Abstract
Transverse basilar cleft (TBC) is an extremely rare variation of the clivus or the basilar part of the occipital bone. In this report, a unilateral transverse basilar fissure was found at the clivus in a head computed tomography of an 18-year-old female patient diagnosed with hemifacial microsomia (HFM). Image analysis of this patient showed shortening of the ramus of the right mandible along with medial displacement of the right temporomandibular joint and hypoplastic right maxilla. In addition, observation of the clivus showed a cleft between the basioticum and basioccipital bones at the level of the pharyngeal tubercle on the right side. This cleft was identified as TBC. Clival variations, TBC included, attributed to HFM have never been reported. This report draws attention to the complex relationship between abnormal development of clivus and HFM syndrome, and sheds light on a possible genetic and molecular association between these two conditions.
Hemifacial microsomia (HFM) is a complicated malformation syndrome in which one half of the lower face is underdeveloped. Its estimated prevalence ranges from 1/3,500 to 1/5,600, making it the second most common craniofacial abnormality after cleft lip and palate [1]. Most cases of HFM are sporadic and are characterized by unilateral hypoplasia of the mandible and ear, along with their overlying muscles, cranial nerves, and connective tissue. There can be significant variability in the manifestation of the HFM spectrum depending on the degree to which these structures are affected. Many skeletal deformities associated with the HFM mandible have been described including hypoplasia of the mandibular ramus and fossa, absence of the temporomandibular joint, orbital distortion, hypoplasia of the maxilla and zygomatic arch, and displacement of the temporal bone [2]. However, no other intracranial skeletal defects associated with HFM have been reported.
The clivus is located at the central base of the skull anterior to the foramen magnum, composed of the occipital and sphenoid bones, which fuse at the spheno-occipital synchondrosis [3]. It is also one of the areas in the human body that exhibit diverse anatomical variants. To our understanding, variations of the clivus associated with HFM have never been reported. In this report, we present for the first time a case of skeletal defect in the clivus, namely a transverse basilar cleft (TBC), associated with HFM.
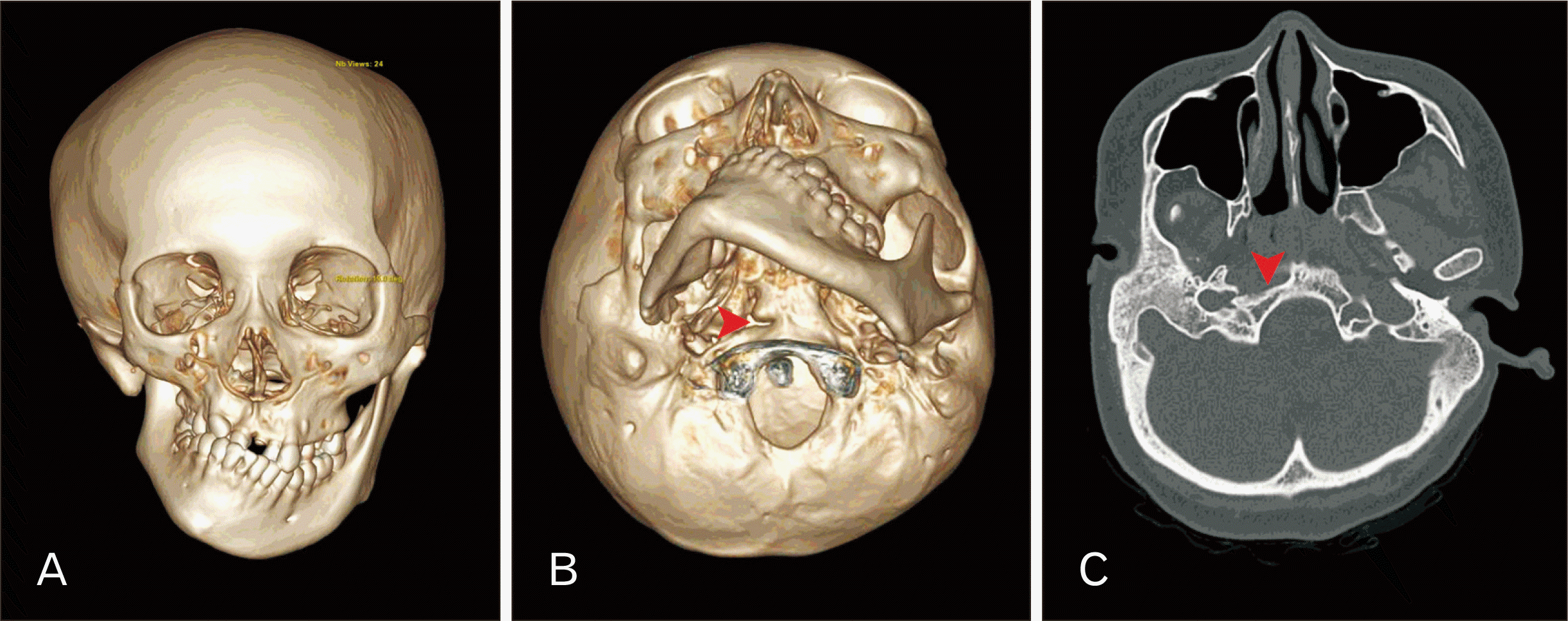
During a retrospective study of anatomical variations in the clivus on computed tomography (CT), TBC was observed in an 18-year-old female patient, who had previously been diagnosed with HFM. Three-dimensional (3D) reconstruction revealed that the ramus of the right mandible was shortened, and there was medial displacement of the mandibular fossa and the temporomandibular joint (Fig. 1A). The right maxilla was also hypoplastic and the zygomatic arch of the temporal bone was misplaced inferiorly. The orbits and nasal septum appeared normal. In addition to these skeletal deformities, the 3D image revealed a cleft between the basioticum and basioccipital bones at the level of the pharyngeal tubercle, dividing the right side of the clivus into two. This cleft was identified as an incomplete TBC (Fig. 1B, C). The present study was approved by Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University (COA. MURA2023/191).
This is the first report of TBC in an HFM patient. TBCs have been reported together with other anomalies or variations of skulls including atlas assimilation [4] and condylus tertius [5]. There are also syndromic cases of TBC such as those associated with CHARGE syndrome [6], Cornelia de Lange syndrome [7] and Chiari malformation [4]. The prevalence of TBC from ranges from 0% to 6%; most studies have reported a prevalence of less than 1% [8], although it is described more frequently in prehistoric populations [8]. It is occasionally observed on radiographs of paranasal sinuses [9] and could be misinterpreted as fractures or synchondroses. It is believed to be caused by disruption of the normal development of the clivus. The basilar part of the occipital bone is formed by fusion of the four occipital sclerotomes and parachordal cartilage [10]. Although earlier anatomists suggested there was a single ossification center in the basilar portion of the occipital bone, there could possibly be two ossification centers including praebasioccipital (anterior) and basioccipital (posterior) ossification centers [11].
Hence, it can be speculated that a TBC arises within the borderline between these two ossification centers, as mentioned by Psenner [12] (as reported by Lang [10]). Nevertheless, it remains to be investigated how clival deformities or variations are formed, particularly when HFM is involved.Even though the exact cause of HFM is uncertain, previous studies have shown that disruption of fetal development, genetic mutations, and environmental factors could be involved [13]. Most of the craniofacial structures affected in patients with HFM are derivatives of the first and second pharyngeal arches. The neural crest cells undergo an epithelial to mesenchymal transition from the neural tube to populate the pharyngeal arches, and cranial neural crest cells form the first and second pharyngeal arches. Those in the first pharyngeal arch form the zygomatic bone, maxilla, mandible, malleus and incus. Those in the second form the stapes and the facial nerve. The clivus, on the other hand, develops from the occipital sclerotomes and parachordal cartilage. Although the development of anterior cranial base has direct genetic links with development of facial elements, the involvement of posterior cranial base including the clivus is less understood. Distortion of the clivus is commonly identified in patients with cleidocranial dysplasia [14], a birth defect associated with RUNX2 mutation [15]. These data [14] indicated that underlying genetic mechanisms involving RUNX2 interactions may be implicated. Nevertheless, further research is needed to better explain the link between HFM and clival variations.
In conclusion, we report for the first time a TBC associated with HFM. Further studies are needed to explain the coexistence of these two conditions. Surgeons and radiologists should be aware of TBC as well as other clival variations or defects when presented with unfamiliar radiological findings.
Notes
References
1. Hartsfield JK. 2007; Review of the etiologic heterogeneity of the oculo-auriculo-vertebral spectrum (Hemifacial microsomia). Orthod Craniofac Res. 10:121–8. DOI: 10.1111/j.1601-6343.2007.00391.x. PMID: 17651128.
2. Murray JE, Kaban LB, Mulliken JB. 1984; Analysis and treatment of hemifacial microsomia. Plast Reconstr Surg. 74:186–99. DOI: 10.1097/00006534-198408000-00003. PMID: 6463144.
3. Jinkins JR. Atlas of neuroradiologic embryology, anatomy, and variants. Lippincott Williams & Wilkins;2000.
4. List CF. 1941; Neurologic syndromes accompanying developmental anomalies of occipital bone, atlas and axis. Arch Neurol Psychiatry. 45:577–616. DOI: 10.1001/archneurpsyc.1941.02280160009001.
5. Barth J. Norrønaskaller: crania antiqua in parte orientali Norvegiae meridionalis inventa: en studie fra universitetets anatomiske institut. A.W. Brøggers bogtrykkeri;1896.
6. Mahdi ES, Whitehead MT. 2018; Clival malformations in CHARGE syndrome. AJNR Am J Neuroradiol. 39:1153–6. DOI: 10.3174/ajnr.A5612. PMID: 29622552. PMCID: PMC7410616.
7. Whitehead MT, Nagaraj UD, Pearl PL. 2015; Neuroimaging features of Cornelia de Lange syndrome. Pediatr Radiol. 45:1198–205. DOI: 10.1007/s00247-015-3300-5. PMID: 25701113.
8. Tur SS, Svyatko SV, Rykun MP. 2019; Transverse basilar cleft: two more probable familial cases in an archaeological context. Int J Osteoarchaeol. 29:144–8. DOI: 10.1002/oa.2692.
9. Köhler A, Zimmer EA, Schmidt H. Grenzen des normalen und anfänge des pathologischen im röntgenbild des skeletts. Thieme;1989.
10. Lang J. Skull base and related structures: atlas of clinical anatomy. Schattauer;1995.
11. Hofmann E, Prescher A. 2012; The clivus: anatomy, normal variants and imaging pathology. Clin Neuroradiol. 22:123–39. DOI: 10.1007/s00062-011-0083-4. PMID: 21710384.
12. Psenner L. 1951; Die anatomischen varianten des hirnschädels. Röfo. 75:197–214. DOI: 10.1055/s-0029-1231984.
13. Taiwo AO. 2020; Classification and management of hemifacial microsomia: a literature review. Ann Ib Postgrad Med. 18:S9–15. PMID: 33071690. PMCID: PMC7513375.
14. Jensen BL. 1994; Cleidocranial dysplasia: craniofacial morphology in adult patients. J Craniofac Genet Dev Biol. 14:163–76. PMID: 7852545.
15. Lou Y, Javed A, Hussain S, Colby J, Frederick D, Pratap J, Xie R, Gaur T, van Wijnen AJ, Jones SN, Stein GS, Lian JB, Stein JL. 2009; A Runx2 threshold for the cleidocranial dysplasia phenotype. Hum Mol Genet. 18:556–68. DOI: 10.1093/hmg/ddn383. PMID: 19028669. PMCID: PMC2638795.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download