Abstract
Intrauterine alcohol exposure delays bone maturation and intensifies osteoporosis and fracture risk. As most studies emphasize the neurological aspects of intrauterine alcohol exposure, there is a lack of research on the implications pertaining to osseous tissue. Previous studies investigated these effects in fetuses, with limited studies on postnatal life. Postnatal studies are crucial since peak bone growth occurs during adolescence. This study aimed at assessing the effects of prenatal alcohol exposure on the humerus proximal and distal growth plate chondrocytes in 3-week-old rats. Sprague Dawley rats (n=9) were assigned to either the ethanol group (n=3), saline (n=3), and untreated (n=3) group and time-mated. Once pregnant, as confirmed by the presence of a copulation plug, the former 2 groups were treated with 0.015 ml/g of 25.2% ethanol and 0.9% saline. The untreated group received no treatment. The left humeri belonging to 6 pups per group were used. Serial sections were cut with a microtome at 5 µm thickness. These sections were stained with haematoxylin and eosin for assessment of normal morphology or immunolabeled with anti-Ki-67 and transforming growth factor β-1 (TGFβ-1) antibody. Prenatal alcohol exposure adversely effected the growth plate sizes and the number of cells in the proliferative zone. Fewer TGFβ-1 immunopositive and proliferative chondrocytes were found using the anti-Ki-67 antibody. This may explain the growth retardation in offspring exposed to gestational alcohol, showing that gestational alcohol exposure inhibits cell proliferation, aiding the diminished stature.
Growth inhibition is one of the cardinal features occurring in offspring born to women who consume alcohol during gestation [1-3]. This may belong to an umbrella of disorders recognised as fetal alcohol spectrum disorder (FASD). The most serious of which is fetal alcohol syndrome (FAS). Despite stunted growth being a chief characteristic of FASD, the scientific literature on osseous tissue growth in gestational alcohol exposure has received inadequate consideration. The limited studies that investigate the skeletal aspects of intrauterine alcohol intake [2-6] do not explore how prenatal alcohol exposure exerts its detrimental effects in postnatal life.
Studies [2, 3] have shown that gestational alcohol exposure hinders the longitudinal growth and mineralization of fetal bone. In support of this, Miralles-Flores and Delgado-Baeza (1992) [7], suggested that exposure to alcohol during gestation antagonises long bone epiphyseal plate chondrocyte function in rodents. These authors described the epiphyseal plate in 2-week-old pups exposed to intrauterine alcohol to be shorter. These same researchers report reduced proliferative and hypertrophic zone heights, and with fewer chondrocytes compared to the controls. Other researchers made similar findings in newborn rats [2, 3].
It remains unclear how gestational alcohol exposure affects the epiphyseal plate leading to the observed smaller epiphyseal growth plate with fewer chondrocytes. The current study proposes that the expression of cytokines that promote cell proliferation may be inhibited. One such cytokine is the transforming growth factor β-1 (TGFβ-1) which is responsible for embryonic development as well as skeletogenesis growth postnatally [8]. The TGFβ-1 cytokine promotes cell differentiation into either chondrocytes or osteoblasts [8] and influences bone remodelling in postnatal life [8, 9].
Taking all the above into consideration, this study sought to comprehend whether binge exposure to alcohol during the prenatal duration would affect bone postnatal development and the involvement of TGFβ-1. This was achieved by assessing epiphyseal growth plate chondrocyte proliferation and TGFβ-1 expression using immunohistochemical methods.
The study commenced after receiving ethics approval from the Animal Ethics Screening Committee, University of the Witwatersrand (AESC 2015/07/27C). Animals were handled and treated in alignment with the standards and principles set out by this committee.
The study comprised of nine time-mated Sprague Dawley rats weighing between 260–350 g and aged 13–15 weeks. All study dams were bred and kept at the Central Animal Services (CAS), University of the Witwatersrand. These animals were kept in conditions free of pathogens, with the temperature set as 23°C±2°C and a 12-hour light/dark cycle. Each pregnant dam was accommodated in a plastic cage (L 430 mm×W 220 mm×H 200 mm), with free movement within the respective enclosures. There was unlimited access to tap water and standard rodent chow.
The dams (n=9) were placed into either the ethanol (n=3), saline control (n=3) and untreated control groups (n=3) (Fig. 1). The untreated control group received neither ethanol nor saline. Either 0.015 ml/g body mass of 25.2% ethanol was given in the experimental group or 0.9% saline for the corresponding control group (Fig. 1). This concentration of ethanol (0.015 ml/g body mass of 25.2% ethanol) was chosen because higher concentrations of ethanol could increase risk miscarriages. The ethanol administered was to mimic a chronic binge drinking model. The study used oral gavage with a metal, curved, 16-gauge rounded/bulb-tipped gavage needle as a treatment vehicle. The treatment was for the first 19 days of gestation, the presence of a copulation plug indicated the first day of gestation.
Heparinized microcapillary tubes (100 µl) were used for the collection of whole blood obtained from the tail vein one hour after alcohol administration to measurer blood alcohol levels in the dams. As the rat contains approximately 70 ml/kg of blood [10], 2×100 µl microcapillary tubes were used to collect blood from each dam as this is permissible without impacting physiological functions [11]. To obtain plasma, the microcapillary tubes were spun in a microhaematocrit centrifuge (Haematokrit 210; Hetich) at 3,000 rpm for 10 minutes in an alcohol-free environment. A BioVision Ethanol Colorimetric Assay Kit (BioVision Incorporation) was used to determine plasma alcohol levels. Then, the absorbance values were determined with an iMark Bio-rad Microplate Absorbance Reader (Bio-rad Laboratories Inc.). This colorimetric assay detects 0.1–10 ppm according to the manufacturer and requires as little as 10–30 µl of plasma. While gas chromatography is more accurate, it requires a larger blood sample (100 µl plasma) [12] and more complex equipment. More blood from the rats would have been required to achieve this.
After the dams littered successfully and naturally, the delivered pups were kept with their mothers till 21 days postnatally. From each group based on the treatment and group allocation of the dams, 6 pups were obtained and assigned as follows: untreated controls (n=6), saline-treated (n=6) and ethanol group (n=6) (Fig. 1).
A lethal intraperitoneal dose of 200 mg/kg pentobarbital injection was used to terminate the pups at the of age 3 weeks. Skin incisions were made to expose the humerus, muscles were meticulously dissected away before detaching the bones. Each bone was then individually immersed and stored in 10% buffered formalin until further processing for histological assessment and immunohistochemistry.
Bone samples were removed and fixed in 10% buffered formalin for a minimum of 14 days. They were then decalcified in 14% w/v ethylenediaminetetraacetic acid (pH 7.4) for 10 days in an incubator with regular gentle shaking at 45˚C. The bones were then rinsed and divided into proximal and distal samples and immersed in 10% buffered formalin for 24 hours. An automatic processor (Citadel 2000; Thermo Fischer Scientific) was then used to take the samples through ascending grades of ethanol prior to embedding in paraffin wax. When embedding, care was taken to ensure that the bone samples were orientated longitudinally to minimise differences in the sectioning angle. Bone sections were cut at 5 µm thickness using a microtome (Leica RM2125 RTS; Leica Biosystems) and placed on silane-coated glass slides. Sections in a sequence of 1 in 3 were stained with haematoxylin and eosin to assess epiphyseal growth plate morphology and chondrocytes or immunolocalization of the Ki-67 protein (cell proliferation maker) or TGFβ-1.
Immunolocalization of epiphyseal growth plate chondrocytes expressing the Ki-67 protein and TGFβ-1 was performed using an anti-Ki-67 antibody (ab66155) or anti-TGFβ-1 antibody (ab215715). Both antibodies were sourced from Abcam. In summary, sections were deparaffinized, rehydrated, and subsequently, immersed with citrate buffer solution (pH=6) kept in a water bath set at a temperature of 60°C overnight. The next morning, tissue sections were cooled down to room temperature for 20 minutes before washing for 5 minutes in a phosphate buffer solution (PBS) (pH=7.4). Endogenous peroxidase was blocked with 1% hydrogen peroxide (H2O2), washed with PBS for 3×5 minutes, and incubated with normal goat serum for 10 minutes. The tissue sections were then incubated with primary antibody (anti-Ki-67 in 1:1,200 or TGFβ-1 in 1:250 dilution factor) overnight at 4°C. On the 3rd third day, sections were allowed to reach room temperature, and washed in PBS for 3×5 minutes. A biotinylated secondary antibody (biotinylated goat anti-rabbit IgG [H+L] [ab64256]) was applied for 10 minutes and then washed in PBS for 3×5 minutes. To reduce nonspecific staining background while enhancing antibody binding, sections were incubated with streptavidin horseradish peroxidase for 10 minutes (ab64269) and washed in PBS for 3×5 minutes. For visualisation under a light microscope, sections were incubated with 3-3’ di-aminobenzidine working solution for 5 minutes and then rinsed in running tap water for 5 minutes. Finally, the sections were dehydrated through alcohol grades, cleared in xylene and eventually mounted in entellen.
A light microscope (Zeiss Axioscope 2 plus) fitted with an axiocam HRC digital camera was utilised to acquire photomicrographs of the stained slides. These were taken under ×10 magnification. Photomicrographs were transferred into Fiji Image-J software and the parameters measured were delineated based on cell morphology. The proliferative zone was delineated by the first flattened chondrocyte on the epiphyseal end and the first hypertrophic chondrocyte on the metaphyseal aspect.
The built image J tool for estimation of the area was used to estimate the area of the respective epiphyseal growth plate zones. For the quantification of chondrocytes, the manual counting method from the images was employed.
The data were managed in Microsoft Excel 365 (Microsoft Corporation) and analysed using SPSS® version 28 (IBM Co.). The descriptive data of the growth plate areas, number of chondrocytes and quantity of Ki-67 and TGFβ-1 immunopositive chondrocytes were reported as means and standard deviation. ANOVA with Tukey post-hoc was used for multiple group comparisons of means. The significance level was set at P<0.05.
The ethanol group had a high mean blood ethanol concentration (170 mg/dl) whereas the saline controls had negligible amounts.
All the study groups (untreated control, saline control, and ethanol) displayed similar bone lengths (P=0.29, for all comparisons) (Table 1).
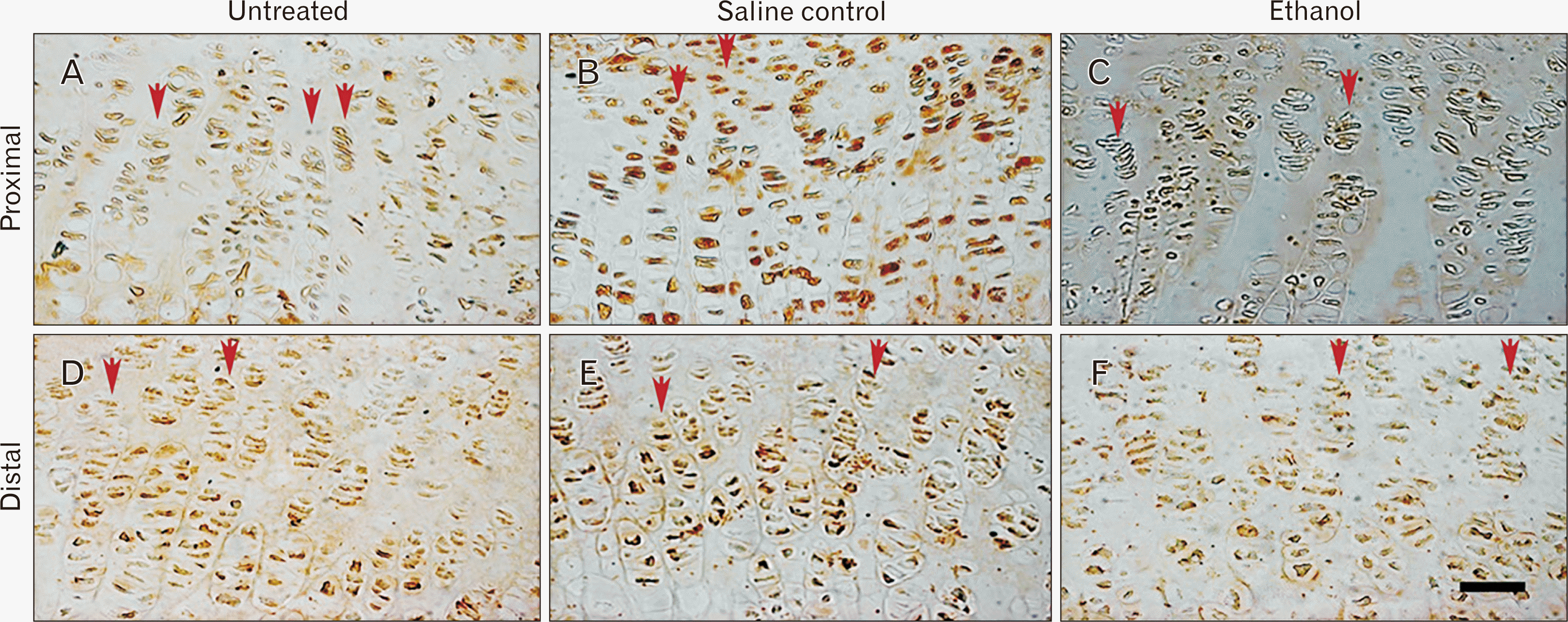
The proliferative zone of both epiphyseal ends (proximal and distal) showed a partly similar group comparison. The untreated group had tightly packed chondrocytes in prominent rows (Fig. 2A) while the saline controls showed the chondrocytes to be evenly distributed with a few gaps (Fig. 2B). In contrast, the chondrocytes in the ethanol group were loosely packed with areas showing huge gaps (Fig. 2C). This pattern continued in the distal region (Fig. 2D–F).
In the proximal region, the epiphyseal plate surface area showed no significant group differences when comparing the untreated with the saline controls. However, the ethanol group was 7.6% smaller than the untreated controls (P=0.029) (Fig. 3A). In contrast, the distal aspect showed differences in epiphyseal plate surface area compared to the untreated. It was decreased by 10.5% in the saline controls and 13% in the ethanol group than the untreated controls. This was statistically significant when comparing the ethanol or saline controls to the untreated (P<0.001 and P=0.004, respectively) (Fig. 3A).
In the proximal epiphysis, the saline group had 13% reduction while the ethanol group had 16.5% fewer chondrocytes than the untreated controls. The ethanol group exhibited significantly fewer chondrocytes than both control groups (P<0.001 for both comparisons) (Fig. 3B). In the distal epiphysis, no significant different difference was detected when comparing the untreated controls with the saline controls (2.3% decrease) and ethanol group (2.3% decrease) number of chondrocytes (P=0.371 and P=0.380, respectively) (Fig. 3B).
Quantification of Ki-67 immuno-positive chondrocytes showed the saline controls to have 6.5% fewer cells whereas the ethanol group had 10.1% reduction in cells than the untreated group (P=0.043 and P=0.001, respectively) (Figs. 3C, 4A–C). In the distal region, the number of Ki-67 was reduced by 11% in the saline controls and by 16.7% in the ethanol group compared to the untreated controls (P=0.001 and P<0.001, respectively) (Figs. 3C, 4D–F).
The proximal plate proliferative zone showed immuno-positive chondrocytes showed the saline controls to have 2.5% fewer cells whereas the ethanol group had 6.3% fewer cells than the untreated group. However, these cell numbers were only significantly different between the ethanol and the untreated group (P=0.033) (Figs. 3D, 5A–C). In the distal epiphysis, TGFβ-1 labelled cells were reduced by 5.1% in the saline controls and 9.2% fewer in the ethanol group compared to the untreated controls. This difference was significant only for the untreated controls compared to ethanol (P=0.005) (Figs. 3D, 5D–F).
We sought to evaluate whether binge intrauterine alcohol exposure would affect the rat humerus epiphyseal growth plate and diaphysis dimensions as well as chondrocyte proliferation and TGFβ-1 immunopositivity. The study used untreated controls as well as a saline control group to control for the impact of psychological stress that may be introduced by animal handling, particularly by oral gavage. Literature suggests that oral gavage causes stress to laboratory animals [13]. Psychological stress may impact bone outcomes [14].
Gestational alcohol exposure resulted in a smaller epiphyseal growth plate with fewer proliferating chondrocytes and reduced TGFβ-1 expression. Enumeration of proliferative cells in the growth plate in postnatal development of rats exposed to alcohol during gestation provides new knowledge about the impact of gestational alcohol exposure on postnatal life chondrocyte proliferation. TGFβ-1 is crucial for bone development [15, 16], maintenance of normal bone health [17], but this study has brought new information which shows that TGFβ-1 is inhibited in postnatal life after gestational alcohol exposure.
No significant differences in bone length were found in the present study, among the three groups. However, previous studies [2, 3, 18-20] observed diminished length in the alcohol group. The contrasting findings may be ascribed to the variability in the concentration of alcohol given to the respective study dams. In the current study, the ethanol group received 25.2% ethanol. This is lower than the ethanol concentration of 30% and 36% in comparative studies [2, 3, 20]. The bone effects of exposure to ethanol during gestation have been reported to be dose-dependent, with 36% ethanol causing more adverse effects than 25% and 15% ethanol [2, 3]. Therefore, it is likely that using a higher alcohol concentration in the present study would have exhibited shorter bone length in the ethanol group.
Previous studies attributed the stunted growth observed in offspring of mothers who conceded to drinking during pregnancy to a smaller epiphyseal growth plate size [7]. Other researchers [8] also found fewer chondrocytes in the epiphyseal growth plate of offspring exposed to gestational alcohol using animal studies. Similarly, in the current study, the epiphyseal plate surface area was smaller for the ethanol group in both the proximal and the distal epiphysis. This supports the proposition that gestational alcohol may affect postnatal bone development through inhibition of the TGFβ-1 pathway. Additionally, this finding shows that the impact of gestational exposure to alcohol may last long into postnatal life as this present study used 3-week-old rat pups.
The ethanol group had a significantly lower chondrocyte number, as well as fewer actively dividing cells (Ki-67 immunopositive). The fewer chondrocytes observed in our study is a similar finding to that of Miralles-Flores and Delgado-Baeza (1992) [7] who found fewer chondrocytes. The authors of this study attributed this to dysregulation in mitochondrial activity that could alter the maturation of the proliferative chondrocytes. While information about markers of cell proliferation was not in the scope of their study [7], this was included in our study. In the present study, we found fewer proliferating cells (Ki-67 immunopositive) together with fewer cells expressing TGFβ-1 in the ethanol group. This suggests that alcohol may interfere with TGFβ-1 signalling pathway for cell proliferation in the growth plate resulting in fewer dividing cells as TGFβ-1 is a major role player in osseous tissue growth and development which affects both bone and cartilage [21].
With these findings, we propose that intrauterine alcohol exposure inhibits TGFβ-1 expression, leading to fewer chondrocytes that also proliferate at a slower rate in the epiphyseal growth plates. We suggest that this is one of the plausible ways in which intrauterine alcohol exposure causes a shorter stature as seen in FAS children.
Previous studies reported on the length of the epiphyseal growth plate and found it to be reduced in the bones of rats exposed to alcohol during gestation [3]. In the present study the epiphyseal growth plate area was reduced in both the proximal and distal aspects among the rats in the ethanol group. Use of the epiphyseal growth plate area in the present study provided a more reliable means of measuring the epiphyseal growth plate size. This reduced epiphyseal growth plate area was accompanied by a reduction in chondrocyte proliferation (Ki-67 immunopositive cells) as well as reduced TGFβ-1 expression. This finding is aligned to finding of growth retardation in children born to mothers who drink alcohol during pregnancy [2].
In conclusion, binge gestational alcohol affects osseous tissue growth by inhibiting cell proliferation and disturbing TGFβ-1 expression in both extremities of the humerus. The authors suggest this may provide an explanation for growth retardation in offspring exposed to binge gestational alcohol exposure. Additionally, this study shows that growth retardation emanating from gestational alcohol exposure may persist until the advanced stages of bone growth and development as this study used 3-week-old rats unlike other studies using newborn rats. Communities need to be enlightened about these poor outcomes of consuming alcohol during pregnancy.
Acknowledgements
Ms. Hasiena Ali provided technical assistance whereas the staff of the University of the Witwatersrand CAS assisted with animal husbandry.
Notes
References
1. Pielage M, El Marroun H, Odendaal HJ, Willemsen SP, Hillegers MHJ, Steegers EAP, Rousian M. 2023; Alcohol exposure before and during pregnancy is associated with reduced fetal growth: the Safe Passage Study. BMC Med. 21:318. DOI: 10.1186/s12916-023-03020-4. PMID: 37612658. PMCID: PMC10463675.
2. Simpson M, Duggal S, Keiver K. 2005; Prenatal ethanol exposure has differential effects on fetal growth and skeletal ossification. Bone. 36:521–32. DOI: 10.1016/j.bone.2004.11.011. PMID: 15777686.
3. Snow ME, Keiver K. 2007; Prenatal ethanol exposure disrupts the histological stages of fetal bone development. Bone. 41:181–7. DOI: 10.1016/j.bone.2007.04.182. PMID: 17532282. PMCID: PMC2039868.
4. Abel EL, Dintcheff BA. 1978; Effects of prenatal alcohol exposure on growth and development in rats. J Pharmacol Exp Ther. 207:916–21. PMID: 731439.
5. Chaudhuri JD. 2000; Alcohol and the developing fetus--a review. Med Sci Monit. 6:1031–41. PMID: 11208451.
6. Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M. 1989; Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 84:536–41. DOI: 10.1542/peds.84.3.536. PMID: 2771556.
7. Miralles-Flores C, Delgado-Baeza E. 1992; Histomorphometric analysis of the epiphyseal growth plate in rats after prenatal alcohol exposure. J Orthop Res. 10:325–36. DOI: 10.1002/jor.1100100304. PMID: 1569495.
8. Kanaan RA, Kanaan LA. 2006; Transforming growth factor beta1, bone connection. Med Sci Monit. 12:RA164–9. PMID: 16865078.
9. Bismar H, Klöppinger T, Schuster EM, Balbach S, Diel I, Ziegler R, Pfeilschifter J. 1999; Transforming growth factor beta (TGF-beta) levels in the conditioned media of human bone cells: relationship to donor age, bone volume, and concentration of TGF-beta in human bone matrix in vivo. Bone. 24:565–9. DOI: 10.1016/S8756-3282(99)00082-4. PMID: 10375198.
10. Kumar M, Dandapat S, Sinha MP, Kumar A, Raipat BS. 2017; Different blood collection methods from rats: a review. Balneo Res J. 8:46–50. DOI: 10.12680/balneo.2017.141.
11. Parasuraman S, Raveendran R, Kesavan R. 2010; Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 1:87–93. Erratum in: J Pharmacol Pharmacother 2017;8: 153. DOI: 10.4103/0976-500X.72350. PMID: 29081629. PMCID: PMC5642135.
12. Tiscione NB, Alford I, Yeatman DT, Shan X. 2011; Ethanol analysis by headspace gas chromatography with simultaneous flame-ionization and mass spectrometry detection. J Anal Toxicol. 35:501–11. DOI: 10.1093/anatox/35.7.501. PMID: 21871160.
13. Balcombe JP, Barnard ND, Sandusky C. 2004; Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 43:42–51. PMID: 15669134.
14. Kelly RR, McDonald LT, Jensen NR, Sidles SJ, LaRue AC. 2019; Impacts of psychological stress on osteoporosis: clinical implications and treatment interactions. Front Psychiatry. 10:200. DOI: 10.3389/fpsyt.2019.00200. PMID: 31024360. PMCID: PMC6465575.
15. Chen G, Deng C, Li YP. 2012; TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 8:272–88. DOI: 10.7150/ijbs.2929. PMID: 22298955. PMCID: PMC3269610.
16. Pillay DS, Ndou R. 2022; Intrauterine alcohol exposure delays growth and disturbs trabecular morphology in 3-week-old Sprague - Dawley rat femur. J Anat Soc India. 71:93–101. DOI: 10.4103/jasi.jasi_183_21.
17. Dlamini GF, Ndou R. 2023; Osteoblastogenesis and osteolysis in the Zucker Diabetic Sprague Dawley rat humerus head. Anat Cell Biol. 56:552–61. DOI: 10.5115/acb.23.166. PMID: 37788886. PMCID: PMC10714090.
18. Pillay D, Ndou R. 2021; Intrauterine alcohol exposure disturbs trabecular morphology in the Sprague Dawley rat humerus epiphysis up to 3-weeks postnatally. Int J Morphol. 39:1436–42. DOI: 10.4067/S0717-95022021000501436.
19. Ndou R, Bello NK, Perry V, Pillay D. 2023; Distal tibial trabecular morphometry in a Sprague Dawley rat model of fetal alcohol syndrome: a micro focus X-ray computed tomography case-control study. Pan Afr Med J. 46:35. DOI: 10.11604/pamj.2023.46.35.37151. PMID: 38145202. PMCID: PMC10746879.
20. Nwaogu IC. 2002; Foetal alcohol syndrome: growth rate of bones in rats. J Appl Anim Res. 22:249–53. DOI: 10.1080/09712119.2002.9706406.
21. Janssens K, ten Dijke P, Janssens S, Van Hul W. 2005; Transforming growth factor-beta1 to the bone. Endocr Rev. 26:743–74. DOI: 10.1210/er.2004-0001. PMID: 15901668.
Fig. 1
Study design. A diagrammatic illustration of the group allocation of dams, pups and humeri for the study.
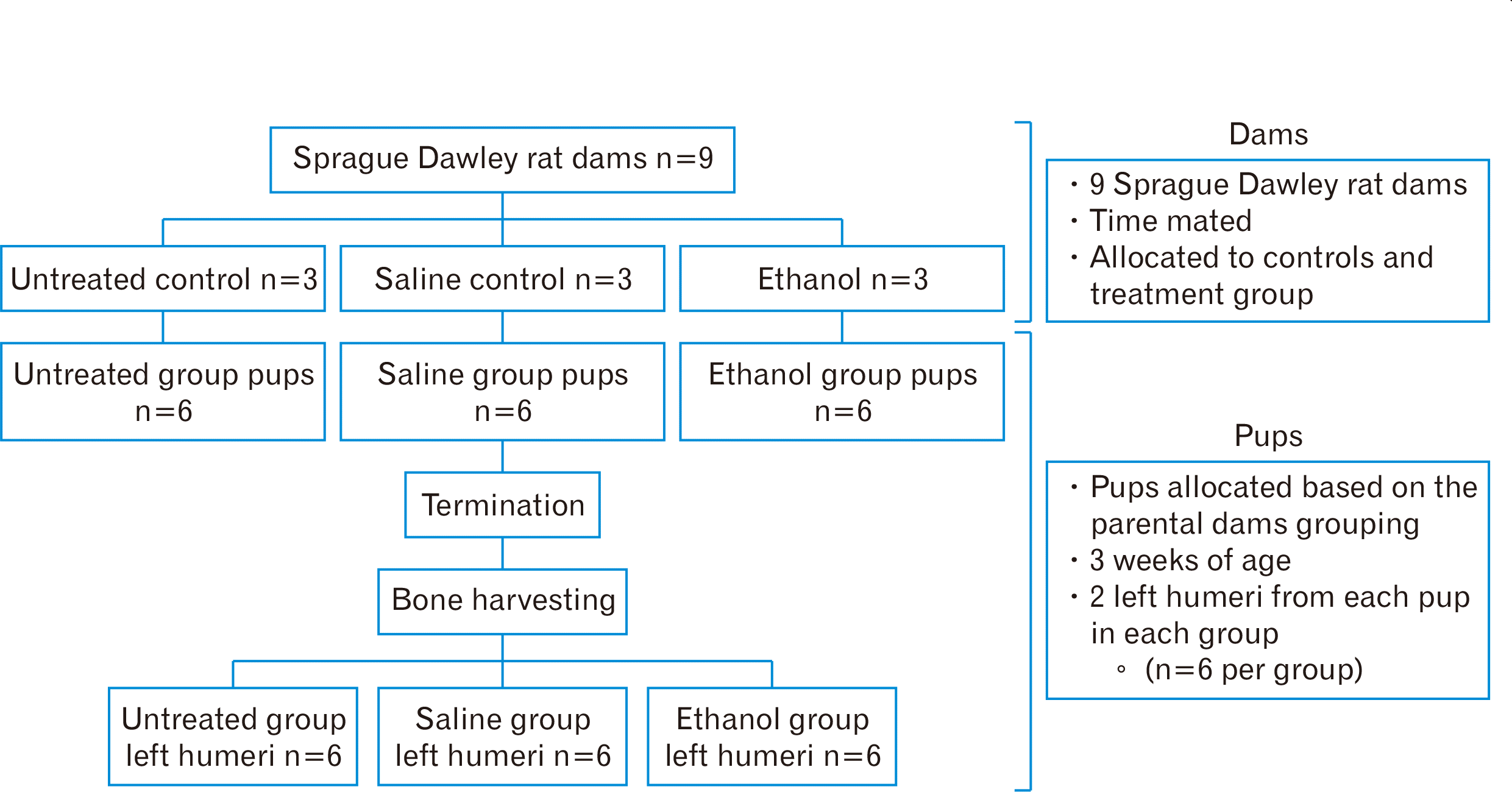
Fig. 2
Photomicrographs of haematoxylin and eosin-stained sections of the epiphyseal growth plate of the humerus. (A–C) are representative micrographs of the proximal while (D–F) show the distal epiphyseal growth plate for the untreated, saline and ethanol groups, respectively. The insert is blown up from the boxed area in (A) to show proliferative zone chondrocytes. The white arrowheads point to the typical stacking of chondrocytes in the proliferative zone and point to examples of this chondrocyte stacking. Scale bar=40 microns and applies to all images.
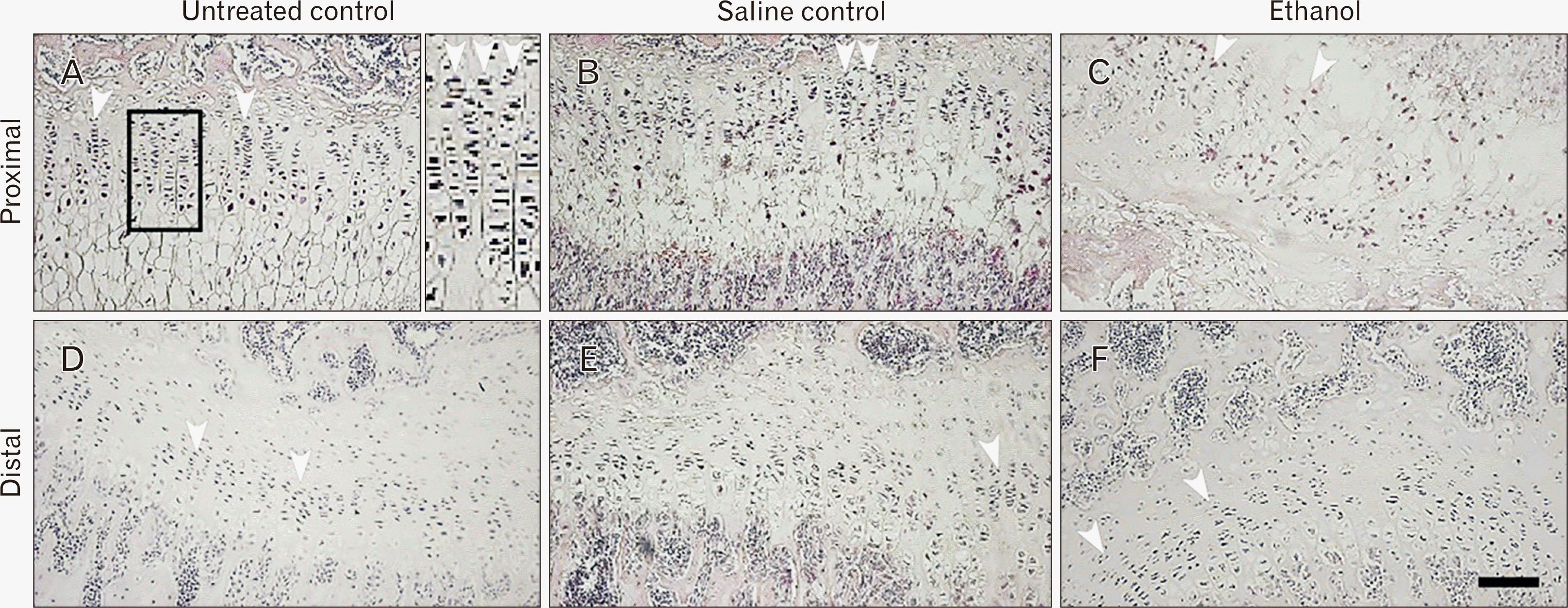
Fig. 3
Epiphyseal growth place area and chondrocyte parameters in the proliferative zone illustrated as a percentage difference relative to the untreated control group. (A) Histomorphometry of the epiphyseal growth plate surface area. (B) Epiphyseal growth plate proliferative zone number of chondrocytes. (C) Ki-67 immuno-positive (proliferative) chondrocytes are shown for the proliferative zone. (D) Transforming growth factor β-1 immuno-positive chondrocytes in the epiphyseal growth plate proliferative zone. The mean percentage difference in relation to the untreated control group is shown for the saline controls and the ethanol group in the proximal and distal epiphyseal growth plate. The P-values are for comparison with the untreated control group. Error bars represent standard error of the mean.
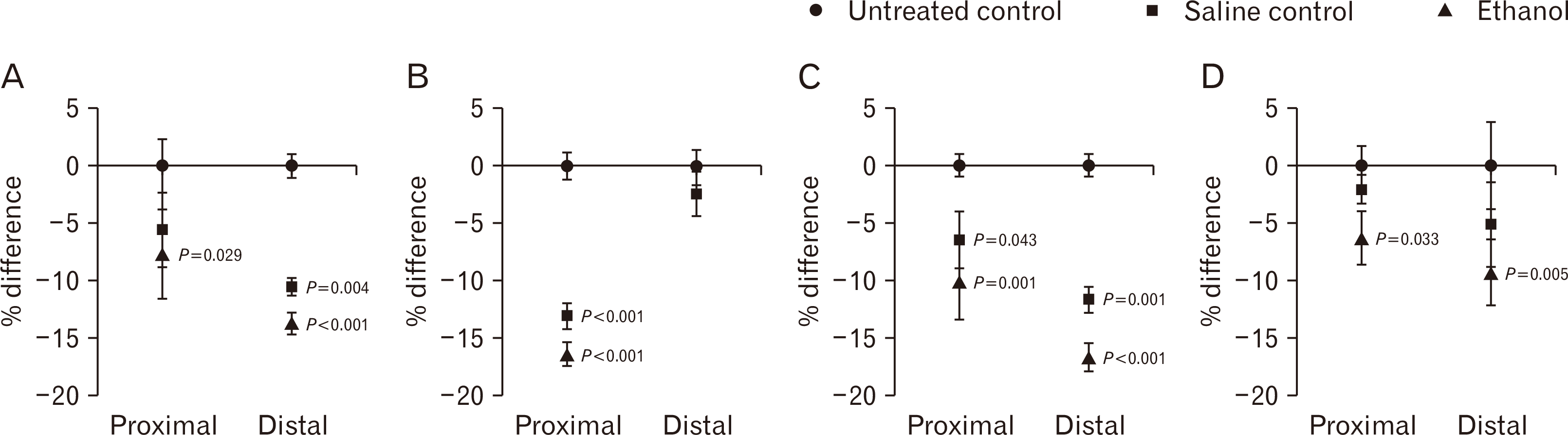
Fig. 4
Photomicrograph of humerus proliferative zone chondrocytes immunolabeled with the anti-Ki-67 antibody. Representative micrographs of the proximal (A–C) and distal (D–F) epiphyseal growth plate for the three groups studied. The arrowheads point to examples of the typical stacking of chondrocytes in the proliferative zone. Scale bar=30 microns and applies to all images.
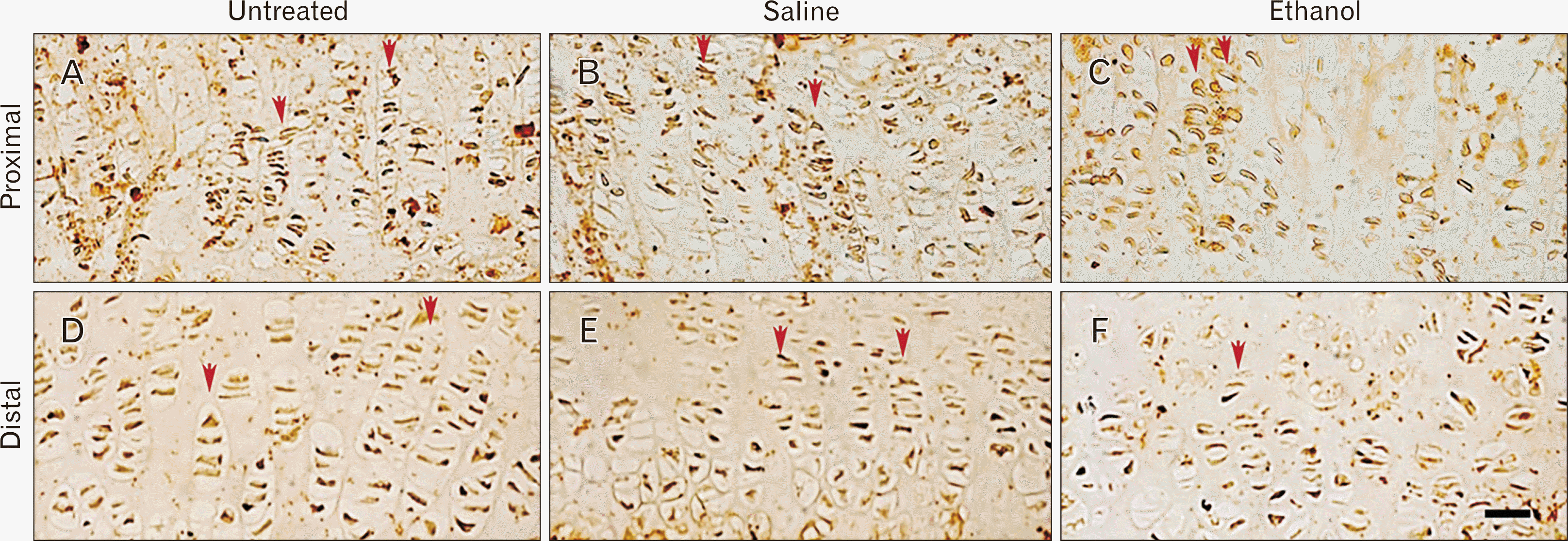
Fig. 5
Photomicrograph of humerus proliferative zone chondrocytes immunolabeled with the anti-transforming growth factor β-1 antibody. Representative micrographs of the proximal (A–C) and distal (D–F) epiphyseal growth plate for the three groups studied. The arrowheads point to examples of the typical stacking of chondrocytes in the proliferative zone. Scale bar=40 microns and applies to all images.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download