Abstract
Vitamin C is a well-known antioxidant with antiviral, anticancer, and anti-inflammatory properties based on its antioxidative function. Aptamin C, a complex of vitamin C with its specific aptamer, has been reported to maintain or even enhance the efficacy of vitamin C while increasing its stability. To investigate in vivo distribution of Aptamin C, Gulo knockout mice, which, like humans, cannot biosynthesize vitamin C, were administered Aptamin C orally for 2 and 4 weeks. The results showed higher vitamin C accumulation in all tissues when administered Aptamin C, especially in the spleen. Next, the activity of natural killer (NK) cells were conducted. CD69, a marker known for activating for NK cells, which had decreased due to vitamin C deficiency, did not recover with vitamin C treatment but showed an increasing with Aptamin C. Furthermore, the expression of CD107a, a cell surface marker that increases during the killing process of target cells, also did not recover with vitamin C but increased with Aptamin C. Based on these results, when cultured with tumor cells to measure the extent of tumor cell death, an increase in tumor cell death was observed. To investigate the signaling mechanisms and related molecules involved in the proliferation and activation of NK cells by Aptamin C showed that Aptamin C treatment led to an increase in intracellular STAT3 activation. In conclusion, Aptamin C has a higher capability to activate NK cells and induce tumor cell death compared to vitamin C and it is mediated through the activation of STAT3.
Vitamin C (L-ascorbic acid) is rapidly oxidized due to light, heat, and in aqueous solutions, so there have been numerous researches into developing stabilized forms of vitamin C to maintain or enhance its activity [1, 2]. There are various derivatives of vitamin C, each with unique characteristics in terms of stability, skin absorption rates, and efficacy. The most common derivatives include ascorbic acid 2-phosphate, magnesium ascorbyl phosphate, tetrahexyldecyl ascorbate, and ascorbyl glucoside [3-6]. These are much more stable than conventional form of vitamin C, but they have been developed mainly for use in the field of skin care such as skin whitening and wrinkle care [7]. For this reason, they are not suitable for medication through orally or intravenously administration to activate immune functions. In some cases, their high stability means that the inherent antioxidant function of vitamin C is relatively low [8, 9], which consequently reduces their effectiveness as a vitamin C, because vitamin C should rapidly be oxidized for its conventional anti-oxidant activity.
Recently, there is study that have focused on aptamers that bind to vitamin C to increase its stability and persistence in the body [10, 11]. Vitamin C in combination with aptamer is called as a “Aptamin C.” The reported efficacies of Aptamin C are mainly based on its anti-inflammatory function, one of the most representative characteristics of vitamin C. Effective anti-inflammatory functions through the suppression of inflammatory cytokines like interleukin (IL)-6 and tumor necrosis factor-α in skin inflammation models using house dust mite (HDM) extracts and the down-regulation of glial cell-derived neurotrophic factor, which is closely related with itching during HDM-induced skin inflammation, have been reported [12]. Moreover, reports suggest it may improve symptoms in degenerative brain disease animal models like Parkinson’s and Alzheimer’s, neuronal diseases that don’t yet have a significant effective treatment, indicating its potential as an effective treatment or supportive therapeutic agent for diseases [13].
However, there has not been research on Aptamin C related to the activation of immune functions, which is a representative function of vitamin C. The immune system is divided into innate and adaptive immunity, based on the specificity, diversity, and memory of immune system [14, 15]. It is further divided into humoral immunity, mediated by bodily fluids like antibodies and complement, and cell-mediated immunity, mediated by immune cells including natural killer (NK) cells and cytolytic T lymphocytes (CTLs) [16]. Cell-mediated immunity by NK cells and CTLs is known to be an important immune system that primarily acts in antiviral immunity against virus-infected cells and antitumor immunity against tumor cells [17]. The activation of these cells is primarily induced by cytokines such as IL-2, IL-15, IL-22, and IL-32 [18, 19]. However, the production of these cytokines is typically increased in pathological situations, and unregulated production can lead to other immune diseases [20, 21]. Therefore, maintaining a minimal state of NK cell and CTL activation before pathological situations develop and responding quickly and effectively when viral infections or tumors occur is crucial.
Nutrients and foods, such as ginseng rich in saponin and mushrooms rich in beta-glucan, are known to play roles in the activation of NK cells and CTLs [22, 23]. Vitamin C is well-known for inducing the activation of NK cells and CTLs, with its effective antiviral and anticancer efficacy reported in both in vitro and in vivo studies involving animals [24]. Despite some controversy, cohort studies involving humans have also reported that vitamin C shows effective antiviral and anticancer efficacy [25-27]. Based on these facts, this study aimed to examine the activation level of immune cells, focusing on NK cells, by orally administering Aptamin C to Gulo knockout (KO) mice, which cannot synthesize vitamin C like humans and thus must receive vitamin C externally to sustain life, in a human-like environment.
Gulo (–/–) KO mice (Strain: C57BL/6) were interbred and housed under specific pathogen-free conditions at the animal facility of the Seoul National University College of Medicine. After vitamin C supplementation was discontinued for 3 weeks, the KO mice were maintained with the oral administration of vitamin C (3.3 g/L) and Aptamin C (vitamin C 3.3 g/L, aptamer 0.033 g/L) for 4 weeks. Animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Seoul National University College of Medicine animal facility (IACUC no. SNU-230601-2-2).
Aptamer were dissolved in phosphate buffered saline (PBS) containing 1 mM of MgCl2 and heated for 30 minutes at 60°C to unfold aptamer. The production of Aptamin C for the purpose of verifying its efficacy in relation to the distribution of vitamin C within the body through oral administration is as follows. L-ascorbic acid (Sigma) was added to the aptamer in a ratio of 1:100 (w/w) and mixture sit for 30 minutes at room temperature (RT) to enable the aptamers to fold into their tertiary structures. L-ascorbic acid (Sigma) was added to the aptamer in a ratio of 1:50 (w/w) and mixture sit for 30 minutes at RT to enable the aptamers to fold into their tertiary structures. Aptamin C were stored at 4°C.
After orally administering vitamin C and Aptamin C, the stomach, lungs, liver, brain, spleen, and adrenal glands were harvested at 2 and 4 weeks, quickly frozen in liquid nitrogen, and then stored at –70°C until use. After measuring the weight, the tissues were homogenized with TissueLyser II (Qiagen) in PBS. The homogenates were centrifuged at 14,000 rpm for 30 minutes at 4°C, and the supernatants were used for measuring vitamin C. The tissue homogenates were diluted in PBS, and vitamin C (ascorbic acid) was converted into its oxidized form, dehydroascorbic acid (DHA), after which the total DHA concentration was measured using a colorimetric microtiter plate assay kit (Immundiagnostik AG) according to the manufacturer’s instructions. The final concentration of vitamin C in each tissue was normalized to tissue weight.
NK cells from Gulo (–/–) KO mice were isolated from splenocytes. NK cells were then purified from splenocytes utilizing the NK cell isolation kit (Miltenyi Biotec). To separate NK cell in splenocyte, negative selection was performed by auto MACS pro separator (Miltenyi Biotec) to obtain NK cell in splenocytes. To examine the purity of NK cells, staining with fluorescein isothiocyanate (FITC)-conjugated anti-CD3e (BD science) and APC-conjugated anti-NK1.1 (BD science) was performed. Purified NK cells were used in cytotoxicity assay.
To observe the proliferation inhibition of Aptamin C treated NK-92, NK-92 was seeded in the 96-well plate at 1×104 cells per well and pretreated with inhibitor STAT3 50 μM (S3I-201; Sigma), inhibitor ERK 20 μM (PD98059; Sigma). NK-92 was treated in presence and absence of 20 μg/ml of aptamin C and treated with IL-2 25 IU/ml for 48 hours. To examine the mechanism of NK-92 proliferation, the Alamar Blue assay was performed. After 48 hours, 10% of the total volume of Alamar Blue reagent (Serotec) was added to each well of the plate. The plate was incubated for 6 hours at 37°C absence of light. The absorbance was measured at 570 and 600 nm using the SpectraMax iD3 and normalized with Softmax Pro software (Molecular Devices). The proliferation formula is as follows: % Reduction=[{(Absorbance 570 nm sample–Absorbance 570 nm background)–(Absorbance 600 nm sample–Absorbance 600 nm background)}/{(Absorbance 570 nm control–Absorbance 570 nm background)– (Absorbance 600 nm control–Absorbance 600 nm background)}]×100.
NK cells were obtained from Gulo (–/–) mice that were administered vitamin C (3.3 g/L) or Aptamin C (vitamin C 3.3 g/L, aptamer 33 mg/L) for 4 weeks following 3 weeks of vitamin C depletion. YAC-1 target cells were labeled by staining PKH-26 (Sigma) for 5 minutes at RT in the dark and then washed with complete RPMI media. NK cells (effector cells) were seeded in 96 well U-bottom plates into 5:1 and 10:1 effector–target (E/T) ratios. After co-culture with YAC-1 for 4 hours at 37°C in the atmosphere of 5% CO2, the co-cultured samples were stained with 7-aminoactinomycin D (BD Science) for detection of dead cells in the samples. Flow cytometry analysis was performed using Flowjo software (BD Science) to analyze the data. The assay was conducted in triplicate for E/T ratios. In these experiments, spontaneous lysis of YAC-1 never exceeded 6%.
Splenocytes were obtained from Gulo (–/–) mice. These mice were given vitamin C (3.3 g/L) or Aptamin C (vitamin C 3.3 g/L, aptamer 33 mg/L) for 4 weeks following 3 weeks of vitamin C depletion. To detect CD107a surface marker expression, splenocytes were incubated with YAC-1 target cells at 100:1 E/T ratio in the presence of FITC-labeled anti-CD107a Ab (BD Science) for 1 hour in 96 well U-bottom plates. Subsequently, Brefeldin A/Monensin cocktail (Invitrogen) was added for an additional 3 hours of incubation. To detect CD69 surface marker expression, splenocytes and YAC-1 were co-cultured at an E/T ratio of 100:1 for 4 hours. After co-culture, cells are stained with FITC-labeled anti-CD69 Ab (BD Science). Expressions of CD69 and CD107a which are known to be activation markers of NK cells were assessed by flow cytometry. To analyze the data, analysis was performed using Flowjo software (BD Science). The assay was conducted in triplicate for E/T ratios.
Human NK cell line, NK-92 and splenocytes from Gulo (–/–) KO mice were cultured with vitamin C (20 μg/ml), aptamer (0.2 μg/ml) and Aptamin C (a mixture of vitamin C [20 μg/ml] with aptamer [0.2 μg/ml] in a ratio of 1:100 [w/w]) for 30 minutes. And then washed three times with PBS and lysed with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP40, 0.25% sodium deoxycholate, and 1 mM EDTA) supplemented with a protease inhibitor cocktails (Sigma). Protein concentration was determined with the BCA protein assay. Equal amounts of protein (30 μg/sample) from cell lysates were electrophoretically separated on 10% polyacrylamide-SDS gel with 100 V for 3 hours. The transfer of proteins from 10% SDS to nitrocellulose membrane (Millipore) was performed with 750 mA for 1 hour 30 minutes on ice. After the transfer, membranes were blocked with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBS-T) for 1 hour at RT. The blocked membrane was incubated with 5% nonfat milk, washed with 0.1% Tween 20-PBS for 1 hour, and then exposed to primary antibody (rabbit anti-human phosphorylation of STAT3 (p-STAT3) antibody [1:2,000; Cell Signaling], rabbit anti-human STAT3 antibody [1:2,000; Cell Signaling] or mouse anti-human β-actin antibody [1:5,000; Santa Cruz Biotechnology]) in 0.1% Tween-20-PBS at RT for 1 hour. After the blots were washed, they were exposed to anti-rabbit IgG antibodies (1:5,000; Cell Signaling Technology) for detection of p-STAT3 and STAT3 or with HRP-conjugated anti-mouse IgG antibody (1:10,000; Cell Signaling) for detection of β-actin as a secondary antibody at RT for 1 hour. The membranes were washed three times for 10 minutes each wash and exposed to X-ray film after treatment with Lumi La, Femto (DoGenBio) detection reagents for visualization of immunoreactive proteins. The bands were analyzed for the density using the Image J software (NIH).
Data are presented as the mean±SD. To evaluate the level of vitamin C levels in tissues, One-way analysis of variance (ANOVA) was performed when multiple groups were compared. Unpaired t-tests were applied to assess the relation between proliferation and Aptamin C in vitro. Statistical analysis was performed using the Prism software (GraphPad Software).
We investigated how the absorption rate and duration of vitamin C in vivo differ when administered as Aptamin C compared to vitamin C in its conventional form. Gulo KO mice, which, like humans, cannot synthesize vitamin C by themselves, were deprived of vitamin C for 3 weeks to lower their internal vitamin C levels. Afterwards, they were orally administered vitamin C and Aptamin C for 2 and 4 weeks. And then, the amount of vitamin C in each organ, stomach, liver, lungs, spleen, and adrenal glands at 2 and 4 weeks after administration was measured. Even in the group given vitamin C in its conventional form, an increase in the amount of vitamin C in each organ was observed at 4 weeks compared to 2 weeks. Interestingly, in the case of administration over 4 weeks, the groups administered with Aptamin C showed higher levels of vitamin C in each organ compared to those given vitamin C in its conventional form (Fig. 1). This suggests that administering vitamin C in the form of Aptamin C results in a longer duration and increased absorption rate of vitamin C in the body compared to a vitamin C in its conventional form.
According to the results presented in Fig. 1, due to the high accumulation of vitamin C in the spleen from the oral administration of Aptamin C, it was desired to examine if the activity of immune cells present in the spleen also increased. We first measured and compared the weights of spleens extracted from the mice in each experimental group. There was a slight trend towards an increase in the groups treated with vitamin C, aptamer, and Aptamin C compared to the control group (control: 0.33±0.07 g, vitamin C: 0.36±0.03 g, aptamer: 0.35±0.03 g, Aptamin C: 0.35±0.03 g), but this was not statistically significant. Furthermore, there were no significant differences observed among these three groups (data not shown). Among the immune cells, changes centered on NK cells and CTLs, which are important for antiviral and anti-cancer immunity, were examined through the changes on the expression of CD69, a marker that increases at the early stage of activation, and CD107a, a marker that increases with the increase of apoptosis capability of immune cells. As a result, the oral administration of vitamin C did not increase the expression of CD69. However, it was confirmed that the oral administration of Aptamin C increased the expression of CD69 (Fig. 2). Similarly, for CD107a, the oral administration of vitamin C did not induce an increase in CD107a, but a significant increase was observed with the oral administration of Aptamin C (Fig. 2).
Based on the results from Fig. 2, the tumor cell-killing ability of NK cells isolated from Gulo KO mice administered with Aptamin C orally was compared with that of NK cells isolated from mice administered with vitamin C. For this, spleen cells from Gulo KO mice administered with vitamin C and Aptamin C were isolated, and then NK cells were isolated and cultured with tumor cells YAC-1 as a target cells at E:T ratios of 5:1 and 10:1, after which the extent of YAC-1 cell apoptosis was analyzed. At both 5:1 and 10:1 E:T ratios, the killing ability of NK cells obtained from mice administered with Aptamin C was observed to be higher than that of NK cells obtained from mice administered with vitamin C (Fig. 3).
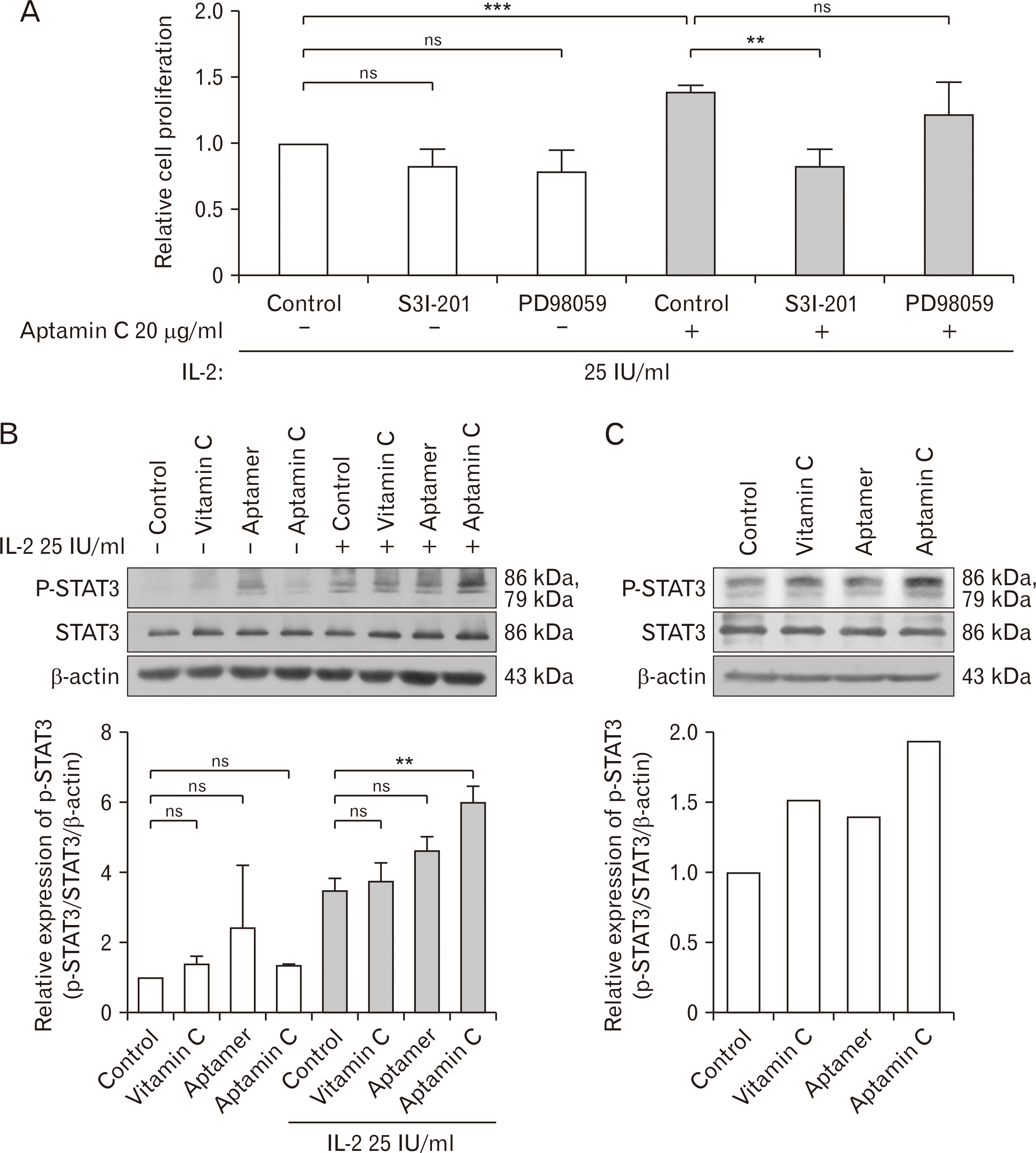
To investigate the signaling mechanisms and related molecules associated with the proliferation of NK cells by Aptamin C, groups treated without IL-2 (a cytokine essential for NK cell proliferation and activation) and groups treated with IL-2 at a concentration that maintains baseline levels of NK cell proliferation were given treatments of vitamin C, an aptamer, and Aptamin C. The results showed an increase in NK cell proliferation in the group treated with both IL-2 and Aptamin C. Subsequently, to identify the intracellular signaling factors involved in NK cell proliferation by Aptamin C, cells were pre-treated with specific inhibitors for STAT3, and ERK for 1 hour before treatment of Aptamin C. As shown in Fig. 4A, NK cell proliferation was inhibited in the cells treated with the STAT3 inhibitor S3I-201, indicating that Aptamin C-induced NK cell proliferation is dependent on STAT3 activation. This was confirmed at the protein level by treating the NK-92 cell line with Aptamin C and performing immunoblotting, which showed increased activation of STAT3 (Fig. 4B). Similarly, immunoblotting of NK cells from Gulo KO mice administered with Aptamin C confirmed increased STAT3 activation (Fig. 4C), consistent with the findings in the NK-92.
Vitamin C, known as ascorbic acid, is a potent antioxidant that plays diverse roles within the human body [28]. It supports the function of the immune system and is crucial in aiding the growth and repair of tissues [29]. Furthermore, vitamin C enhances the absorption of iron and aids in the synthesis of collagen [30-32], essential for maintaining the health of skin, bones, and connective tissues [33]. We have been conducting studies on the antiviral, anticancer, and anti-inflammatory effects of vitamin C over the past years [2, 34]. Vitamin C is known to regulate the production of pro-inflammatory cytokines including IL-6, IL-18, and IL-22 and effectively inhibiting the occurrence and progression of several kinds of inflammatory diseases and cancer [35, 36].
NK cells are part of the innate immune system and are cells that possess potent antiviral and anticancer effects without being dependent on antigens [37-39]. Therefore, inducing NK cell activity is a crucial concept in immunotherapeutic approaches aimed at treating viral infections and cancer. However, excessive induction of NK cell activity can lead to serious autoimmune diseases, accompanied by severe damage to tissues or organs, hence NK cell activity must be induced within appropriate limits [40]. Antioxidants, including vitamin C, are well-known substances that can induce immune activity within these limits, and previous studies have reported that vitamin C can effectively enhance antiviral and anticancer efficacy through the activation of NK cells and overall immune activity [34]. Nevertheless, vitamin C rapidly oxidizes when in solution or exposed to air, and although various studies have been conducted to compensate for this, applying it effectively in vivo still presents challenges. In this regard, Aptamin C used in this study is considered a promising candidate to overcome the limitations of vitamin C.
Aptamers are single-stranded DNA or RNA oligonucleotides with high affinity and specificity for their target molecules [12, 41, 42]. They can selectively bind to specific targets both inside and outside the body, making them useful in diagnostics, therapeutic development, and biological research [43]. Aptamers are particularly valuable due to their high binding specificity, adjustable biocompatibility, and relatively low immunogenicity [42, 44]. An example of an aptamer-based therapeutic is Macugen (pegaptanib), used to treat age-related macular degeneration (AMD) [45, 46]. This drug specifically binds to the vascular endothelial growth factor contributing to vision loss in AMD, inhibiting its function [46, 47]. Thus, Macugen suppresses abnormal blood vessel growth and reduces macular edema, slowing vision loss in AMD patients. Aptamers binding specifically to vitamin C can effectively inhibit the rapid oxidation of vitamin C, neutralizing free radicals and preventing cell damage more effectively [10, 47]. In other words, aptamers form a protective layer around vitamin C, preventing it from reacting with oxygen or other oxidizing agents, enhancing the stability of vitamin C, and maintaining its antioxidant effects over an extended period. This maximizes the health benefits of vitamin C, including its anti-inflammatory, antiviral, and skin-protective properties. The complex of aptamers and vitamin C, termed Aptamin C, could be utilized in developing more stable vitamin C formulations, such as injectables or oral supplements [11].
For vitamin C to be effective, the concentration accumulated in organs is considered very important, perhaps even more so than its concentration in the blood [48]. As shown in Fig. 1, the concentration of vitamin C in various organs of the body demonstrates that orally ingested vitamin C is absorbed through the digestive system and then accumulates in various organs where it is used as needed. Particularly, the accumulation in the spleen, an immune organ, clearly explains the efficacy of vitamin C in activating immune cells including NK cells within these organs. Moreover, administering vitamin C in the form of Aptamin C leads to higher organ accumulation compared to administering vitamin C in its typical form, highlighting the advantage of Aptamin C over vitamin C in activating immune functions, especially the activity of NK cells. Specifically, in Gulo KO mice, which like humans cannot synthesize vitamin C endogenously, when vitamin C and Aptamin C were orally administered, and spleen cells were obtained and NK cells isolated and cultured with tumor cells, the group administered Aptamin C showed higher expression of the NK activation marker CD69 and the NK killing marker CD107a, as well as a higher direct killing capacity of target cells compared to the group given vitamin C. This suggests that intake of Aptamin C could be expected to enhance NK cell activity and its based antitumor capabilities (Figs. 2, 3).
It has been reported that the activation of immune cells, including NK cells, is mediated through the activation of STAT3 or ERK [35, 49], and our previous research has indicated that the anti-inflammatory effects of vitamin C are mediated through the activation of ERK. Based on these findings, additional research was conducted to identify the intracellular mediators involved in the activation of NK cells by Aptamin C. As shown in Fig. 4A, treating the NK cell line, NK-92 with specific inhibitors for STAT3 or ERK showed no significant difference in the proliferation of NK-92. However, when treated with baseline levels of IL-2 and Aptamin C, a remarkable increase in NK cell proliferation was observed, and this proliferation was effectively inhibited by the treatment with a specific inhibitor for STAT3, not by a specific inhibitor of ERK. These results indicate that Aptamin C induces the activation of STAT3 in NK cells, which was also confirmed by immunoblotting using NK-92 treated with Aptamin C and splenocytes isolated from Gulo KO mice administered with Aptamin C.
In conclusion, Aptamin C not only stabilizes vitamin C but also possesses characteristics of higher accumulation in organs compared to vitamin C, and it induces the activation of immune cells, especially NK cells, which play crucial roles in antiviral and anticancer immunity, through the activation of STAT3. Therefore, it is considered that Aptamin C can be effectively used as an adjuvant substance in enhancing immune function targeting NK cells and in NK cell-based anticancer therapies in the future.
Notes
References
1. Caritá AC, Fonseca-Santos B, Shultz JD, Michniak-Kohn B, Chorilli M, Leonardi GR. 2020; Vitamin C: one compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine. 24:102117. DOI: 10.1016/j.nano.2019.102117. PMID: 31676375.
2. Meščić Macan A, Gazivoda Kraljević T, Raić-Malić S. 2019; Therapeutic perspective of vitamin C and its derivatives. Antioxidants (Basel). 8:247. DOI: 10.3390/antiox8080247. PMID: 31357509. PMCID: PMC6721080.
3. Takamizawa S, Maehata Y, Imai K, Senoo H, Sato S, Hata R. 2004; Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biol Int. 28:255–65. DOI: 10.1016/j.cellbi.2004.01.010. PMID: 15109981.
4. Campos PM, Gonçalves GM, Gaspar LR. 2008; In vitro antioxidant activity and in vivo efficacy of topical formulations containing vitamin C and its derivatives studied by non-invasive methods. Skin Res Technol. 14:376–80. DOI: 10.1111/j.1600-0846.2008.00288.x. PMID: 19159387.
5. Swindell WR, Randhawa M, Quijas G, Bojanowski K, Chaudhuri RK. 2021; Tetrahexyldecyl ascorbate (THDC) degrades rapidly under oxidative stress but can be stabilized by acetyl zingerone to enhance collagen production and antioxidant effects. Int J Mol Sci. 22:8756. DOI: 10.3390/ijms22168756. PMID: 34445461. PMCID: PMC8395926.
6. Yamamoto I, Tai A, Fujinami Y, Sasaki K, Okazaki S. 2002; Synthesis and characterization of a series of novel monoacylated ascorbic acid derivatives, 6-O-acyl-2-O-alpha-D-glucopyranosyl-L-ascorbic acids, as skin antioxidants. J Med Chem. 45:462–8. DOI: 10.1021/jm010379f. PMID: 11784150.
7. Ravetti S, Clemente C, Brignone S, Hergert L, Allemandi D, Palma S. 2019; Ascorbic acid in skin health. Cosmetics. 6:58. DOI: 10.3390/cosmetics6040058.
8. Weeks BS, Perez PP. 2007; Absorption rates and free radical scavenging values of vitamin C-lipid metabolites in human lymphoblastic cells. Med Sci Monit. 13:BR205–10. PMID: 17901843.
9. Pinnell SR, Yang H, Omar M, Monteiro-Riviere N, DeBuys HV, Walker LC, Wang Y, Levine M. 2001; Topical L-ascorbic acid: percutaneous absorption studies. Dermatol Surg. 27:137–42. DOI: 10.1097/00042728-200102000-00008. PMID: 11207686.
10. Song MK, Lee JH, Kim J, Kim JH, Hwang S, Kim YS, Kim YJ. 2021; Neuroprotective effect of NXP031 in the MPTP-induced Parkinson's disease model. Neurosci Lett. 740:135425. DOI: 10.1016/j.neulet.2020.135425. PMID: 33075422.
11. Choi S, Han J, Kim JH, Kim AR, Kim SH, Lee W, Yoon MY, Kim G, Kim YS. 2020; Advances in dermatology using DNA aptamer "Aptamin C" innovation: oxidative stress prevention and effect maximization of vitamin C through antioxidation. J Cosmet Dermatol. 19:970–6. DOI: 10.1111/jocd.13081. PMID: 31353789. PMCID: PMC7154658.
12. Lee D, Kim Y, Jo H, Go C, Jeong Y, Jang Y, Kang D, Park K, Kim YS, Kang JS. 2021; The anti-inflammatory effect of Aptamin C on house dust mite extract-induced inflammation in keratinocytes via regulation of IL-22 and GDNF production. Antioxidants (Basel). 10:945. DOI: 10.3390/antiox10060945. PMID: 34208021. PMCID: PMC8230602.
13. Kong AH, Wu AJ, Ho OK, Leung MM, Huang AS, Yu Y, Zhang G, Lyu A, Li M, Cheung KH. 2023; Exploring the potential of aptamers in targeting neuroinflammation and neurodegenerative disorders: opportunities and challenges. Int J Mol Sci. 24:11780. DOI: 10.3390/ijms241411780. PMID: 37511539. PMCID: PMC10380291.
14. Delves PJ. Rose NR, Mackay IR, editors. Innate and adaptive systems of immunity. The Autoimmune Diseases. 6th ed. Academic Press;2020. p. 45–61. DOI: 10.1016/B978-0-12-812102-3.00004-X.
15. Vivier E, Malissen B. 2005; Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nat Immunol. 6:17–21. DOI: 10.1038/ni1153. PMID: 15611777. PMCID: PMC7097365.
16. Khan MM. Khan MM, editor. Overview of the immune response. Immunopharmacology. Springer;2016. p. 1–55. DOI: 10.1007/978-3-319-30273-7_1.
17. Nutt SL, Huntington ND. Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Cytotoxic T lymphocytes and natural killer cells. Clinical Immunology. 5th ed. Elsevier;2019. p. 247–59.e1. DOI: 10.1016/B978-0-7020-6896-6.00017-X.
18. Guglielmo A, Zengarini C, Agostinelli C, Motta G, Sabattini E, Pileri A. 2024; The role of cytokines in cutaneous T cell lymphoma: a focus on the state of the art and possible therapeutic targets. Cells. 13:584. DOI: 10.3390/cells13070584. PMID: 38607023. PMCID: PMC11012008.
19. Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. 2011; Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 127:701–21. e1–70. DOI: 10.1016/j.jaci.2010.11.050. PMID: 21377040.
20. Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Röcken M. 1994; Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 180:1961–6. DOI: 10.1084/jem.180.5.1961. PMID: 7525845. PMCID: PMC2191757.
21. Yoshimoto T, Yoshimoto T. Cytokine frontiers: regulation of immune responses in health and disease. Springer;2013. DOI: 10.1007/978-4-431-54442-5.
22. Kim H, Jang M, Kim Y, Choi J, Jeon J, Kim J, Hwang YI, Kang JS, Lee WJ. 2016; Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J Pharm Pharmacol. 68:406–20. DOI: 10.1111/jphp.12529. PMID: 26898166.
23. Kim JH, Kim DH, Jo S, Cho MJ, Cho YR, Lee YJ, Byun S. 2022; Immunomodulatory functional foods and their molecular mechanisms. Exp Mol Med. 54:1–11. DOI: 10.1038/s12276-022-00724-0. PMID: 35079119. PMCID: PMC8787967.
24. Jeong YJ, Kim JH, Hong JM, Kang JS, Kim HR, Lee WJ, Hwang YI. 2014; Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8(+) memory T cell production capacity of these cells in vivo. Immunobiology. 219:554–64. DOI: 10.1016/j.imbio.2014.03.006. PMID: 24698552.
25. Grudzien M, Rapak A. 2018; Effect of natural compounds on NK cell activation. J Immunol Res. 2018:4868417. DOI: 10.1155/2018/4868417. PMID: 30671486. PMCID: PMC6323526.
26. Toliopoulos I, Simos Y, Verginadis I, Oikonomidis S, Karkabounas S. 2012; NK cell stimulation by administration of vitamin C and Aloe vera juice in vitro and in vivo: a pilot study. J Herb Med. 2:29–33. DOI: 10.1016/j.hermed.2012.04.002.
27. Huijskens MJ, Walczak M, Sarkar S, Atrafi F, Senden-Gijsbers BL, Tilanus MG, Bos GM, Wieten L, Germeraad WT. 2015; Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy. 17:613–20. DOI: 10.1016/j.jcyt.2015.01.004. PMID: 25747742.
28. Mandl J, Szarka A, Bánhegyi G. 2009; Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 157:1097–110. DOI: 10.1111/j.1476-5381.2009.00282.x. PMID: 19508394. PMCID: PMC2743829.
29. Carr AC, Maggini S. 2017; Vitamin C and immune function. Nutrients. 9:1211. DOI: 10.3390/nu9111211. PMID: 29099763. PMCID: PMC5707683.
30. Jacob RA, Sotoudeh G. 2002; Vitamin C function and status in chronic disease. Nutr Clin Care. 5:66–74. DOI: 10.1046/j.1523-5408.2002.00005.x. PMID: 12134712.
31. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. 1981; Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 78:2879–82. DOI: 10.1073/pnas.78.5.2879. PMID: 6265920. PMCID: PMC319462.
32. Stone N, Meister A. 1962; Function of ascorbic acid in the conversion of proline to collagen hydroxyproline. Nature. 194:555–7. DOI: 10.1038/194555a0. PMID: 13917472.
33. Davey MW, Montagu MV, Inzé D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJ, Strain JJ, Favell D, Fletcher J. 2000; Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric. 80:825–60. DOI: 10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6.
34. Kim Y, Kim H, Bae S, Choi J, Lim SY, Lee N, Kong JM, Hwang YI, Kang JS, Lee WJ. 2013; Vitamin C Is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 13:70–4. DOI: 10.4110/in.2013.13.2.70. PMID: 23700397. PMCID: PMC3659258.
35. Jo H, Lee D, Go C, Jang Y, Chu N, Bae S, Kang D, Im JP, Kim Y, Kang JS. 2022; Preventive effect of vitamin C on dextran sulfate sodium (DSS)-induced colitis via the regulation of IL-22 and IL-6 production in Gulo(-/-) mice. Int J Mol Sci. 23:10612. DOI: 10.3390/ijms231810612. PMID: 36142515. PMCID: PMC9505994.
36. Boretti A, Banik BK. 2020; Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 12:100190. DOI: 10.1016/j.phanu.2020.100190. PMID: 32322486. PMCID: PMC7172861.
37. Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. 1999; Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 17:189–220. DOI: 10.1146/annurev.immunol.17.1.189. PMID: 10358757.
38. Bald T, Krummel MF, Smyth MJ, Barry KC. 2020; The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. 21:835–47. DOI: 10.1038/s41590-020-0728-z. PMID: 32690952. PMCID: PMC8406687.
39. Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. 2015; NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 6:368. DOI: 10.3389/fimmu.2015.00368. PMID: 26284063. PMCID: PMC4515552.
40. Poggi A, Zocchi MR. 2014; NK cell autoreactivity and autoimmune diseases. Front Immunol. 5:27. DOI: 10.3389/fimmu.2014.00027. PMID: 24550913. PMCID: PMC3912987.
41. Röthlisberger P, Hollenstein M. 2018; Aptamer chemistry. Adv Drug Deliv Rev. 134:3–21. DOI: 10.1016/j.addr.2018.04.007. PMID: 29626546.
42. Wu Z, Tang LJ, Zhang XB, Jiang JH, Tan W. 2011; Aptamer-modified nanodrug delivery systems. ACS Nano. 5:7696–9. DOI: 10.1021/nn2037384. PMID: 22023403. PMCID: PMC3245875.
43. Iliuk AB, Hu L, Tao WA. 2011; Aptamer in bioanalytical applications. Anal Chem. 83:4440–52. DOI: 10.1021/ac201057w. PMID: 21524128. PMCID: PMC3115435.
44. Song KM, Lee S, Ban C. 2012; Aptamers and their biological applications. Sensors (Basel). 12:612–31. DOI: 10.3390/s120100612. PMID: 22368488. PMCID: PMC3279232.
45. Kalathingal M, Rhee YM. 2023; Molecular mechanism of binding between a therapeutic RNA aptamer and its protein target VEGF: a molecular dynamics study. J Comput Chem. 44:1129–37. DOI: 10.1002/jcc.27070. PMID: 36625560.
46. Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR. 2004; Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 351:2805–16. DOI: 10.1056/NEJMoa042760. PMID: 15625332.
47. Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP. 2006; Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 5:123–32. DOI: 10.1038/nrd1955. PMID: 16518379.
48. Kim H, Bae S, Yu Y, Kim Y, Kim HR, Hwang YI, Kang JS, Lee WJ. 2012; The analysis of vitamin C concentration in organs of gulo(-/-) mice upon vitamin C withdrawal. Immune Netw. 12:18–26. DOI: 10.4110/in.2012.12.1.18. PMID: 22536166. PMCID: PMC3329599.
49. Jung SA, Lee DH, Moon JH, Hong SW, Shin JS, Hwang IY, Shin YJ, Kim JH, Gong EY, Kim SM, Lee EY, Lee S, Kim JE, Kim KP, Hong YS, Lee JS, Jin DH, Kim T, Lee WJ. 2016; L-ascorbic acid can abrogate SVCT-2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Free Radic Biol Med. 95:200–8. DOI: 10.1016/j.freeradbiomed.2016.03.009. PMID: 27012422.
Fig. 1
Comparison of vitamin C levels in the tissues of Gulo knockout (KO) mice treated with vitamin C and Aptamin C. Vitamin C levels were measured in the stomach, spleen, brain, liver, lung, and adrenal gland of Gulo KO mice (n=5) supplemented with vitamin C (3.3 g/L) and Aptamin C (vitamin C 3.3 g/L, aptamer 33 mg/L) for 2 and 4 weeks. Vitamin C level was measured by using a colorimetric microtiter plate assay kit as described in Materials and Methods. Data were analyzed by one-way ANOVA with Tukey’s multiple comparison test and presented as the mean±SD. Three independent experiments were performed. ns, not significant; WT, wild type. *P<0.05, **P<0.01, ***P<0.001.
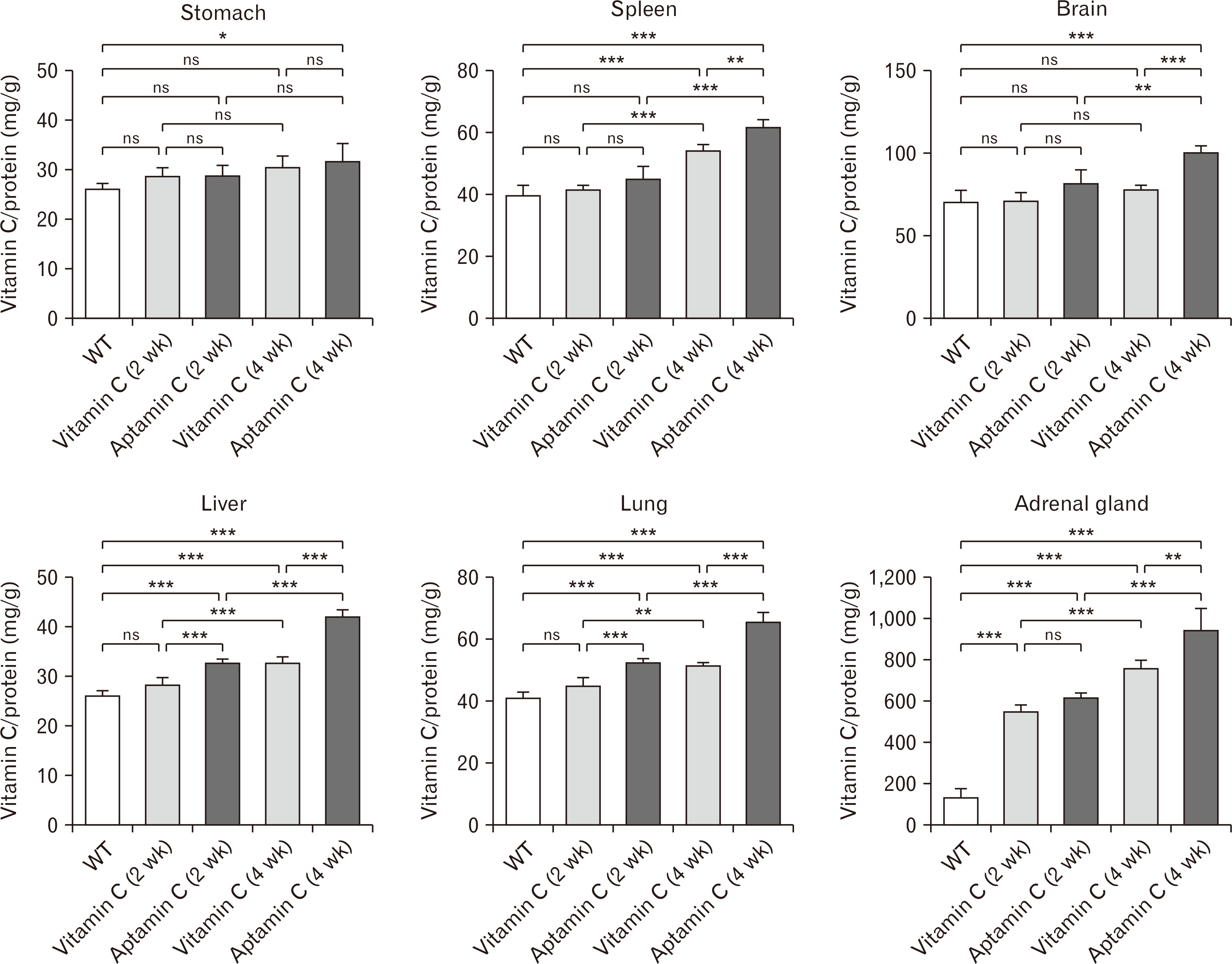
Fig. 2
Effect of Aptamin C on expression of surface activation marker in immune cell. Splenocytes were isolated from Gulo knockout (KO) mice (n=4) supplemented vitamin C (3.3 g/L) and Aptamin C (vitamin C 3.3 g/L, aptamer 33 mg/L) for 2 and 4 weeks. Splenocytes were stained with antibodies specific to CD69 and CD107a as described in Materials and Methods. Representative flow cytometry graph is shown in CD69 expression (A) and CD107a expression (B). The CD69 and CD107a positive cell percentage represents on flow cytometry histogram. The presented data represent data analyzed from splenocytes of the four mice used in the experiment. Data were analyzed by one-way ANOVA with Tukey’s multiple comparison test and presented as the mean±SD. ns, not significant. *P<0.05, **P<0.01.
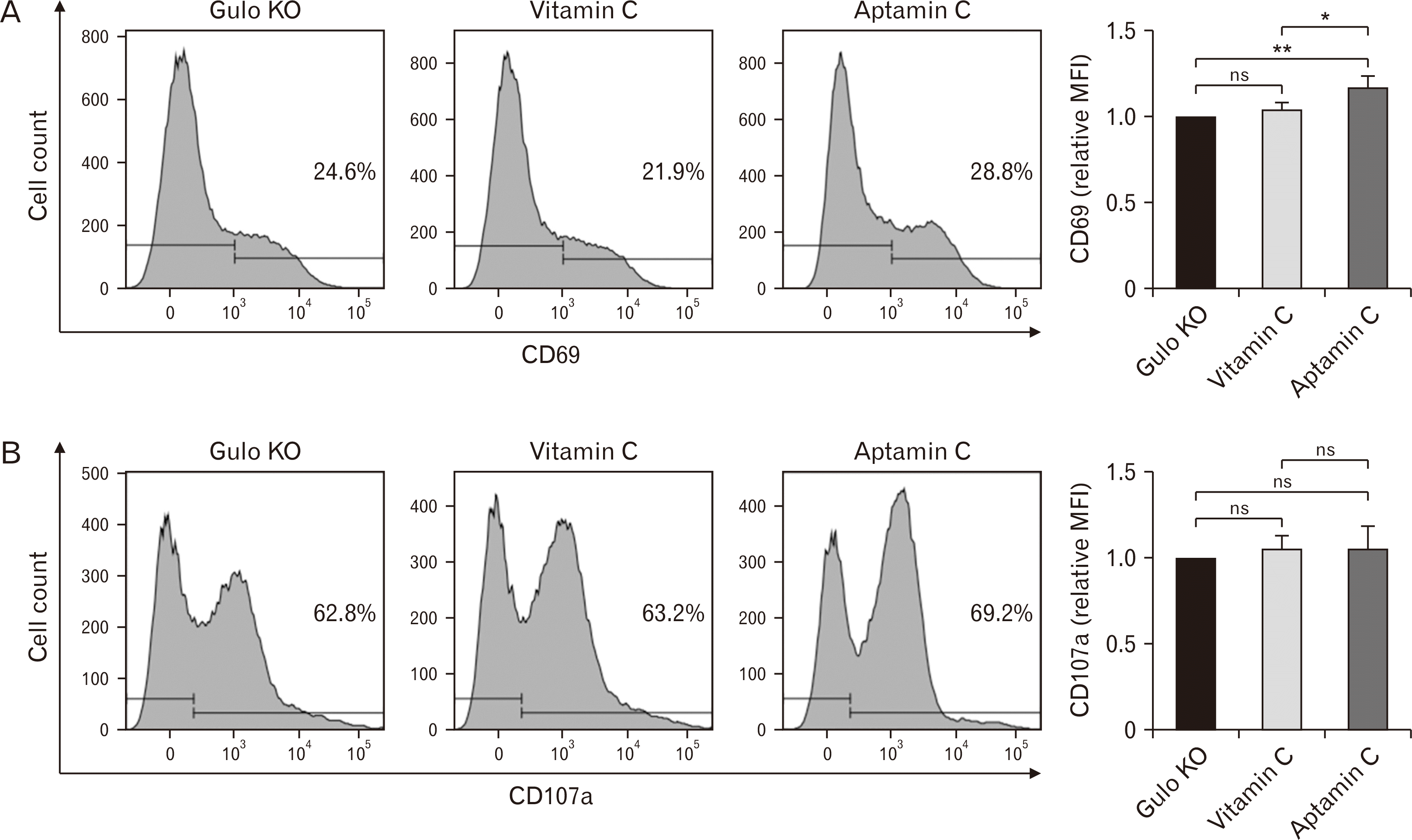
Fig. 3
Cytotoxicity of natural killer (NK) cells in Gulo knockout (KO) mice supplemented with vitamin C and Aptamin C. Splenocytes were isolated from Gulo KO mice (n=4) supplemented vitamin C (3.3 g/L) and Aptamin C (vitamin C 3.3 g/L, aptamer 33 mg/L) for 2 or 4 weeks. And then NK cells were purified from the isolated splenocytes as described in Materials and Methods. Target cell, YAC-1 were prepared after labelling by staining with PKH-26 to distinguish YAC-1 from NK in flow cytometry analysis. Four hours co-culture of PKH26 labelled YAC-1 with NK cells with 5:1, 10:1 effector–target ratio, 7-aminoactinomycin D (AAD) was stained for detection dead cell in the samples. Double positive cells with PKH26 and 7-AAD were gated and analyzed flow cytometry analysis. Dead cells were selected based on the YAC-1 target cells. The presented data represent data analyzed from splenocytes of the four mice used in the experiment.
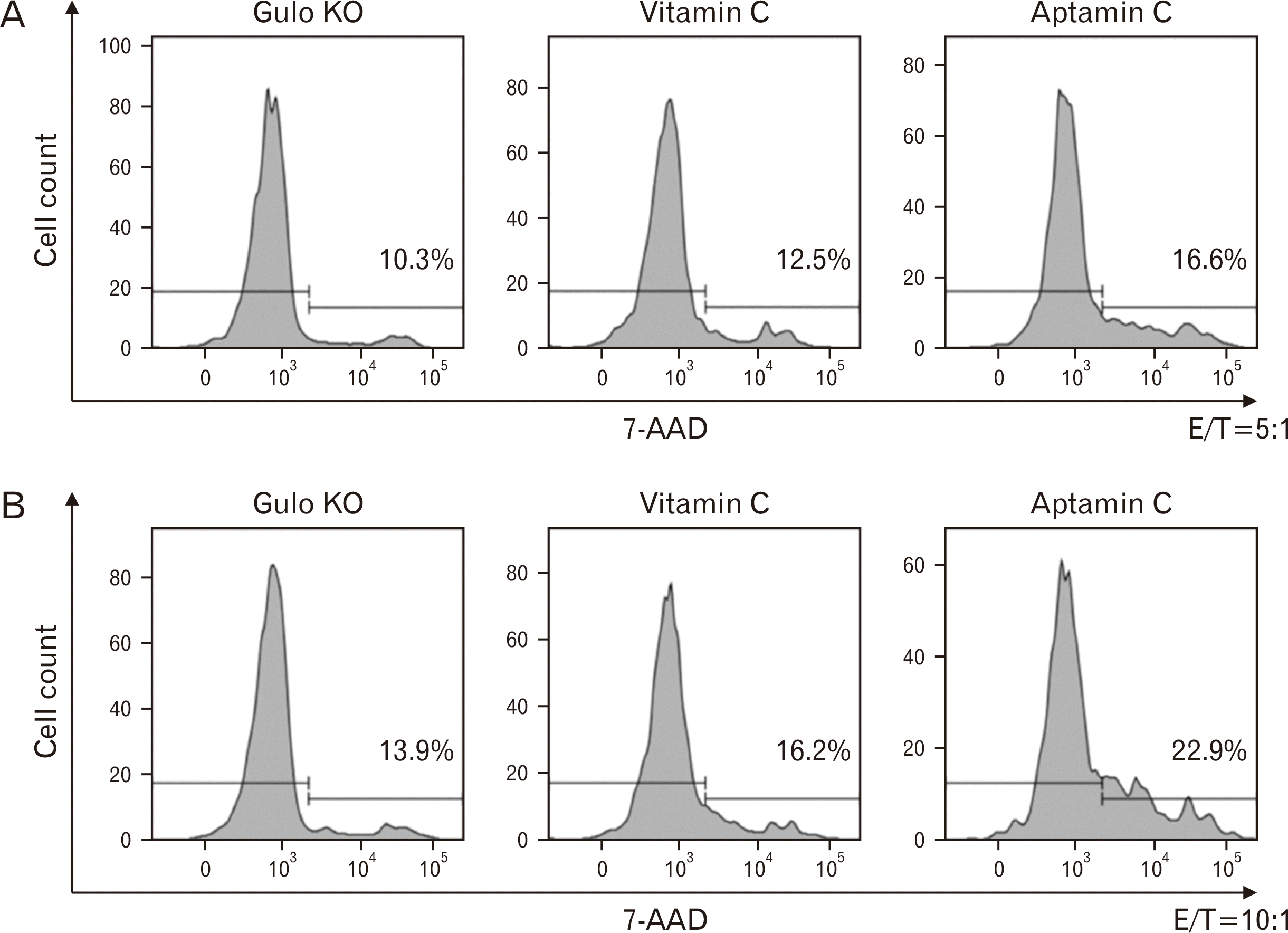
Fig. 4
Enhanced effect of Aptamin C on activation of STAT3 in NK cells. (A) Human natural killer (NK) cell line, NK-92 was pretreated with specific inhibitor for STAT3 (S3I-201, 50 μM) and ERK inhibitor (PD98059, 20 μM) for 1 hour, and then cells were incubated in the presence or absence of Aptamin C (mixture of vitamin C [20 μg/ml] with aptamer [0.2 μg/ml]) in medium containing 25 IU/ml of IL-2. NK-92 cell proliferation was assessed by Alamar Blue assay as described in Materials and Methods. (B) The phosphorylation of STAT3 (p-STAT3) in NK-92 upon the treatment of Aptamin C was analyzed by Western blot analysis. After cells were treated with Aptamin C for 30 minutes, cell lysates were prepared and the p-STAT3 was analyzed as described in Materials and Methods. (C) Based on the results with NK-92, the p-STAT3 in murine NK cells upon the treatment of Aptamin C for 30 minutes was analyzed by Western blot analysis. Unpaired two-tailed Student’s t-test and one-way ANOVA with Tukey’s multiple comparison test were performed. Data is presented as mean±SD. ns, not significant. **P<0.01, ***P<0.001.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download