to recurrent GVH (mo) |
3, 25, 129 |
|
TRAE per treatment (n=25)b)
|
2 (8.0) |

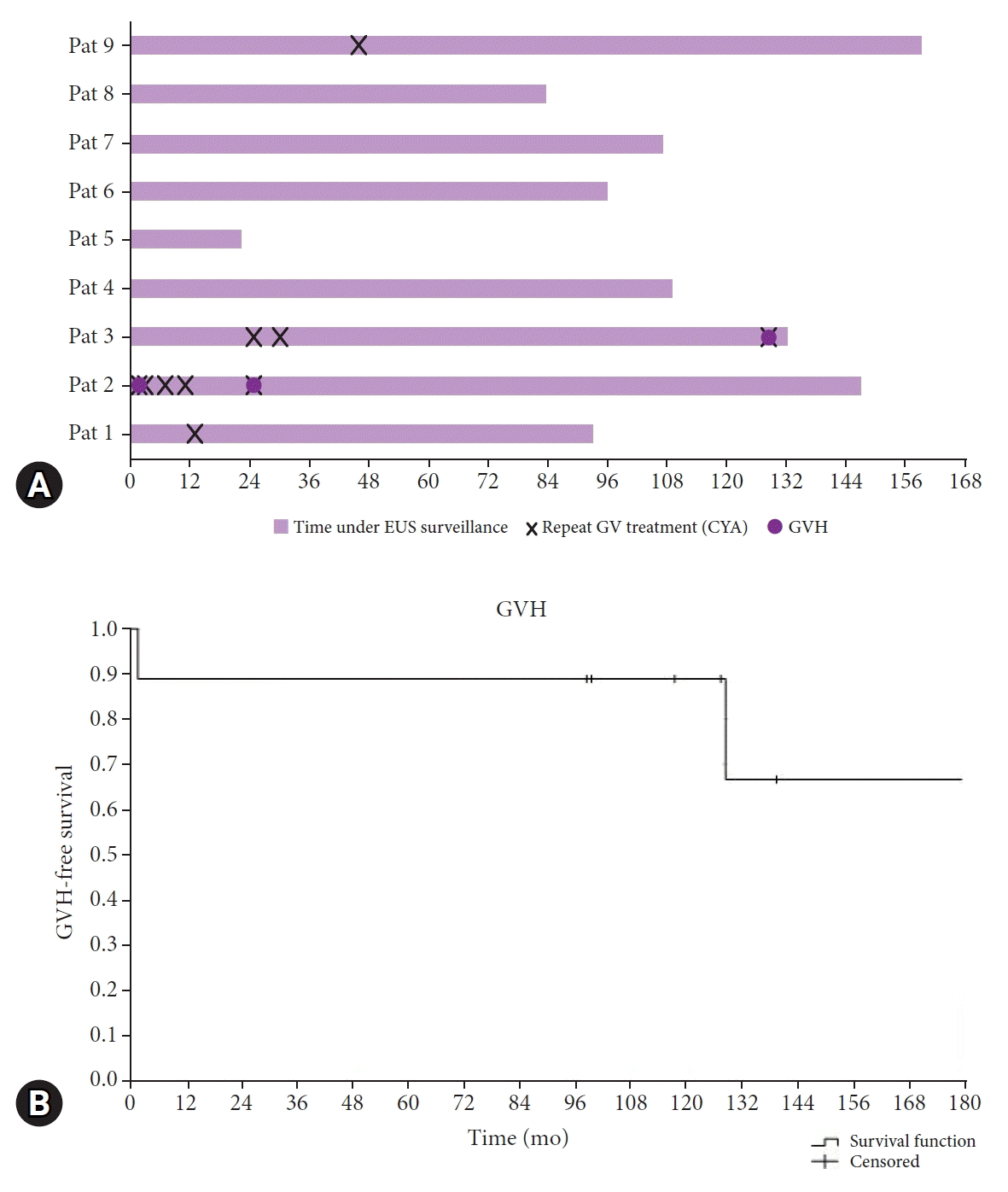
In total, three episodes of rebleeding were observed in two patients (22%) during a median follow-up of 140 months (89–258) and a median time under EUS surveillance of 107 months (22–159) (
Table 1,
Fig. 1). Neither of the two patients had a treatable cause of the underlying NCPH; one patient had congenital liver fibrosis, and the other developed chronic portal vein thrombosis during pregnancy. One episode occurred three months after the initial GV treatment. The other two occurred after 25 and 129 months, respectively. All re-bleedings occurred despite repeated CYA treatments and documented eradication of any clinically relevant intramural vessel. Each recurrent GVH was successfully retreated with CYA injections, and patients recovered without any sequelae in each case. Patients were discharged after a brief hospital stay (1, 3, and 4 days). No mortality due to GVH existed in this cohort.
Fig. 1.
(A) Individual patient timelines after initial gastric varices treatment and endoscopic ultrasound (EUS) confirmation of obliteration. (B) Cumulative survival (Kaplan-Meier estimates) without recurrent gastric variceal hemorrhage (GVH). Patients 2 and 3 had GVH during follow-up, despite EUS surveillance and treatment of recurrent varices. Patients 1 and 9 had a single repeat intervention whereas patients 4 to 8 did not have recurrent varices.

Furthermore, the authors undertook a comprehensive literature review using the PubMed database in May 2023; the
Supplementary Figure 1 depicts the search strategy and selection process. Total 485 articles were identified using the following search terms: gastric varices AND long-term follow-up NOT review NOT meta-analysis NOT case report.
6,
7 Studies that exclusively described the long-term follow-up of cirrhotic or pediatric patients, together with studies that used no or other treatments such as balloon-occluded retrograde transvenous obliteration, were excluded. Investigations with heterogeneous patient populations were excluded if the results were insufficiently stratified according to the underlying cause of PH.
As depicted in the
Supplementary Table 1, the comprehensive review of the literature revealed only two relevant studies.
6,
7 The most comprehensive study from Spaander et al.
8 was excluded since it combined esophageal and gastric variceal bleeding in patients with NCPH. Notably, of the 27 consecutive patients in this study, nine patients (33%) had GVH at initial presentation. No bleeding-related death was recorded during a median follow-up of 8.6 years. One study predominantly included patients with schistosomiasis,
6 an etiology of NCPH rarely encountered in Europe. Collectively, few case series described the long-term clinical course of patients with NCPH and GVH. None of the studies mentioned above describe EUS as a method to surveil these patients.
The present cohort confirms that GVH-related mortality in adequately treated NCPH patients appears to be low. Nonetheless, rebleeding may occur in a relevant proportion of these patients. In the present cohort, three re-bleedings were observed in two patients even though every potentially relevant intramural vessel had been treated. All re-bleedings arose from small varices (2–3 mm) and were treated successfully with additional CYA injections. Furthermore, in all nine patients, EUS surveillance beyond 48 months resulted in no additional prophylactic treatments. This questions the efficiency of EUS surveillance beyond three to four years.
To the best of the authors’ knowledge, the current cohort encompasses the longest follow-up described in the literature, giving important insight regarding the clinical course of patients with NCPH and GVH. Furthermore, the regular use of EUS to survey these patients may be regarded as another strength of this report since the role of EUS in this context has not been conclusively defined.
There are shortcomings of this study that need mentioning. As in comparable studies,
6-
8 the number of patients was small, and no strict treatment or surveillance protocol could be sustained during the numerous years of treatment and surveillance. Furthermore, advances in methods resulted in inconsistent standard operating procedures, explaining why some of the CYA treatments were performed under direct vision and Doppler ultrasound was inconsistently used. However, establishing a significantly larger cohort with a comparable observation period would be challenging.
Altogether, endoscopic surveillance after adequately treating GVH in patients with NCPH rarely results in repeat secondary-prophylactic interventions beyond four years, and rebleeding occurs despite EUS surveillance and secondary-prophylactic CYA treatments. However, late rebleeding may be associated with a good prognosis. Larger prospective cohorts would be of significant value to confirm the current findings.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download