Abstract
Anticipating a wide range of morphological variations of arterial anatomy of foregut derivatives beyond the classical pattern, a precise understanding is pertinent to preoperative diagnosis, operative procedure and to avoid potentially devastating post-operative outcome during various traumatic and non-traumatic vascular insult of foregut. The study aimed to revisit the morphological details and update unusual configurations of arteries of foregut to establish clinico-anatomical correlations. This study described the detailed branching pattern of coeliac trunk (CT) as principal artery of foregut with source & course of hepatic, gastric, duodenal and pancreatic branches in 58 cadaveric dissections. Based on morphology, different types and subtypes were made. The descriptions were explained using figures and pertinent tables. Among classical branches of CT, splenic artery was found as most stable whereas other two branches were found to be most variable with missing common hepatic artery in 11 cases. In addition to classical trifurcation (65.52%), different types of bifurcation (12.07%) and tetrafurcations (22.41%) of CT were observed. Regarding variations of hepatic arteries (27.59%), both non-classical origin and accessory hepatic branches were found. In case of gastric branches, more variant origins were seen with right gastric (50%) as compared to left gastric artery (34.48%). Other morphological variations included non-classical origin of gastro-duodenal artery (18.96%) along with presence of accessory pancreatic (17.13%) and duodenal arteries (6.38%). Awareness of anatomical variations regarding circulatory dynamics of foregut is worth knowing in order to facilitate successful planning of surgery involving upper abdominal organs with least complications.
Upper abdominal surgeries like hepatobiliary, pancreatic, gastro-duodenal and splenic surgery often require significant knowledge about vascular anatomy of foregut. As foregut is differentiated from primitive gut tube, coeliac trunk (CT) is conventionally preserved for perfusion of its derivatives. The variation of the arterial system of human anatomy still keeps its importance regarding the surgical and clinical interest. The variations can be a reasoning for a complication or serve for another chance to supply the area [1]. Classically CT appears as tripod with left gastric (LGA), splenic artery (SA), and common hepatic arteries (CHA) to supply their embryological segmental territories. Subsequently, stomach is supplied by anastomosis of all three branches of CT, spleen by SA and liver, gall bladder, gastro-duodenal region and head of pancreas by CHA [2].
Unanticipated anatomical variations may increase undesirable clinical sequalae like graft ischemia time with associated risk of post-operative graft dysfunction and emphasizes the need of additional anastomosis [3]. Compression of CT by median arcuate ligament may be associated with visceral ischemia with symptoms of chronic abdominal pain or jaundice [4]. Previous literature mentioned that artery arising as common trunk with other artery often lacks the protective benefit over vessel of single origin and therefore might increase the risk of vascular compromise during major abdominal interventions [5].
Standard hepatic artery persists in 50%–75% patients; however, appreciation of variant hepatic arterial anatomy is important in liver transplantation, intra-arterial chemotherapy & radio-embolization of liver etc. [5-7]. Hiatt et al. [8] and Michels [9] have highlighted hepatic arterial variations. But, replaced hepatic artery as substitution and accessory hepatic artery as additional artery are variations regarding aberrant hepatic artery described in previous literature is crucial with evolution of placement of intra-arterial infusion pump for the treatment of primary cancer or hepatic metastasis [10, 11]. Also, peri-hepatic arterial variation may interfere with surgical outcome following pancreaticoduodenectomy [4]. In this context, in terms of origin of common (C), right (R), and left (L) hepatic arteries, recently CRL classification being the abbreviation of their initials has been presented based on three-dimensional CT technology to cover wide range of hepatic arterial variations [12].
Nowadays, different invasive and non-invasive imaging studies are employed to explore and document arterial variations. However, introduction of minimally invasive methods remains challenging as surgical view is limited to delineate arterial anatomy and for accurate and reliable interpretation of radiological analysis often needs dependency on anatomical references [5-7, 13]. Considering this, in an attempt to validate the morphological details of arterial pattern of foregut in eastern Indian population and updating the unusual configurations along with its clinic-anatomical correlations, present study was done.
The present study was carried out on fifty-eight embalmed cadavers of either sex (male-43, female-15), aged 50–80 years present in Department of Anatomy which were voluntarily donated by deceased person (by pledge form) or by family members for scientific research purpose.
Cadavers exhibiting any kind of deformities, pathological conditions, traumas, upper abdominal surgeries, previous dissections in the area of interest and all that may modify the vascular anatomy were excluded from the study. Abdominal dissection was made according to standard dissection technique following the guidelines of Cunningham’s manual. All arteries were traced up to the target organs to acquire knowledge about the feeder arteries. Every attempt was made carefully to preserve anomalous vessels and documented with photographs. The data analyses were done taking into account the morphology and branching pattern. Each variant type was again sub-typed and named according to pattern of arteries.
In the present study, following data has been noted regarding different vessels of foregut.
Origin was from abdominal aorta in all cases; but variations were noted in its branching pattern. Conventional three branches were observed in 38 (65.52%) cases (Fig. 1). In rest, SA was comparatively more stable than LGA & CHA. In 11 cases CHA was missing also. Among variant branching pattern, prevalence of tetrafurcation was more (13 cases; 22.41%) than bifurcation (7 cases; 12.07%). In case of Type I bifurcation in 4 (6.90%) cases, left hepatic artery (LHA) and LGA arose as common trunk (hepatogastric trunk) and CHA & SA arose from another common trunk (hepatosplenic trunk). In Type II bifurcation in 3 (5.17%) cases, apart from LGA, hepatosplenic trunk was found which again branched into proper hepatic artery (PHA) and SA. Regarding tetrafurcation, SA was constant in both types but other branches varied. In Type II tetrafurcation in 5 (8.62%) cases, LGA arose directly from aorta instead of CT. Detail about normal and variant branches of CT have been summarized in Table 1 and photographed with schematic representation of each variant type in Fig. 2 and Fig. 3 respectively.
Found in 47 cases. Classical origin as CT trifurcation was noted in 38 cases and in remaining 9 (19.15%) cases, variant origins was from CT bifurcation (Type I) & tetrafurcation (Type II). In 11 (18.96%) cases, PHA replaced CHA (Fig. 2; Bifurcation Type II, Tetrafurcation Type I). Regarding its branches, other than its typical bifurcation as gastro-duodenal artery (GDA) & PHA, variant bifurcation was observed in 4 (8.51%) cases in which right hepatic artery (RHA) was arising directly from CHA instead of PHA (Fig. 2; Bifurcation Type I). Other nonclassical branching patterns of CHA were studied in terms of trifurcation and tetrafurcation in 19 (40.43%) and 10 (21.28%) cases respectively. In these cases, branching arteries varied widely but GDA was constant. Detail branching characteristics of CHA have been summarized Table 2 and variable patterns were depicted in Fig. 3 and Fig. 4 with schematic representation of each variant type.
During exploration of individual hepatic artery, classical origins of both right and LHAs were observed in 42 (72.41%) cases from PHA. In case of missing PHA (16 cases) either one or both arteries were originated directly from CHA (Fig. 2; Bifurcation Type I & Fig. 4; Trifurcation Type II, III, Tetrafurcation Type III). In 4 (6.90%) cases, LHA was also observed from common hepatogastric trunk along with LGA (Fig. 2; Bifurcation Type I). Moreover, middle hepatic artery (MHA) was present in 5 (10.64%) cases arising as common trunk with LHA (Fig. 4; Trifurcation Type II). In the present study, though we found variant LHA in 4 cases (6.90%) from hepatogastric trunk (Fig. 2; Bifurcation Type I), but both right and left accessory hepatic arteries from RHA as well as LHA respectively in 4 (6.90%) cases also (Fig. 2; Bifurcation Type I & Fig. 4; Trifurcation Type III) and from LGA (Fig. 2; Bifurcation Type II, Tetrafurcation Type I) in 11 (18.97%) cases respectively.
Classical origin of LGA artery were more in compare to right gastric artery. Regarding variant sources, right gastric artery arose from GDA, CT, RHA, LHA, CHA etc. whereas LGA was from abdominal aorta only apart from CT. Details about variant origins of both arteries were tabulated in Table 3.
Few other additional arteries were also noticed in the form of pancreatic (Fig. 2; Tetrafurcation Type II & Fig. 3; Tetrafurcation Type I) and duodenal branches (Fig. 4; Tetrafurcation Type II).
In this study, we found concurrent variations of CT with hepatic and gastric arteries. Variations of RGA were highest among both sexes followed by LHA, CT, and LGA. Fig. 3 represents schematic diagram of arterial variations.
The complex arterial anatomy of foregut is highlighted since 1756 when Haller [14] first described variations of coeliac axis as ‘Haller’s tripod’ followed by other extensive researches focusing further classifications like Adachi [15] in 1928.
It is important to appreciate arterial variations of gut tube in the light of embryological explanations. Development involves origin from six pairs of ventral splanchnic vessels followed by spanning of these pairs and subsequent disappearance. CT is differentiated as artery of foregut and originates initially from dorsal aorta at 7th cervical segment followed by caudal migration to 12th thoracic segment as a result of differential growth and descent of abdominal viscera. Among other factors attributing to vascular variations include alteration of mid-gut rotation, physiological hernia, left-ward movement of spleen and hemodynamic changes of abdominal viscera during developmental process [16]. According to another hypothesis there is a longitudinal anastomosis between the primitive segmental arteries, which later regresses to give rise to develop normal vessel. However, in most cases vascular anomalies or formation of new atypical vessel often results from either persistence or complete/incomplete or failure of regression of parts of different primitive vessels [17].
A wide range of variations of CT have been reported in both cadaveric as well as radiological series among different populations [18-27]. Santos et al. [23] recorded CT bifurcations as many as 66.7% cases, although lesser incidences were noticed among other studies [18, 21, 22, 25-27] which is mostly similar to our observations as in 12.07% cases. In 18.97% cases, we found, PHA arising directly from CT or indirectly as common trunk with SA. It has been reported that such variations could influence surgical outcome among gastric cancer patients [28]. Some ‘atypical trifurcation’ was documented in previous reports [19] even in high frequency as 66% which was totally missing in our report. Rather, we found tetrafurcation in 22.41% cases. Incidence of complete absence was mentioned previously as high as 41.7% [23] which we failed to document as similar as other reports [18, 22]. Table 4 summarizes the comparison of such variations with present study [18-27].
Hepatic arterial variations have been observed more frequently than CT variations. Variation of CHA has been recorded as high as 41% and surprisingly, absence of CHA has been considered as clinically most relevant variations of CT branching which we found in 18.97% cases [29]. Variant CHA branching was seen in high incidence 70.21% in the form of bifurcation, trifurcation, and tetrafurcation. Additionally, we observed variant origin of GDA in 18.96% cases which has definitive pre-operative relevance in placement of catheter in different arterial interventions.
Moreover, presence replaced RHA in comparison to replaced or accessory LHA often exhibits an increased risk of hepatic complications [6]. Critical evaluation of aberrant LHA is also required for esophagectomy [10]. Unfortunately, it is also reported that pre-treatment computed tomography failed to identify aberrant LHA in 31% cases [30]. In this context, surgical decision to ligate LGA as a source should be distal to origin of replaced hepatic artery to avoid iatrogenic hypoperfusion of liver [6]. However, risk of vascular injury often gets enhanced during laparoscopic procedures making pre-operative clarification of aberrant LHAs mandatory before arterial ligation for patients undergoing laparoscopic assisted gastrectomy to avoid risk of bleeding, ischemia, necrosis, embolism and hypovolemic shock [16]. It is important to mention that origin of MHA either from proper or RHA or LHA does not require special attention as it often corroborates persistence of fetal pattern for supplying fourth hepatic segment [6]. But in present study, MHA was arising as common trunk with LHA from CHA.
Variation of gastric arteries have definitive clinical values in upper gastro-intestinal interventions. Accurate identification of source and course of gastric artery often helps in arterial catheterization to control gastric hemorrhage [10]. In the present study, RGA was most common variant form (52.02%) than LGA (34.48%).
In the present study, we observed concurrent arterial variations affecting different parts of foregut with variable frequencies. So, it is imperative that the clinicians, surgeons and radiologists working on this area must be well versed with the detail of anatomical knowledge of arterial anatomy of foregut.
In conclusion, literature on vascular supply of foregut has not proved to be adequate. Acquaintance about variations of arteries is not only crucial for diagnostic error but also for decision making during upper abdominal surgeries. In this context, cadaveric dissections need to be highlighted to provide new insight. Present data shows considerable variations in branching of CT and CHA in 20 (34.48%) and 33 (70.21%) cases respectively along with PHA replacing CHA in 11 (18.96%) cases, whereas variant origins of RHA & LHA in 16 (27.59%) cases each and RGA & LGA in 52.02%, and 34.48% cases respectively which might facilitate surgeons to achieve best possible results in surgical techniques in the challenging area of post-pharyngeal part of foregut in abdomen.
Acknowledgements
Authors sincerely thank those who donated their bodies to Department of Anatomy and gave consent to conduct studies for anatomical research purposes. We also acknowledge the great help received from Department of Anatomy.
Notes
References
1. Hemamalini . 2018; Variations in the branching pattern of the celiac trunk and its clinical significance. Anat Cell Biol. 51:143–9. DOI: 10.5115/acb.2018.51.3.143. PMID: 30310705. PMCID: PMC6172596.
2. Standring S, Gray H. Gray's anatomy: the anatomical basis of clinical practice. 40th ed. Churchill Livingstone/Elsevier;2008.
3. Walker TG. 2009; Mesenteric vasculature and collateral pathways. Semin Intervent Radiol. 26:167–74. DOI: 10.1055/s-0029-1225663. PMID: 21326561. PMCID: PMC3036491.
4. Ochoa JE, Pointer DT Jr, Hamner JB. 2016; Vascular anomalies in pancreaticoduodenectomy: a lesson learned. Case Rep Surg. 2016:5792980. DOI: 10.1155/2016/5792980. PMID: 27200204. PMCID: PMC4856910.
5. Malviya KK, Verma A, Nayak AK, Mishra A, More RS. 2021; Unraveling variations in celiac trunk and hepatic artery by CT angiography to aid in surgeries of upper abdominal region. Diagnostics (Basel). 11:2262. DOI: 10.3390/diagnostics11122262. PMID: 34943499. PMCID: PMC8700197.
6. Jalamneh B, Nassar IJ, Sabbooba L, Ghanem R, Nazzal Z, Kiwan R, Awadghanem A, Maree M. 2023; Exploring anatomical variations of abdominal arteries through computed tomography: classification, prevalence and implications. Cureus. 15:e41380. DOI: 10.7759/cureus.41380. PMID: 37546145. PMCID: PMC10400811.
7. Zaki SM, Abdelmaksoud AHK, Khaled BEA, Abdel Kader IA. 2020; Anatomical variations of hepatic artery using the multidetector computed tomography angiography. Folia Morphol (Warsz). 79:247–54. DOI: 10.5603/FM.a2019.0090. PMID: 31436302.
8. Hiatt JR, Gabbay J, Busuttil RW. 1994; Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 220:50–2. DOI: 10.1097/00000658-199407000-00008. PMID: 8024358. PMCID: PMC1234286.
9. Michels NA. 1966; Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 112:337–47. DOI: 10.1016/0002-9610(66)90201-7. PMID: 5917302.
10. Ande T, Makani TK, Nannam K, Velichety SD, Kumar JA. 2023; Left gastric artery variants: a cadaveric, postmortem and radiological investigation. Scr Med. 54:157–61. DOI: 10.5937/scriptamed54-44773.
11. Mazurek A, Juszczak A, Walocha JA, Pasternak A. 2021; Rare combined variations of the coeliac trunk, accessory hepatic and gastric arteries with co-occurrence of double cystic arteries. Folia Morphol (Warsz). 80:460–6. DOI: 10.5603/FM.a2020.0052. PMID: 32459367.
12. Wu X, Kang J, Liu Y, Sun G, Shi Y, Niu J. 2022; A rare hepatic artery variant reporting and a new classification. Front Surg. 9:1003350. DOI: 10.3389/fsurg.2022.1003350. PMID: 36105121. PMCID: PMC9465518.
13. Ugurel MS, Battal B, Bozlar U, Nural MS, Tasar M, Ors F, Saglam M, Karademir I. 2010; Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol. 83:661–7. DOI: 10.1259/bjr/21236482. PMID: 20551256. PMCID: PMC3473504.
14. Haller AV. [Anatomical icons with which some of the main parts of the human body are outlined are presented & the arteries especially the history is contained]. Vandenhoeck;1756. Latin.
15. Adachi B. [The Arterial System of the Japanese]. Kaiserlich-Japanischen Universitat zu Kyoto, Maruzen Publishing Co;1928. Japanese.
16. Agarwal S, Pangtey B, Vasudeva N. 2016; Unusual variation in the branching pattern of the celiac trunk and its embryological and clinical perspective. J Clin Diagn Res. 10:AD05–7. DOI: 10.7860/JCDR/2016/19527.8064. PMID: 27504274. PMCID: PMC4963634.
17. Sadler TW. Sadler TW, editor. Cardiovascular system. Langman's Medical Embryology. 12th ed. Wolters Kluwer;2012. p. 189.
18. Chitra R. 2010; Clinically relevant variations of the coeliac trunk. Singapore Med J. 51:216–9. PMID: 20428743.
19. Juszczak A, Mazurek A, Walocha JA, Pasternak A. 2021; Coeliac trunk and its anatomic variations: a cadaveric study. Folia Morphol (Warsz). 80:114–21. DOI: 10.5603/FM.a2020.0042. PMID: 32301103.
20. Mburu KS, Alexander OJ, Hassan S, Bernard N. 2010; Variations in the branching pattern of the celiac trunk in a Kenyan population. Int J Morphol. 28:199–204. DOI: 10.4067/S0717-95022010000100028.
21. Panagouli E, Venieratos D, Lolis E, Skandalakis P. 2013; Variations in the anatomy of the celiac trunk: a systematic review and clinical implications. Ann Anat. 195:501–11. DOI: 10.1016/j.aanat.2013.06.003. PMID: 23972701.
22. Pinal-Garcia DF, Nuno-Guzman CM, Gonzalez-Gonzalez ME, Ibarra-Hurtado TR. 2018; The celiac trunk and its anatomical variations: a cadaveric study. J Clin Med Res. 10:321–9. DOI: 10.14740/jocmr3356w. PMID: 29511421. PMCID: PMC5827917.
23. Santos PVD, Barbosa ABM, Targino VA, Silva NA, Silva YCM, Barbosa F, Oliveira ASB, Assis TO. 2018; Anatomical variations of the celiac trunk: a systematic review. Arq Bras Cir Dig. 31:e1403. DOI: 10.1590/0102-672020180001e1403. PMID: 30539978. PMCID: PMC6284376.
24. Srivastava AK, Sehgal G, Sharma PK, Kumar N, Singh R, Parihar A, Aga P. 2012; Various types of branching patterns of celiac trunk. FASEB J. 26(Suppl 1):722.5. DOI: 10.1096/fasebj.26.1_supplement.722.5.
25. Sureka B, Mittal MK, Mittal A, Sinha M, Bhambri NK, Thukral BB. 2013; Variations of celiac axis, common hepatic artery and its branches in 600 patients. Indian J Radiol Imaging. 23:223–33. DOI: 10.4103/0971-3026.120273. PMID: 24347852. PMCID: PMC3843330.
26. Torres K, Staśkiewicz G, Denisow M, Pietrzyk Ł, Torres A, Szukała M, Czekajska-Chehab E, Drop A. 2015; Anatomical variations of the coeliac trunk in the homogeneous Polish population. Folia Morphol (Warsz). 74:93–9. DOI: 10.5603/FM.2014.0059. PMID: 25792402.
27. Venieratos D, Panagouli E, Lolis E, Tsaraklis A, Skandalakis P. 2013; A morphometric study of the celiac trunk and review of the literature. Clin Anat. 26:741–50. DOI: 10.1002/ca.22136. PMID: 22886953.
28. Huang Y, Mu GC, Qin XG, Chen ZB, Lin JL, Zeng YJ. 2015; Study of celiac artery variations and related surgical techniques in gastric cancer. World J Gastroenterol. 21:6944–51. DOI: 10.3748/wjg.v21.i22.6944. PMID: 26078572. PMCID: PMC4462736.
29. Cirocchi R, D'Andrea V, Amato B, Renzi C, Henry BM, Tomaszewski KA, Gioia S, Lancia M, Artico M, Randolph J. 2020; Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance. Surgeon. 18:100–12. DOI: 10.1016/j.surge.2019.06.002. PMID: 31337536.
30. van den Hoven AF, Smits ML, de Keizer B, van Leeuwen MS, van den Bosch MA, Lam MG. 2014; Identifying aberrant hepatic arteries prior to intra-arterial radioembolization. Cardiovasc Intervent Radiol. 37:1482–93. DOI: 10.1007/s00270-014-0845-x. PMID: 24469409.
Fig. 1
Classical picture of coeliac trunk. 1, coeliac trunk; 2, splenic artery; 3, left gastric artery; 4, common hepatic artery; 5, proper hepatic artery; 6, gastro-duodenal artery.
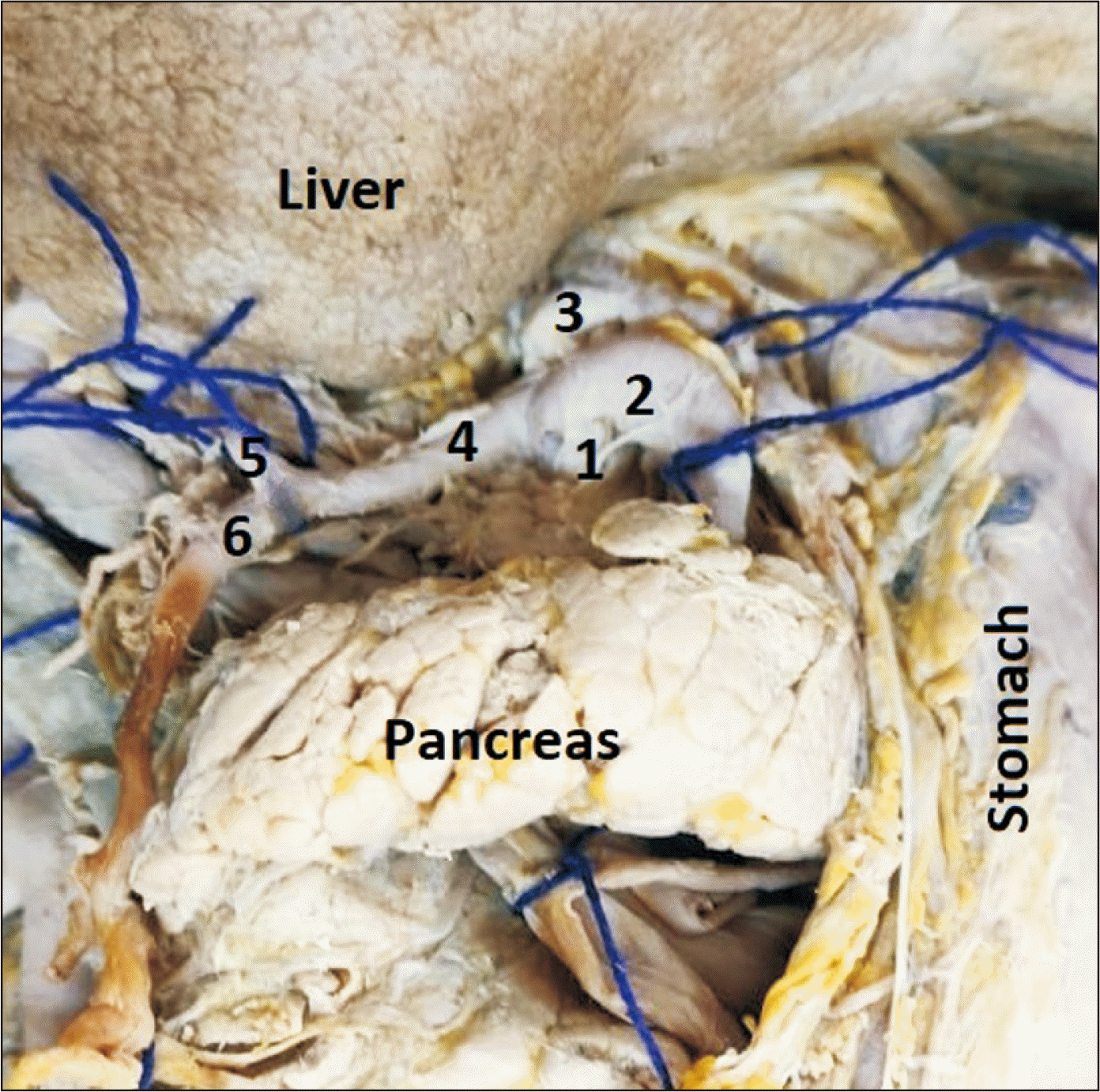
Fig. 2
Variations of branching pattern of coeliac trunk. (A) Bifurcation Type I. (B) Tetrafurcation Type I. (C) Bifurcation Type II. (D) Tetrafurcation Type II. 1, coeliac trunk; 2, hepatosplenic trunk; 3, hepatogastric trunk; 4, common hepatic artery; 5, splenic artery; 6, left hepatic artery; 7, left gastric artery; 8, proper hepatic artery; 9, gastro-duodenal artery; 10, superior pancreatico-duodenal artery; 11, accessory right hepatic artery; 12, right gastric artery; 13, accessory left hepatic artery; 14, right hepatic artery; 15, pancreatic branch; 16, abdominal aorta.
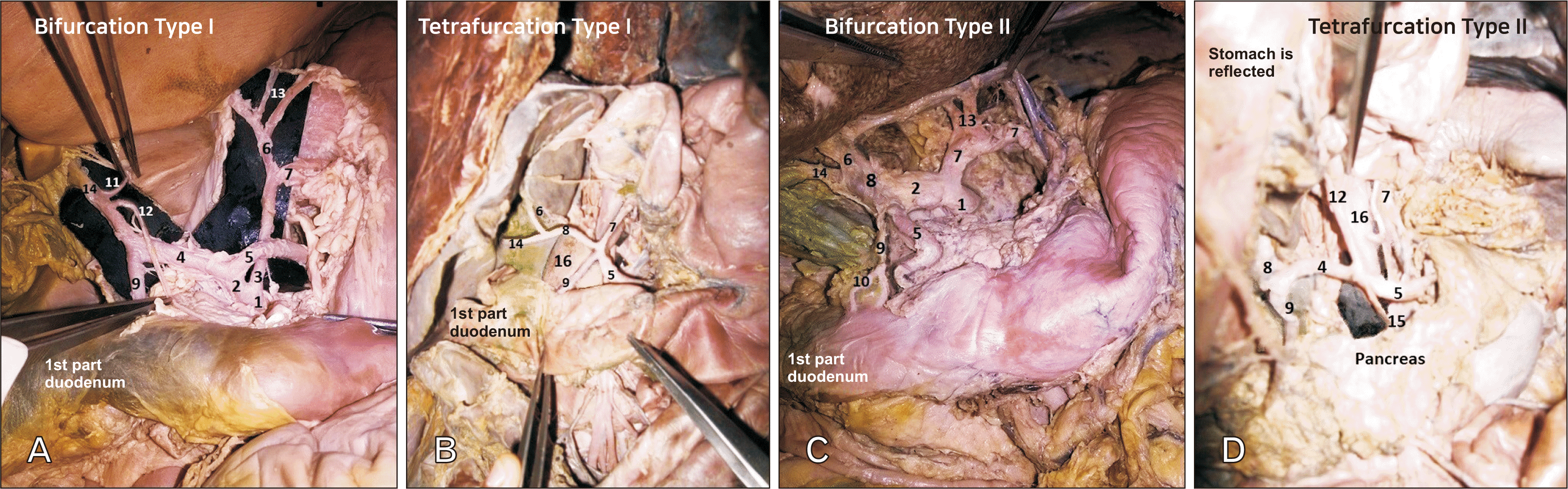
Fig. 3
Schematic diagram of variations of branching pattern of (A) coeliac trunk and (B) hepatic artery. 1, coeliac trunk; 2, hepatosplenic trunk; 3, hepatogastric trunk; 4, common hepatic artery; 5, splenic artery; 6, left hepatic artery; 7, left gastric artery; 8, proper hepatic artery; 9, gastro-duodenal artery; 10, superior pancreaticoduodenal artery; 11, accessory right hepatic artery; 12, right gastric artery; 13, accessory left hepatic; 14, right hepatic artery; 15, pancreatic branch; 16, abdominal aorta; 17, middle hepatic artery; 18, common trunk for middle and left hepatic artery; 19, common trunk for supraduodenal and superior pancreatoduodenal artery; 20, accessory duodenal artery; 21, supraduodenal artery.
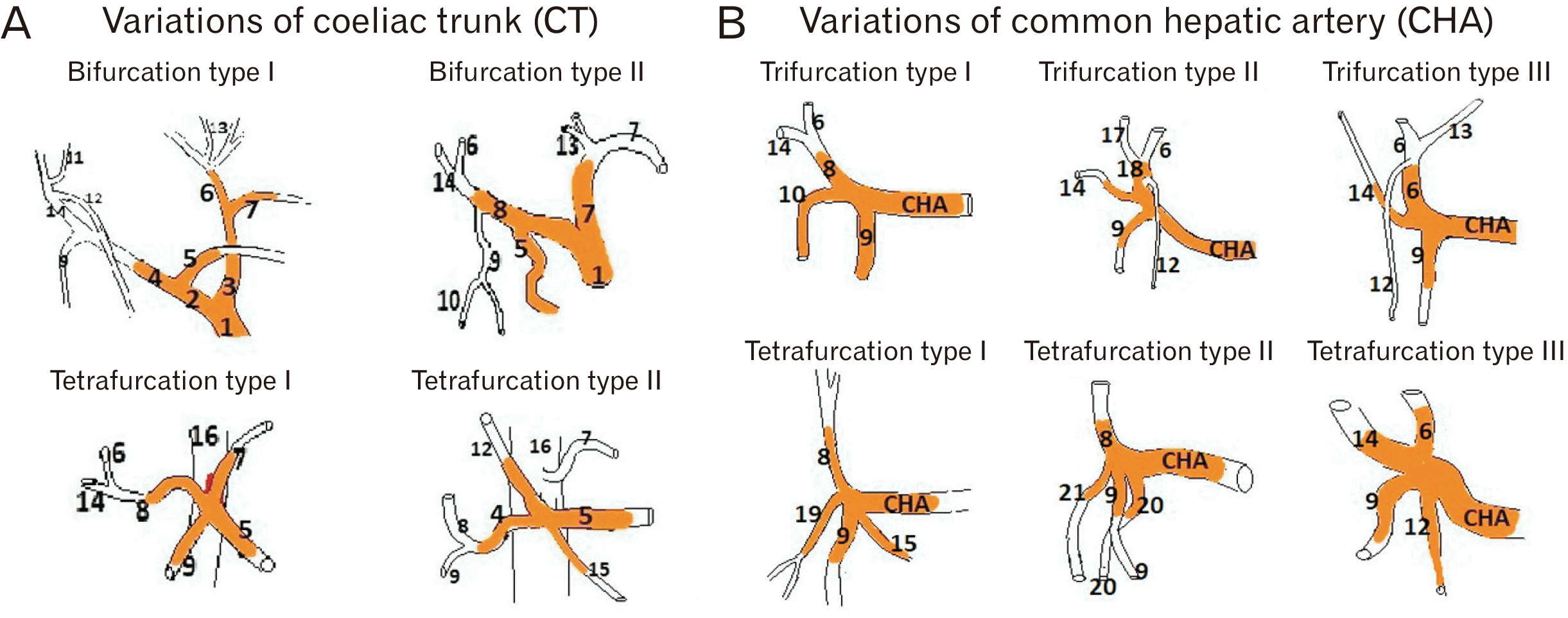
Fig. 4
Variations of branching pattern of common hepatic artery. (A) Trifurcation Type I. (B) Trifurcation Type II. (C) Trifurcation Type III. (D) Tetrafurcation Type I. (E) Tetrafurcation Type II. (F) Tetrafurcation Type III. a, proper hepatic artery; b, gastro-duodenal artery; c, left hepatic artery; d, right hepatic artery; e, superior pancreatico-duodenal artery; f, middle hepatic artery; g, common trunk for left and middle hepatic artery; h, right gastric artery; i, accessory left hepatic artery; j, common trunk for supra duodenal and superior pancreatico-duodenal; k, pancreatic branch; l, accessory duodenal branch; m, supra duodenal artery; CHA, common hepatic artery.

Table 1
Branching pattern of coeliac trunk
Table 2
Branching pattern of common hepatic artery (n=47)
Table 3
Incidence of classical and variant origin of gastric arteries
Table 4
Comparison of frequency of coeliac trunk variations between previous and present study
| Author | Country | Total sample (n) | Type of study | Trifurcation (%) | Bifurcation (%) | Tetrafurcation (%) | Absent (%) | |
|---|---|---|---|---|---|---|---|---|
| Classical | Atypical | |||||||
| Srivastava et al. [24] | India | 50 | CT scan | 8 | - | 28 | 36 | 4 |
| Panagouli et al. [21] | - | 12,196 | Review article | 89.42 | - | 7.40 | - | 0.38 |
| Torres et al. [26] | Poland | 1,569 | CT scan | 92.7 | - | 2.4 | - | 0.1 |
| Santos et al. [23] | - | - | Systemic review | 75 | - | 66.7 | 8.33 | 41.7 |
| Sureka et al. [25] | India | 600 | MDCT | 91 | 4 | 0.82 | - | - |
| Mburu et al. [20] | Kenya | 123 | Cadavers | 61.7 | - | 17.9 | - | - |
| Chitra [18] | India | 50 | Cadavers | 40 | - | 0.06 | - | 0 |
| Venieratos et al. [27] | Caucasus | 77 | Cadavers | 74 | 16.9 | 1.3 | - | 2.6 |
| Pinal-Garcia et al. [22] | Mexico | 140 | Cadavers | 7.1 | 36.4 | 7.1 | 12.9 | 0 |
| Juszczak et al. [19] | Poland | 50 | Cadavers | 16 | 66 | - | - | - |
| Present study | Eastern India | 58 | Cadavers | 65.51 | 0 | 12.07 | 22.41 | 0 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download