Abstract
Background/Aims
Advanced pancreatic and biliary tract cancers can invade the duodenum and cause duodenal hemorrhagic stenosis. This study aimed to evaluate the efficacy of covered self-expandable metal stents in the treatment of cancer-related duodenal hemorrhage with stenosis.
Methods
Between January 2014 and December 2016, metal stents were placed in 51 patients with duodenal stenosis. Among these patients, a self-expandable covered metal stent was endoscopically placed in 10 patients with hemorrhagic duodenal stenosis caused by pancreatobiliary cancer progression. We retrospectively analyzed the therapeutic efficacy of the stents by evaluating the technical and clinical success rates based on successful stent placement, degree of oral intake, hemostasis, stent patency, and overall survival.
Results
The technical and clinical success rates were 100%. All 10 patients achieved a gastric outlet obstruction scoring system score of three within two weeks after the procedure and had no recurrence of melena. The median stent patency duration and overall survival after stent placement were 52 days (range, 20–220 days) and 66.5 days (range, 31–220 days), respectively.
Approximately 20% of pancreatic cancers (PCs) and some periampullary cancers, such as biliary tract cancer (BTC), are associated with duodenal stenosis and obstruction,1-3 which should be treated either surgically or endoscopically when accompanied by gastrointestinal (GI) obstruction symptoms. However, owing to a poor performance status (PS) and prognosis, several patients are considered ineligible for gastrojejunostomy but are often considered eligible for endoscopic stent placement. Additionally, anemia and GI hemorrhage are observed in some patients due to tumor bleeding. Regarding bleeding from the tumor site, the National Comprehensive Cancer Network Guidelines on PC state that either angiography with embolization or therapeutic endoscopy should be performed if clinically indicated; alternatively, radiation therapy should be employed if not previously performed.4 However, there is currently no detailed method for performing therapeutic endoscopy. As arterial bleeding is rarely associated with such tumors, transarterial embolization usually provides little benefit. Additionally, direct endoscopic hemostasis (e.g., using endoclips or coagulation forceps) can increase tumor necrosis-related bleeding. Only a few studies to date have reported the use of covered self-expandable metal stents (cSEMS) to treat cancer-related duodenal hemorrhage5-7; however, these stents are rarely used to treat duodenal wall invasion in patients with PC and BTC. Hemostatic peptide preparations have recently become available to facilitate endoscopic hemostasis of GI bleeding.8 However, their efficacy in treating tumor bleeding from the GI invasion site in cases of pancreatobiliary cancer remains unknown. At the National Cancer Center Hospital, Tokyo, Japan, we have successfully placed a cSEMS while performing compression hemostasis in several patients with duodenal hemorrhagic stenosis.
This study aimed to retrospectively evaluate the efficacy of nitinol (nickel–titanium) cSEMS placement in the treatment of malignant hemorrhagic duodenal stenosis in patients with PC and BTC.
Between January 2014 and December 2016, 51 patients underwent stent placement for duodenal stenosis due to unresectable PC or BTC at the National Cancer Center Hospital, Tokyo, Japan. An uncovered SEMS (ucSEMS) or cSEMS was selected for placement based on the state of the stenosis. Notably, ucSEMSs were placed in 26 patients, and cSEMSs were placed in 22 patients at risk of overgrowth after stent placement or bleeding from a stenotic lesion. Ten of these patients with duodenal hemorrhagic stenosis who underwent nitinol cSEMS placement were retrospectively examined (Fig. 1).
Abdominal computed tomography and esophagogastroduodenoscopy (EGD) were performed to evaluate duodenal invasion and stenosis. Initially, the type of duodenal stenosis was assessed in all patients and categorized as follows: type I, stenosis occurring at the level of the duodenal bulb or upper duodenal genu but without involvement of the papilla; type II, stenosis affecting the second part of the duodenum and involving the papilla; and type III, stenosis affecting the third part of the duodenum distal to the papilla but without involvement of the papilla (Fig. 2).9,10 Patients 4, 8, and 9 had type II stenosis, patient 10 had type III stenosis, and the other six patients had type I stenosis. All of the patients exhibited decreased hemoglobin levels and melena at least once before the examination (Table 1). All of the patients, except patient 6, received a blood transfusion before the procedure; furthermore, all 10 patients required resolution of the hemorrhage. After the procedure, hemostasis was evaluated based on the absence of melena, as other causes related to secondary anemia (e.g., cancer progression, cachexia, and nutritional disorders) can alter hemoglobin levels.
A nitinol cSEMS (Niti-S Combi Gastroduodenal Stent; Taewoong Medical) was constructed using a braided nitinol wire partially covered with a silicone membrane (Fig. 3). The distal end of the stent had a 1 cm uncovered portion, and the proximal end had a flared portion. The stent was 20 mm in diameter and 8, 10, or 12 cm long. The size of the stent-delivery system was 10.5 Fr.
A therapeutic forward-viewing endoscope (GIF 1T240; Olympus Medical Systems) was used for stent placement shortly after the onset of vomiting, hematemesis, and/or melena. Before the procedure, we performed an EGD to determine the positional relationship between the papilla of Vater and the expected site of duodenal stent placement. If the duodenal stent could cover the papilla of Vater, as in type I and III stenoses, we placed the duodenal stent so that the papilla of Vater was not covered and bile flow was not compromised. In patients with type II stenosis and some patients with type I stenosis, if there was a risk of covering the papilla of Vater due to the duodenal stent placement, the following steps were performed in all patients, except for those in whom the biliary stent had previously been endoscopically placed: (1) In cases where one stent was insufficiently long, two long-type biliary stents were serially inserted via the percutaneous transhepatic biliary drainage (PTBD) route and placed toward the duodenal anal side beyond the PC or BTC stenosis because it is difficult to endoscopically place a biliary stent toward the anal side. (2) A duodenal stent was placed to position these stents in parallel such that the biliary and duodenal stents were in a side-by-side position and did not interfere with each other (Fig. 4). This is a novel approach to treat biliary and duodenal obstructions.
After explaining the stent placement procedure, written informed consent was obtained from all of the patients before starting the procedure. The patients were placed in the left lateral decubitus position under moderate sedation with propofol. After confirming the duodenal stenosis via endoscopy, a catheter (PR-V614M, ERCP Cannula; Olympus Medical Systems) was passed through the stenosis over a guidewire (RevoWave–J type 0.035 inch hard; PIOLAX Medical Device) and placed at a site beyond the stenosis proximal to the jejunum. The through-the-scope method11,12 was used to place the Niti-S Combi gastroduodenal stent, which was passed over the guidewire through the forceps channel of the endoscope from the distal end of the stenosis to the desired site of placement. Figure 5 shows the endoscopic procedures performed for patient 1.
The day after stent placement, the clinical condition of the patients was monitored, and plain abdominal radiography and routine blood tests were performed. The patients were allowed to drink water if melena and GI symptoms were absent, their blood tests revealed no apparent abnormalities, and the stent was patent and well positioned on radiography. Patients who remained asymptomatic after drinking water were permitted to consume a liquid diet. Gradually, a semi-liquid low-residue diet was introduced. When the patients tolerated a normal diet, they were discharged or transferred to a palliative care unit. After discharge and transfer to another hospital, a follow-up survey was conducted to assess the ability of the patients to eat normally and the presence of rebleeding, including melena.
The technical and clinical success rates of the procedures were evaluated. The technical success rate was defined as successful stent placement and deployment across the stricture. The clinical success rate was defined by the degree of oral intake, which was assessed using the adapted gastric outlet obstruction
scoring system (GOOSS; no oral intake, 0; liquids only, 1; soft solids, 2; low-residue or full diet, 3).13 All of the patients except for patient 4 could eat, with a GOOSS score of 3 two weeks before the procedure. As patient 4 had pancreatitis due to biliary stent placement, fluids were administered intravenously for more than two months, during which the patient exhibited improvement. Accordingly, patient 4 had a GOOSS score of 2 prior to the procedure. All of the patients had advanced cancer, and their activities of daily living were evaluated before and after the procedure based on the Eastern Cooperative Oncology Group PS14 (Table 1).
After the procedure, any occurrences of recurrent stenosis; rebleeding, including melena; or other adverse events were recorded. Stent patency was defined as the number of days that the patients could tolerate oral intake after stent placement. Furthermore, the date of death was determined via follow-up and surveys, and overall survival (OS) was defined as the duration that the patients survived after the procedure. To evaluate the survival duration, deaths before the recurrence of duodenal obstruction were defined as censored cases.
To compare the efficacies of duodenal ucSEMS and cSEMS placement, we investigated the duration of stent patency, survival after stent placement, and complications in 26 patients with pancreaticobiliary cancer who underwent ucSEMS placement during the study period.
The clinical features and disease courses of the study participants are summarized in Table 1. This study included 10 patients (seven men and three women; median age of 70 years), eight of whom had PC and two had BTC. The pre-treatment PS of the participants ranged from 1 to 3. All of the patients had biliary stenosis and underwent biliary stent placement. Eight patients underwent biliary stent placement prior to duodenal stent placement. One patient (patient 7) had previously undergone duodenal stent placement and restenting for hemorrhagic stenosis accompanied by tumor overgrowth. All of the patients exhibited melena or tarry stools, with evidence of progressive anemia, and some had GI hemorrhage, as indicated by EGD. All of the patients exhibited duodenal stenosis, difficulty eating in the two weeks before the procedure, and anemia related to tumor bleeding. Of the 10 patients, nine received blood transfusions before the procedure.
The technical success rate of duodenal stent placement was 100%, even in patients who underwent biliary stent placement before the duodenal stent placement. A metal stent was placed in the bile duct across the duodenal papilla via the PTBD route in nine patients (Table 1). Although mild acute pancreatitis was observed in three of these nine patients (33.3%) (Patients 7–9), the condition of all of the patients promptly and conservatively improved. Only patient 8 required puncture of the nondilated bile duct at the time of PTBD, which was successfully performed without any notable complications.
The median duration between duodenal stent placement and diet resumption was 2 days (range, 1–7 days). Within two weeks after the procedure, all of the patients achieved GOOSS scores of 3, and the clinical success rate was 100%. None of the patients experienced a worsening of PS after the procedure. Two patients (Patients 1 and 9) exhibited melena only on the first day after the procedure, indicating blood accumulation in the duodenum. As none of these patients exhibited definite symptoms of melena recurrence, their hemostatic condition was not assessed via endoscopy after the procedure. Two patients (Patients 6 and 9) experienced mild acute pancreatitis on the day after duodenal stent placement, which resolved the day after conservative treatment. No recurrence of pancreatitis or jaundice was observed. Moreover, no stent migration was observed during the follow-up. Two patients (Patients 4 and 9) exhibited stent occlusion due to tumor overgrowth and required new duodenal stents. The median follow-up period was 47.5 days (range, 13–220 days). The median stent patency duration and OS after stent placement were 52 (range, 20–220) and 66.5 (range, 31–220) days, respectively. Three patients (Patients 2–4) resumed chemotherapy.
The median stent patency duration and OS in 26 patients with pancreatobiliary cancer who underwent duodenal ucSEMS placement at the National Cancer Center Hospital were 44 (range, 1–173) and 47.5 (range, 13–185) days, respectively. The complications noted among these patients were five cases (19.2%) of stent dilation failure, as well as stent migration, acute pancreatitis, and enteritis in one case (3.8%) each.
In the present study, nitinol cSEMS effectively achieved hemostasis in patients with hemorrhagic duodenal stenosis due to PC or BTC, with resolution of both the obstruction and GI hemorrhage during the endoscopic procedure. After stent placement, all of the patients were able to resume oral intake and eventually tolerated a solid diet, which was gradually reintroduced depending on their condition. This is the first comprehensive report of hemostasis using an undocumented method for hemorrhagic duodenal stenosis caused by pancreatobiliary cancer.
The hemostatic mechanism for hemorrhagic duodenal stenosis is related to compression hemostasis and silicone stent covering, which creates a barrier in the duodenal wall against the mechanical stimuli of food and digestive enzymes, as shown in a case series on hemostasis in patients with uncontrolled post-endoscopic sphincterotomy bleeding.15 Based on our findings, we hypothesize that the silicone stent covering helps prevent both hemorrhage recurrence and tumor ingrowth.
In the present study, tumor overgrowth was noted in two (20%) of the 10 patients. According to several studies with larger sample sizes (16–366 individuals), the rate of tumor overgrowth after the placement of covered duodenal stents ranged from 0% to 13%.16-20 However, only a few studies to date have reported overgrowth rates without an ingrowth rate. Although previous studies have indicated that the rate of duodenal cSEMS migration is 9.5% to 56%, which is higher than that of ucSEMS migration,16-18,20-24 no duodenal stent migration was observed in our study. Several studies have reported a median duodenal cSEMS patency duration of 68 to 139 days16,17,19,21,23,24; however, the patency duration in our retrospective study was only 52 days. Although most patients in our study did not experience recurrence of duodenal obstruction, the stent patency duration in our study was shorter than that reported in earlier studies, which may be attributed to the shorter median OS period (66.5 days) in our patients. Although these data appear to be better than the ucSEMS data at the National Cancer Center Hospital, we could not confirm this because we did not compare them in a prospective study.
PC and BTC are associated with a poor prognosis. Most patients with PC or BTC who undergo duodenal stent placement are at an advanced cancer stage. Our study supports this finding because only three of the 10 patients could resume chemotherapy. Although the patency duration in our study was shorter than that in previous studies, we treated patients with pancreatobiliary cancer who had a poor prognosis and all of the patients had previously undergone cancer treatment for various durations. Furthermore, none of the patients died of tumor hemorrhage. Our patients benefited from stent patency and hemostasis.
In our study, in the three patients with type II stenosis, duodenal and biliary stents were placed side-by-side, and these patients did not experience difficulty with eating or biliary drainage, confirming the absence of stent-related adverse events after the procedure. To the best of our knowledge, no reports have been published to date on side-by-side positioning of stents in the duodenum. Such side-by-side placement of stents is technically easier than the stent-in-stent method25-29 because it facilitates direct control of each device. Moreover, the advantage of direct viewing offered by an endoscope in the stenotic segment cannot be utilized in the stent-in-stent method. The stent-in-stent method can occasionally be challenging because of the difficulty of viewing the papilla of Vater through the mesh from the endoscope inside the duodenal stent. Additionally, if a biliary stent cannot be placed under endoscopic guidance, the patient may eventually require PTBD. In the present study, acute pancreatitis developed in three (Patients 7–9) out of nine (33.3%) patients who underwent bile duct stent placement across the papilla of Vater via the PTBD route and in two of 10 patients (20%) after duodenal stent placement; however, all cases were mild and rapidly and conservatively improved. Previous reports have indicated that the incidence rates of acute pancreatitis due to percutaneous biliary stent and duodenal stent placement are 3.8% to 24.2%30-33 and 1.1% to 2.4%,19,20 respectively. Although the incidence rate of complications of acute pancreatitis was slightly higher in the present study, only a small number of cases were included, making simple comparisons challenging.
This retrospective study has several limitations. First, the number of patients included was small. Second, we did not endoscopically assess the GI bleeding status after the procedure. However, none of the patients had melena or tarry stools, except for two who had tarry stools only on postprocedure day 1. Therefore, we believe that the presence of these stool samples indicates their accumulation in the duodenum. Finally, we did not follow-up all of the patients until death because some patients with pancreatobiliary cancer were transferred to a palliative care center. However, to the best of our knowledge, the patients in this study who were transferred to another hospital did not experience rebleeding.
Our procedure improved the quality of life of all 10 patients, and oral intake was resumed. Seven of the 10 patients were already receiving palliative care at the time of duodenal stent placement, but the other three patients were able to resume chemotherapy. After treatment for acute pancreatitis, patient 4 could not receive anticancer therapy for an extended period before achieving hemostasis; however, this patient resumed chemotherapy with gemcitabine approximately five weeks after hemostasis. Although her survival time was short, she was discharged from the hospital and resumed chemotherapy. Moreover, one patient (patient 3) survived for 220 days without stent occlusion. Unfortunately, all of the patients succumbed to cancer progression, but none of them died of GI hemorrhage. Therefore, duodenal cSEMS placement appears to be a promising approach for achieving hemostasis in patients with hemorrhagic duodenal stenosis. However, as this retrospective study included only ten patients, our findings need to be confirmed by large-scale prospective studies on hemostasis in patients with duodenal hemorrhagic stenosis due to PC and BTC.
REFERENCES
1. Maire F, Hammel P, Ponsot P, et al. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006; 101:735–742.
2. Maetani I, Nambu T, Omuta S, et al. Treating bilio-duodenal obstruction: combining new endoscopic technique with 6 Fr stent introducer. World J Gastroenterol. 2010; 16:2828–2831.
3. Andtbacka RH, Evans DB, Pisters PW. Surgical and endoscopic palliation for pancreatic cancer. Minerva Chir. 2004; 59:123–136.
4. National Comprehensive Cancer Network (NCCN). NCCN clinical plactice guidelines in oncology; pancreatic adenocarcinoma, version 1 [Internet]. NCCN;2023. [cited 2023 May 4]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
5. D'Souza PM, Sandha GS, Teshima CW. Refractory bleeding from a malignant duodenal ulcer treated with placement of a fully-covered gastroduodenal stent. Dig Dis Sci. 2013; 58:3359–3361.
6. Orii T, Karasawa Y, Kitahara H, et al. Efficacy of self-expandable metallic stent inserted for refractory hemorrhage of duodenal cancer. Case Rep Gastroenterol. 2016; 10:151–156.
7. Daiku K, Ikezawa K, Maeda S, et al. A case of refractory tumor bleeding from an ampullary adenocarcinoma: compression hemostasis with a self-expandable metallic stent. DEN Open. 2022; 2:e23.
8. Subramaniam S, Kandiah K, Chedgy F, et al. A novel self-assembling peptide for hemostasis during endoscopic submucosal dissection: a randomized controlled trial. Endoscopy. 2021; 53:27–35.
9. Mutignani M, Tringali A, Shah SG, et al. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007; 39:440–447.
10. Tonozuka R, Itoi T, Sofuni A, et al. Endoscopic double stenting for the treatment of malignant biliary and duodenal obstruction due to pancreatic cancer. Dig Endosc. 2013; 25 Suppl 2:100–108.
11. Maetani I, Isayama H, Mizumoto Y. Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: a multicenter study. Gastrointest Endosc. 2007; 66:355–360.
12. Maetani I, Ukita T, Nambu T, et al. Comparison of ultraflex and niti-s stents for palliation of unresectable malignant gastroduodenal obstruction. Dig Endosc. 2010; 22:83–89.
13. Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002; 97:72–78.
14. Division of Cancer Treatment and Diagnosis (DCTD)/National Cancer Institute (NCI)/National Institutes of Health (NIH)/U.S. Department of Health and Human Services (DHHS). Common toxicity criteria, version 2.0 [Internet]. DCTD/NCI/NIH/DHHS;1999. [cited 2023 Aug 28]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.
15. Itoi T, Yasuda I, Doi S, et al. Endoscopic hemostasis using covered metallic stent placement for uncontrolled post-endoscopic sphincterotomy bleeding. Endoscopy. 2011; 43:369–372.
16. Lim SG, Kim JH, Lee KM, et al. Conformable covered versus uncovered self-expandable metallic stents for palliation of malignant gastroduodenal obstruction: a randomized prospective study. Dig Liver Dis. 2014; 46:603–608.
17. Maetani I, Mizumoto Y, Shigoka H, et al. Placement of a triple-layered covered versus uncovered metallic stent for palliation of malignant gastric outlet obstruction: a multicenter randomized trial. Dig Endosc. 2014; 26:192–199.
18. Waidmann O, Trojan J, Friedrich-Rust M, et al. SEMS vs cSEMS in duodenal and small bowel obstruction: high risk of migration in the covered stent group. World J Gastroenterol. 2013; 19:6199–6206.
19. Takahara N, Isayama H, Nakai Y, et al. A novel partially covered self-expandable metallic stent with proximal flare in patients with malignant gastric outlet obstruction. Gut Liver. 2017; 11:481–488.
20. Yamao K, Kitano M, Chiba Y, et al. Endoscopic placement of covered versus uncovered self-expandable metal stents for palliation of malignant gastric outlet obstruction. Gut. 2021; 70:1244–1252.
21. Kim CG, Choi IJ, Lee JY, et al. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc. 2010; 72:25–32.
22. Oh D, Lee SS, Song TJ, et al. Efficacy and safety of a partially covered duodenal stent for malignant gastroduodenal obstruction: a pilot study. Gastrointest Endosc. 2015; 82:32–36.
23. Woo SM, Kim DH, Lee WJ, et al. Comparison of uncovered and covered stents for the treatment of malignant duodenal obstruction caused by pancreaticobiliary cancer. Surg Endosc. 2013; 27:2031–2039.
24. Kim JW, Jeong JB, Lee KL, et al. Comparison between uncovered and covered self-expandable metal stent placement in malignant duodenal obstruction. World J Gastroenterol. 2015; 21:1580–1587.
25. Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc. 2003; 17:457–461.
26. Kim KO, Kim TN, Lee HC. Effectiveness of combined biliary and duodenal stenting in patients with malignant biliary and duodenal obstruction. Scand J Gastroenterol. 2012; 47:962–967.
27. Maetani I, Ogawa S, Hoshi H, et al. Self-expanding metal stents for palliative treatment of malignant biliary and duodenal stenoses. Endoscopy. 1994; 26:701–704.
28. Profili S, Feo CF, Meloni GB, et al. Combined biliary and duodenal stenting for palliation of pancreatic cancer. Scand J Gastroenterol. 2003; 38:1099–1102.
29. Moon JH, Choi HJ, Ko BM, et al. Combined endoscopic stent-in-stent placement for malignant biliary and duodenal obstruction by using a new duodenal metal stent (with videos). Gastrointest Endosc. 2009; 70:772–777.
30. Kim ET, Gwon DI, Kim JW, et al. Acute pancreatitis after percutaneous insertion of metallic biliary stents in patients with unresectable pancreatic cancer. Clin Radiol. 2020; 75:57–63.
31. Sugawara S, Arai Y, Sone M, et al. Frequency, severity, and risk factors for acute pancreatitis after percutaneous transhepatic biliary stent placement across the papilla of vater. Cardiovasc Intervent Radiol. 2017; 40:1904–1910.
32. Yang Y, Liu RB, Liu Y, et al. Incidence and risk factors of pancreatitis in obstructive jaundice patients after percutaneous placement of self-expandable metallic stents. Hepatobiliary Pancreat Dis Int. 2020; 19:473–477.
33. Zu QQ, Zhang JX, Wang B, et al. Percutaneous transpapillary biliary stent placement for distal malignant biliary obstruction: outcomes and survival analysis. Turk J Gastroenterol. 2019; 30:714–721.
Fig. 1.
Number and details of duodenal stent placement cases from January 2014 to December 2016. The bold boxes indicate the cases included in the study. cSEMS, covered self-expandable metal stent; ucSEMS, uncovered self-expandable metal stent.
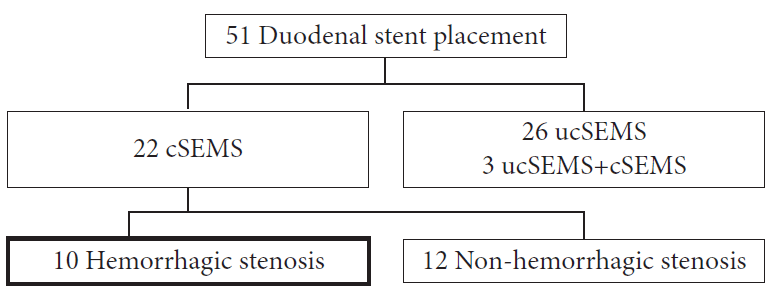
Fig. 2.
Types of duodenal stenosis due to tumor. Type I: Stenosis occurring at the level of the duodenal bulb or upper duodenal genu, but without papillary involvement (see light blue marker area). Type II: Stenosis affecting the second part of the duodenum and involving the papilla (pink marker). Type III: Stenosis affecting the third part of the duodenum distal to the papilla, without involvement of the papilla (yellow marker area).
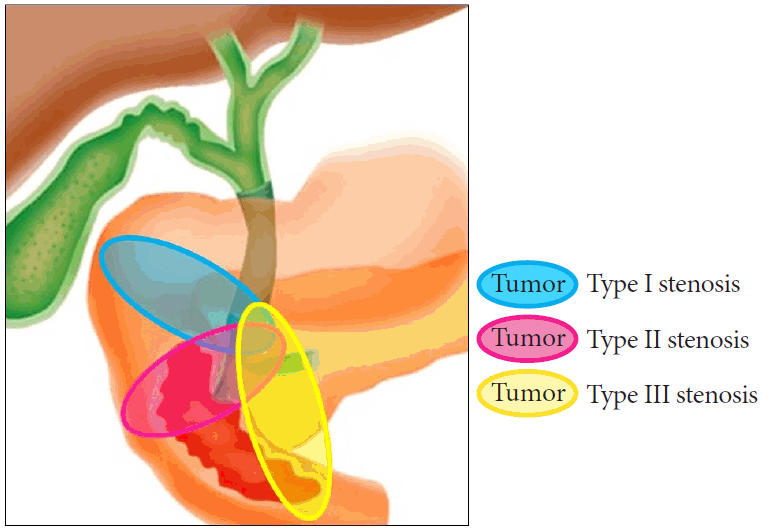
Fig. 3.
Nitinol-covered self-expandable metal stent (Niti-S Combi gastroduodenal stent; Taewoong Medical).
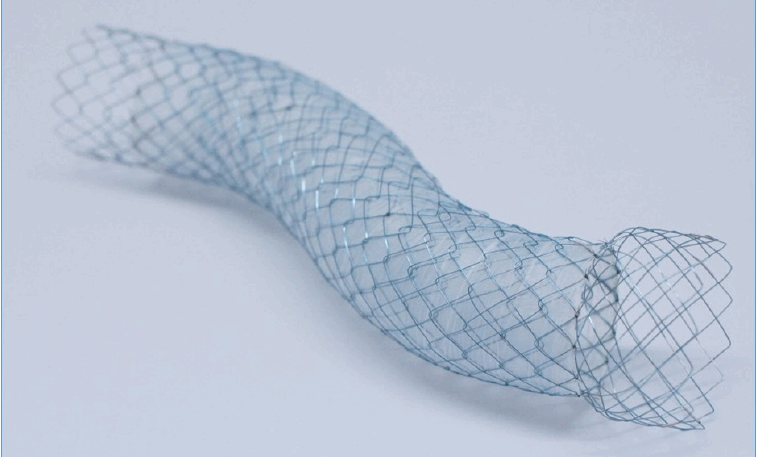
Fig. 4.
Schematic outline of stent placement in a side-by-side position. We positioned the biliary and duodenal stents next to each other to avoid any interference.
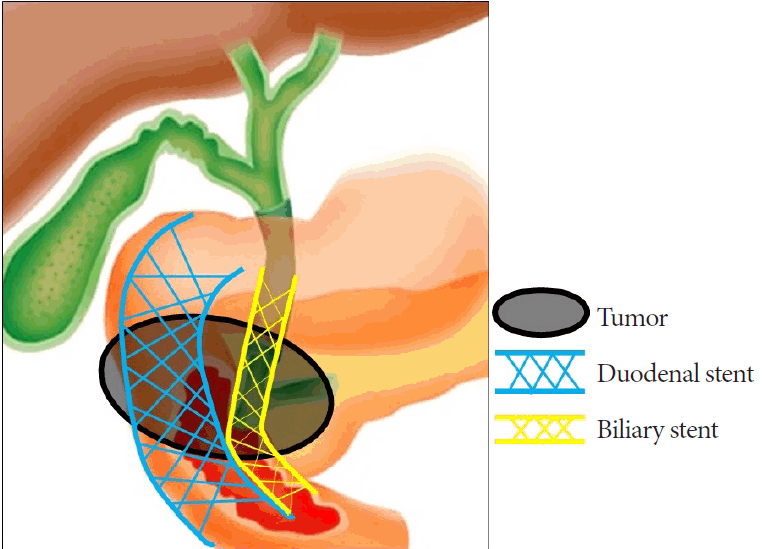
Fig. 5.
Patient 1: Simultaneous placement of biliary and duodenal stents. (A) X-ray showing the simultaneous placement of biliary and duodenal stents. (B) Endoscopic image showing stenosis and bleeding in the duodenum immediately after the stent placement.
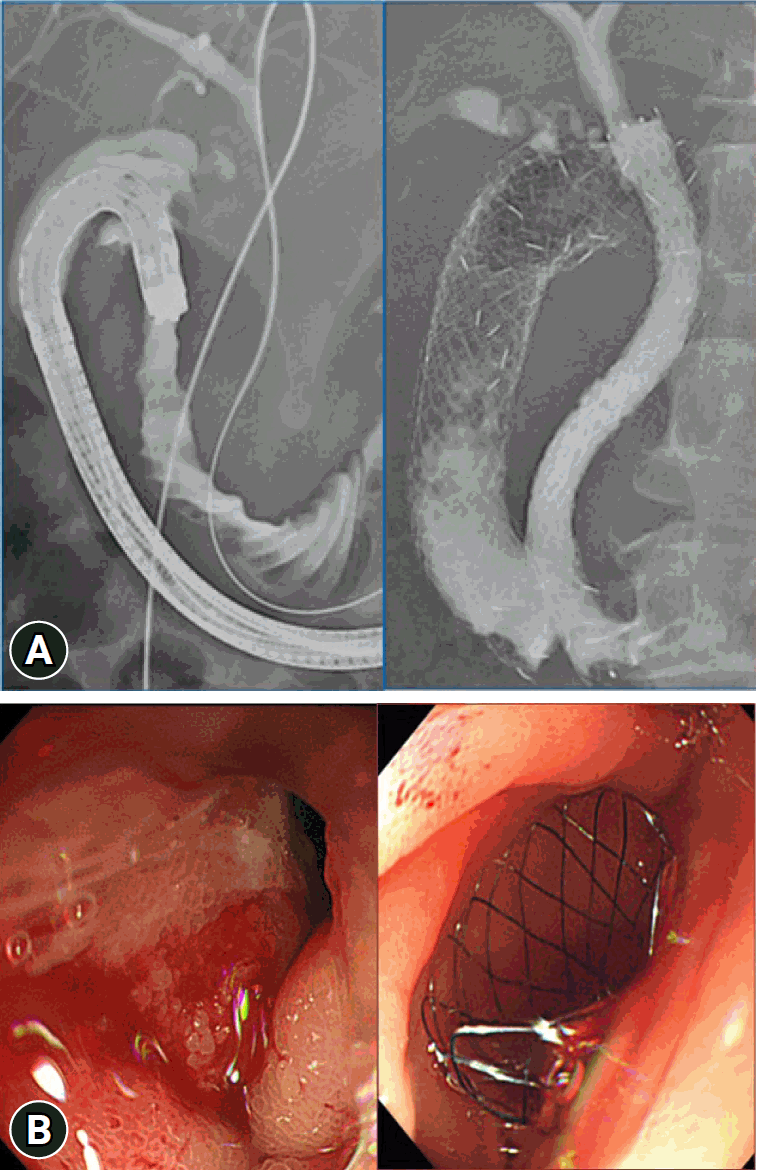
Table 1.
Clinical features and clinical course of the patients
| No. | Age (yr) | Sex | PS | Cancer site | Stenosis type | Duodenal cSEMS length (mm) | Biliary stent placement/route |
Symptom |
Level of Hb (g/dL) |
Transfusion (unit) |
Days after duodenal stent placement |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset | Postb) | Prea) | Postb) | Prea) | Postb) | To refeed | Follow-up | Patency | Survival | ||||||||
| 1 | 73 | M | 2 | Ph | 1 | 120 and 80 | Samec)/PTBD | Anemia | Once melena | 6.5 | 7.8 | 4 | 4 | 7 | 54 | 52 | 54 |
| 2 | 70 | F | 2 | Ph | 1 | 100 | Priord)/PTBD | Anemia | None | 3.1 | 8.8 | 6 | None | 2 | 117 | 164 | 166 |
| Tarry stool | |||||||||||||||||
| 3 | 77 | F | 1 | Ph | 1 | 80 | Priord)/PTBD | Hemorrhage | None | 6.2 | 9.5 | 2 | None | 2 | 193 | 220 | 220 |
| 4 | 73 | F | 2 | Ph | 2 | 100 | Samec)/SBS/PTBD | Hemorrhage | None | 6.3 | 10.1 | 2 | None | 2 | 77 | 52 | 77 |
| Tarry stool | |||||||||||||||||
| 5 | 45 | M | 1 | Ph | 1 | 100 | Priord)/EBD | Tarry stool | None | 8.6 | 8.9 | 2 | None | 4 | 16 | 42 | 56 |
| 6 | 58 | M | 2 | Hilar BD | 1 | 120 | Priord)/PTBD | Tarry stool | None | 7.2 | 8.1 | None | 2 | 3 | 94 | 92 | 102 |
| 7 | 64 | M | 2 | GB | 1 | 120 | Priord)/PTBD | Hemorrhage | None | 6.3 | 7.8 | 8 | 8 | 1 | 32 | 31 | 32 |
| Tarry stool | |||||||||||||||||
| 8 | 82 | M | 3 | Pb | 2 | 120 and 80 | Priord)/SBS/PTBD | Hematemesis | None | 8.4 | 8.7 | 2 | None | 1 | 13 | 93 | 93 |
| 9 | 47 | M | 2 | Ph | 2 | 120 and 100 | Priord)/SBS/PTBD | Tarry stool | Once melena | 7.6 | 6.5 | 4 | 2 | 3 | 41 | 20 | 41 |
| 10 | 62 | M | 2 | Ph | 3 | 120 | Priord)/PTBD | Tarry stool | None | 6.4 | 10.3 | 4 | None | 2 | 28 | 28 | 31 |
| Med | 67 | 6.45 | 8.75 | 2 | 47.5 | 52 | 66.5 | ||||||||||
PS, (Eastern Cooperative Oncology Group) performance status; cSEMS, covered self-expandable metal stent; Hb, hemoglobin; M, male; F, female; Ph, pancreas head; Pb, pancreas body; BD, bile duct; GB; gallbladder; PTBD, percutaneous transhepatic biliary drainage; SBS, side by side; EBD, endoscopic biliary drainage; med, median.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download