Abstract
Stem cells transplantation (SCT) is known as a newfound strategy for multiple sclerosis (MS) treatment. Human umbilical cord mesenchymal stem cells (hUCMSCs) contain various regenerative features. Experimental autoimmune encephalomyelitis (EAE) is a laboratory model of MS. This meta-analysis study was conducted to assess the overall therapeutic effects of hUCMSCs on reduction of clinical score (CS) and restoration of active movement in EAE-induced animals. For comprehensive searching (in various English and Persian databases until May 1, 2024), the main keywords of “Experimental Autoimmune Encephalomyelitis”, “Multiple Sclerosis”, “Human”, “Umbilical Cord”, “Mesenchymal”, and “Stem Cell” were hired. Collected data were transferred to the citation manager software (EndNote x8) and duplicate papers were merged. Primary and secondary screenings were applied (according to the inclusion and exclusion criteria) and eligible studies were prepared for data collection. CS of two phases of peak and recovery of EAE were extracted as the difference in means and various analyses including heterogeneity, publication bias, funnel plot, and sensitivity index were reported. Meta-analysis was applied by CMA software (v.2), P<0.05 was considered a significant level, and the confidence interval (CI) was determined 95% (95% CI). Six eligible high-quality (approved by ARRIVE checklist) papers were gathered. The difference in means of peak and recovery phases were –0.775 (–1.325 to –0.225; P=0.006; I2=90.417%) and –1.230 (–1.759 to –0.700; P<0.001; I2=93.402%), respectively. The overall therapeutic effects of SCT of hUCMSCs on the EAE cases was –1.011 (95% CI=–1.392 to –0.629; P=0.001). hUCMSCs transplantation through the intravenous route to the animal MS model (EAE) seems a considerably effective procedure for the alleviation of motor defects in both phases of peak and recovery.
Multiple sclerosis (MS) is a chronic inflammatory disease damaging to the myelin sheath in the central nervous system (CNS). This pathology disrupts the transmission of neural signals, leading to a variety of symptoms. Although MS is an immune-mediated pathology, it is a progressive neurodegeneration with an unknown cause of etiology [1]. In this condition, the immune system attacks the myelin structure in the CNS. MS is more commonly diagnosed in females than males, and it is typically diagnosed between the age ranges of 20–40. Environmental factors have been implicated in the epidemiology of MS such as sunlight exposure and vitamin D deficiency which are associated with the increased risk of developing MS. 2.8×106 MS patients were diagnosed until 2020 and 35.9×105 individuals are susceptible to MS [2].
Experimental autoimmune encephalomyelitis (EAE) is an animal model of neural inflammation which is widely studied as a laboratory model of human CNS demyelinating diseases, including MS. EAE is an inflammatory demyelinating disease of CNS induced by active sensitization using neural antigens. EAE is also the prototype for T-cell-mediated autoimmune disease. This pathology has been established in numerous species and is induced by priming with a large number of CNS-derived antigens. As a consequence, the pathogenesis, pathology, and clinical signs vary significantly between experimental protocols [3].
Stem cells (SCs) are unspecialized microorganisms with the remarkable ability to self-renew indefinitely and differentiate into more mature cells with specialized features. They play a crucial role in tissue homeostasis, repair, and regeneration [4]. There are two main types of SCs, embryonic stem cells (ESCs) and adult stem cells (ASCs). ESCs are derived from the inner cell mass of the early embryo, while the ASCs are found in adult tissues such as bone marrow, skin, and brain. ESCs are pluripotent with differentiation ability into cells of all three germ layers. Another type of pluripotent SC is induced pluripotent stem cells (iPSCs) which are generated by reprogramming of adult cells into an embryonic-like state. SCs possess the remarkable potential to differentiate into specialized cell types, such as muscle cells, blood cells, and neural cells. This ability makes SC a valuable resource for regenerative medicine and potential treatments for various diseases and injuries [5].
The umbilical cord is a tube-like structure connecting the fetus to the placenta. It is considered both physical and emotional attachment between mother and fetus. This structure contains SCs found in the blood of umbilical cord and placenta. These cells are a rich source of hematopoietic SCs with the ability to differentiate into various types of blood cells [6]. Umbilical cord SCs have been used for treatment of various diseases, including leukemia, lymphoma, and other hematopoietic disorders [7]. The application of umbilical cord SCs is considered a safe and effective alternative than bone marrow transplantation. The collection of umbilical cord SCs is a non-invasive procedure with no possible damage to the mother or baby. The blood of umbilical cord contains an abundant population of SCs, including hematopoietic SCs, which can differentiate into various types of blood cells. These cells have been used to treat a wide range of diseases, including leukemia, lymphoma, and other blood disorders. Umbilical cord SCs are a valuable resource for regenerative medicine and potential treatments for various diseases and injuries [8]. Umbilical cord SCs are used for treatment of EAE, an animal model of MS. Human umbilical cord mesenchymal stem cells (hUCMSCs) have been shown to regulate the immune system function in EAE models through the suppression of pro-inflammatory cytokines production and increasing the anti-inflammatory molecules production. These cells also promote remyelination in the EAE model by enhancement of oligodendrocyte precursor differentiation into mature oligodendrocytes [9]. Besides these cells can infiltrate into the spinal cord of EAE animals leading to the reduction of demyelination [10]. Some studies showed the therapeutic role of transplanted umbilical cord MSCs on the monkey model of MS [11].
Systematic reviews and meta-analyses are important tools for making evidence-based decisions in healthcare, including in clinical settings. Experimental animal studies are often conducted to analyze the safety and efficacy of treatments for human clinical trials. Although various studies have been conducted on the potential therapeutic effects of hUCMSCs with regenerative characteristics on reduction of motor defects in animals with EAE, no comprehensive conclusion regarding the efficacy of this protocol has been published. The efficacy of xenograft transplantation of hUCMSCs on the reduction of clinical score (CS) of EAE phases, including onset, peak, or recovery, was assessed in the present study. Meta-analyses of experimental animal studies, including those on EAE, can provide more robust results than single animal studies, but the methodology for such meta-analyses needs to be adapted and optimized for animal intervention studies.
All assessments were conducted on in accordance with ethical principles and under the supervision of the Ethics Committee of Baqiyatallah University of Medical Scineces (Ethic no. IR.BMSU.BAQ.REC.1402.031).
In the present systematic review and meta-analysis study, the authors aimed to evaluate the efficacy levels of hUCMSCs transplantation in reduction of motor defects in EAE animals. The assessment of efficacy was applied in two MS phases of peak, and recovery. In this era, the CS was considered a measurable effect size in studies. Following designation of the research question, the relevant keywords were identified using medical subject headings (MeSH) terms and text words including “Experimental autoimmune encephalomyelitis”, “Multiple Sclerosis”, “Human”, “Umbilical cord”, “Mesenchymal”, “Stem Cell”, “Rat”, and “Mice”. The search strategy was implemented in various English (PubMed, Scopus, Web of Science, ScienceDirect, and Islamic World Science Citation Center) and Persian (MagIran and scientific information database) databases, focusing on the title, abstract, and keywords of published papers. Additionally, the Google Scholar search engine and citations of included articles were also screened carefully to achieve a comprehensive searching procedure. Operators of “OR” and “AND” were utilized to combine the keywords for searching strategy generation. For paper searching, no time limitation was imposed, and all relevant papers were considered in the searching until May 1, 2024. The utilized searching strategies are listed as Table 1. Following paper collection, the associated citations were exported to the Citation Manager Software (EndNote x8; Clarivate Analytics), and duplicate articles were detected and merged. By the use of this software, the primary screening of articles was conducted based on the assessment of the title and abstract. In this stage, the irrelevant papers were excluded and the full text of final included papers was prepared for deep secondary screening. In secondary stage of searching, all experimental investigations conducted on EAE animals (rats or mice) and treated with hUCMSCs transplantation were included and the main findings and special procedures of each study were summarized in Table 2. Three independent authors conducted the article assessments blindly during the screenings. In each stage of the screening, the corresponding author managed and resolved any possible conflicts or disagreements between the reports. In whole stages of primary and secondary screenings, the process of paper sorting was conducted based on the PRISMA 2020 flowchart (Fig. 1) [12], including; identification, screening, eligibility, and included. The Animal Research: Reporting of In Vivo Experiments (ARRIVE) checklist was also used for assessment of the study quality (Table 2) [13]. In this guideline, all components of the article were considered for quality assessment. The final ARRIVE score <25 was considered inadequate quality, 25–50 as low, 50–75 as medium, and the quality score >75 was a high-quality study. After eligible paper collection and summarizing the main tips of each article (Table 2), the CS index as a measurable value was extracted for meta-analysis procedure. CSs were categorized from 0 to 5 as follows; 0: normal, 1: loss of animals’ tail tonicity, 2: full animals’ tail paralysis, 3: paralyses of one hind leg or partial paralysis of both hind legs, 4: paralyses of both hind legs, and 5: moribund. To resolve the heterogeneity among the study, subgrouping was applied, and I2 index was also reported. Collected data were categorized into two different subgroups of peak (of disease), and recovery (after the treatment). Publication bias assessment was considered, and the related funnel plot was reported. Finally, one-study removed test was used to report the sensitivity index. Significance value was considered P<0.05, and the confidence interval (CI) level was determined 95% in Cochran’s formula (95% CI). All statistical analysis was conducted using comprehensive meta‐analysis software (version 2) [14].
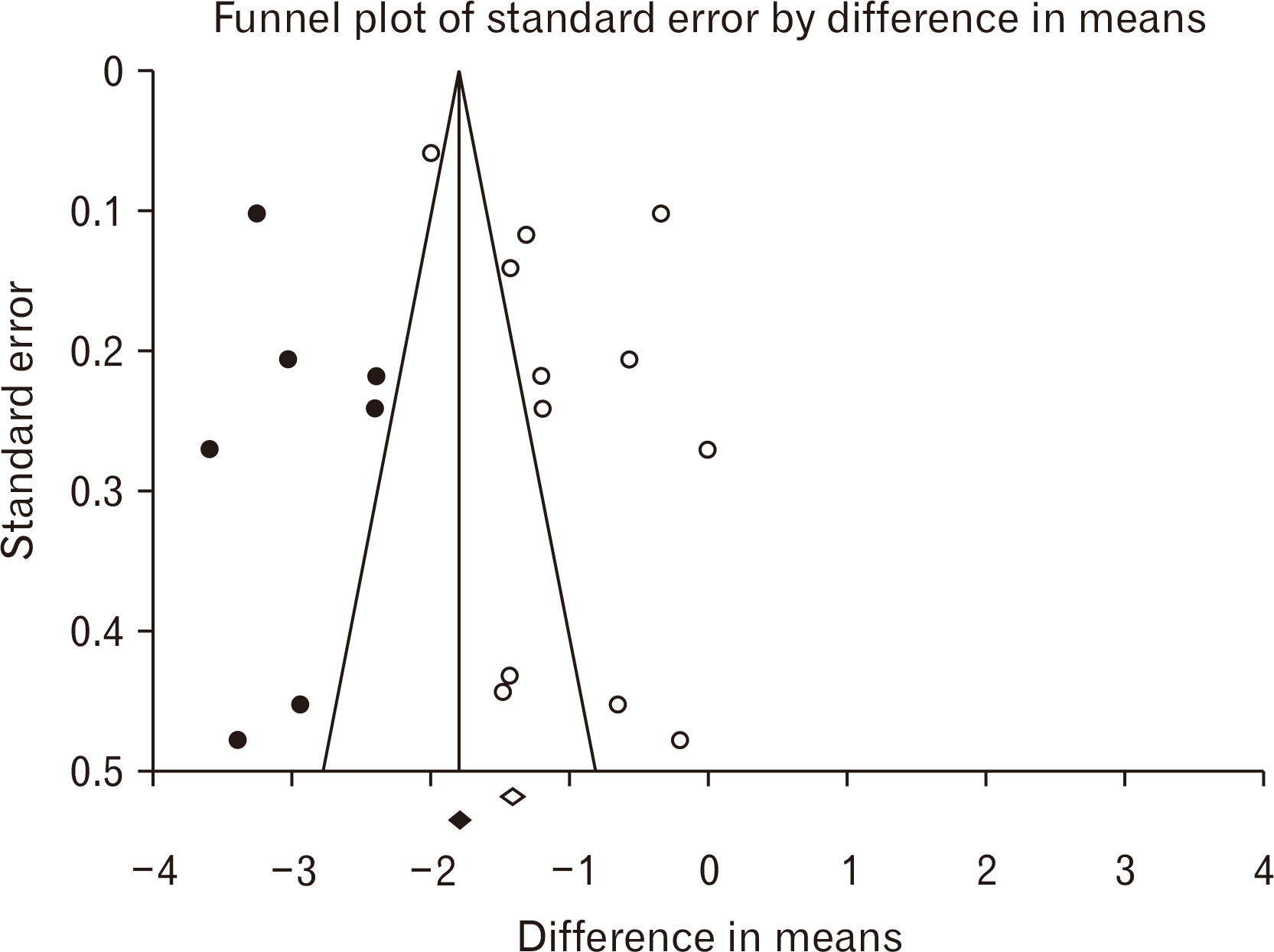
According to the Fig. 1 representing the PRISMA flowchart, 404 papers were selected during various database searching. In this era, 282 duplicate investigations were merged. Following primary assessment of the articles, only 6 ones were found eligible for data extraction and meta-analysis including Ahmadvand Koohsari et al. [15], Zhou et al. [16], Torkaman et al. [17], Donders et al. [18], Mikaeili Agah et al. [19], and Liu et al. [9]. It is also noteworthy to state that following Google Scholar-based searching, 6 non-eligible articles were also found. For the quality assessment of six included papers, the ARRIVE procedure was used. According to the findings of the ARRIVE assessment, all studies represented high-quality investigations. The ARRIVE score for the study of Ahmadvand Koohsari et al. [15] was 89 and this value for Zhou et al. [16], Torkaman et al. [17], Donders et al. [18], Mikaeili Agah [19], and Liu et al. [9] was calculated 75 (Table 2, Fig. 2) [20, 21]. According to the Table 2, surface markers detection and flowcytometry procedure are critical laboratory steps for increasing the injection levels of hUCMSCs. Also, SCs differentiation into the intended types of cells were used in some studies for better treatment outcomes. In most studies, the SCs were injected intravenously and in a study, the cerebroventricular approach was hired. Optimization was also another main step of this cellular transplantation which was mainly conducted using IFN-γ administration. The difference in means was –0.775 (–1.325 to –0.225; P=0.006) and –1.230 (–1.759 to –0.700; P<0.001) for both subgroups of peak and recovery. The overall effect was also detected –1.011 (–1.392 to –0.629; P<0.001) (Fig. 3). The I2 index was hired for heterogeneity assessment in both respective subgroups of peak and recovery including 90.417% (Q=53.847; P<0.001) and 93.402% (Q=75.779; P<0.001). In this era, the overall heterogeneity was reported 95.8% (Q=261.892; P<0.001). In publication bias assessment, the P-value (2-tailed) index for Egger’s regression intercept and Begg rank correlation were respectively 0.78 and 0.09 representing no publication bias (Fig. 4). Also, the number of missing studies (α=0.05) was detected 1,649 according to the test of classic Fail-Safe N test indicating the stability of collected data. The sensitivity evaluation (using the one-study removed option) was also found P<0.001 (Fig. 5).
Following assessment of 6 eligible studies representing the role of hUCMSCs transplantation to the animals with a laboratory model of MS showed that this type of cellular therapeutic procedure can potentially alleviate the CS and restore motor ability considerably. hUCMSCs grafting is effective on both MS phases of peak and recovery with the respective difference in means including –0.775 and –1.230. Totally the overall effect of the application of hUCMSCs on EAE reduction was reported at –1.011 with a P-value of <0.001 indicating the significant therapeutic role of hUCMSCs on EAE.
The umbilical cord is a rich source of SCs with the potential ability for differentiation into various cell types. These cells offer more advantages than SCs available in bone marrow with no requirement for human leukocyte antigen (HLA) tissue matching [22]. Researchers have discovered the SCs in amniotic fluid as well as the blood of umbilical cord [23]. The umbilical cord is also known as a rich source of MSCs. Due to the cryopreservation of umbilical cord immediately after birth, the available SCs can be successfully stored for future therapies of life-threatening diseases. hUCMSCs have been found to have a wide range of medicinal applications. These types of cells contain the powerful ability to differentiate into various cell types, including bone [24], cartilage [25], and germ-like cells [26]. They also have immunosuppressive properties [27] with useful applications in treatment of autoimmune diseases and prevention of transplant rejection. In other studies, transplantation of hUCMSCs has been found to have hepatoprotective effects [28] decreasing hepatocellular necrosis following liver injury. The potential ability for treatment of neurological disorders such as spinal cord injury [29], stroke [30], and Parkinson’s disease [31] is another characteristic of hUCMSCs.
Cell therapy is a type of medicinal treatment using viable cells injection, grafted, or implanted for patients. The cells used in cell therapy can be obtained from a variety of sources, including the patient’s body (autograft), a human donor (allograft), an animal donor (xenograft), or a cultured cell line. The goal of cell therapy is to replace or repair damaged cells or tissues, modulate the function of the patient’s organ through, or remove disease-causing or dysfunctional cells using immune cells. There are many different types of cell therapies, including hematopoietic stem cells transplantation (SCT) [32], CAR T-cell therapy [33], and tumor-infiltrating lymphocyte therapy [34]. Cell therapy has shown great promise in the treatment of a wide range of diseases and conditions, including cancer, autoimmune diseases, and infectious diseases [35].
EAE is a laboratory model of MS that is commonly used to study the pathophysiology and treatment of disease. EAE is induced in animals, usually mice or rats, by injection of myelin antigens or activated T-cells that recognize myelin antigens. This phenomenon leads to an autoimmune response which causes inflammation and demyelination of CNS, similar to what is seen in MS patients. The inflammatory features of EAE include activation of T lymphocytes, infiltration of immune cells (macrophages, T cells, and B cells), and demyelination. EAE models recapitulate the chronic inflammatory features of MS. While much work remains to be applied in laboratories and clinics to understand how to use these cells for cell-based therapies to treat disease, SC therapy offers immense potential for the remedy of various diseases.
Recent studies have shown that the hUCMSCs contain potential to alleviate motor defects in animals with EAE. In one study, researchers found that hUCMSCs could restore behavioral functions and attenuate the histopathological features of EAE in mice [15]. Another study found that this type of cell therapy could also promote neuroregeneration and improve motor function in rats with EAE [16]. The molecular mechanism underlying the therapeutic effects of hUCMSCs in reduction of motor defects and treatment of MS is not yet fully understood. However, several studies have suggested that the immunomodulatory and neuro-regenerative [36] properties of these cells. hUCMSCs have been found to induce immunomodulatory effects leading to regulation of immune system and prevention of autoimmune responses probably through the secretion of cytokines and chemokines suppressing the activation and proliferation of immune cells [36]. hUCMSCs can generate a range of cytokines and neurotrophic factors promoting neuroregeneration and repair [37]. These factors may stimulate the growth and differentiation of neural SCs, promote axonal regeneration, and enhance synaptic plasticity [38]. These MSCs also promote remyelination [39] in CNS through the secretion of factors affecting stimulation of oligodendrocyte differentiation and myelin formation. This main characteristic can be achieved through the secretion of factors inhibiting the activation of microglia and astrocytes. Totally, the therapeutic effects of hUCMSCs in reduction of motor defects in MS cases are likely to be multifactorial and involve complex cellular and molecular mechanisms. Further research is needed to fully understand these mechanisms and optimize the use of hUCMSCs in clinical settings.
Ahmadvand Koohsari et al. [15] investigated the impact of extracellular vesicles derived from hUCSC-extracellular vesicles (EVs) on EAE. The results showed that treatment with hUCSC-EVs results in lower CS. This finding suggests that hUCSC-EVs have a therapeutic effect in EAE treatment, similar to other sources of MSCs. The study also analyzed the levels of inflammatory and anti-inflammatory cytokines. The results indicated that treatment with hUCSC-EVs led to reduced levels of pro-inflammatory cytokines and increased levels of anti-inflammatory agents. This suggested that hUCSC-EVs could represent immunomodulatory properties, regulating the inflammatory response associated with EAE. The researchers also examined the extent of leukocyte infiltration in spinal cord. The study found that treatment with hUCSC-EVs resulted in decreased leukocyte infiltration. This indicates that hUCSC-EVs may inhibit the migration of immune cells to the spinal cord, potentially reducing inflammation and tissue damage. This evaluation also assessed the expression of glial fibrillary acidic protein (GFAP) and myelin basic protein (MBP) in spinal cord. The results showed that hUCSC-EV treatment induces an effective impact on expression of these proteins. Further details about the specific effects of hUCSC-EVs on GFAP and MBP were not provided. These findings suggest that hUCSC-EVs have therapeutic potential for treatment of EAE. They demonstrate the effectiveness of hUCSC-EVs in reduction of CS, modulation of cytokine levels, inhibition of leukocyte infiltration, and potential influence of expression of key proteins in spinal cord. Further research and clinical trials may be needed to fully understand the mechanisms underlying these effects and to explore the potential of hUCSC-EVs as a treatment for MS. Zhou et al. [16] investigated the probable therapeutic effects of IFN-γ primed human umbilical cord mesenchymal stem cell (IFN-γ-hUCMSCs) transplantation to EAE-induced mice. In this study, it was approved that IFN-γ-hUCMSCs transplantation considerably improves the outcomes of EAE in terms of body weight, clinical symptoms, nerve conduction, and inflammatory cytokine levels. Thus, the results suggest that IFN-γ-hUCMSCs have great potential for the treatment of EAE. The purity of the cultured hUCMSCs was over 95% at the 4th passage, indicating that they were suitable for SCT. Flow cytometry analysis also showed that the cultured cells expressed CD73, CD90, and CD105 >95%, while the expression of CD11b, CD19, CD34, CD45, and HLA-DR were <3%. This further confirmed that the cultured cells were hUCMSCs. The IFN-γ-hUCMSCs group showed a significant reduction in behavior scores compared to the hUCMSCs group, indicating alleviation of clinical symptoms. Transplantation of IFN-γ-hUCMSCs could considerably alleviate body weight loss and impairment of CS in EAE mice, performing better than the hUCMSCs group. In summary, this paper suggested that transplantation of IFN-γ-hUCMSCs has a positive impact on the outcomes of EAE in mice, demonstrating potential therapeutic benefits for the treatment of this autoimmune disease. In another study, Torkaman and coworkers [17] conducted an experimental study entitled “The effect of transplanted human Wharton’s jelly MSCs treated with IFN-c on experimental autoimmune encephalomyelitis mice”. The study evaluated the therapeutic effects of IFN-c primed WJ-MSCs (Wharton’s jelly MSCs) in EAE mice. The injected cells reduced cell infiltration, symptoms, and secretion of inflammatory cytokines in treated group. The relative expression of inflammatory genes such as interleukin (IL)-17, RoRg-t, IFN-c, and t-bet, was significantly decreased in the IFN-c primed hWJ-MSCs treated group compared to the unprimed hWJ-MSCs and HFFF-PI6 treated group. The expression of immunomodulatory genes IL-10 and Foxp3 mRNA was significantly increased in IFN-c primed hWJ-MSCs treated group compared to the unprimed hWJ-MSCs and HFFF-PI6 treated group. There was no significant difference between the groups regarding the fold change of IL-4 and GATA3 expression. Overall, the study suggests that IFN-c can enhance the immunomodulatory properties of WJ-MSCs in EAE treatment. The injected IFN-c primed WJ-MSCs showed promising results in reducing inflammation and symptoms in mice. Donders and coworkers [18] also investigated the use of SCs derived from Wharton’s jelly, which is a substance found in umbilical cords. These SCs have immunomodulatory properties. The study focused on the treatment of MS, specifically in a rat model of the disease called EAE. The SCs derived from Wharton’s jelly were found to temporarily improve symptoms in rats with EAE. This suggests that these types of SCs have potential therapeutic benefits for MS. Histological analyses of various tissues, including the CNS, lungs, and lymphoid tissue were performed to understand the underlying processes contributing to the observed differences in CS between the animals treated with Wharton’s jelly-derived SCs and those treated with saline. In the present study, it was also found that the SCs extracted from Wharton’s jelly were trapped primarily in the lungs and spleen of the animals. It’s important to note that these findings are based on the provided summary and further details may be available in the full document. Mikaeili Agah et al. [19] in a study discussed the pathological hallmarks of MS, which include the failure of the CNS to remyelination of MS lesions. This failure leads to irreversible functional decline in patients. The study also investigated the use of SCT as a potential treatment for dysmyelinating and demyelinating diseases, including MS. The researchers focus specifically on the potential of transplanted human Wharton’s jelly SCs-derived oligodendrocyte progenitor cells (hWJ-MSC-derived OPCs) as a cell source for transplantation therapy. The cells underwent three stages before fixation with paraformaldehyde. Immunocytochemical analysis was performed using primary antibodies (Nestin, platelet-derived growth factor receptor α [PDGFR α], and A2B5) and secondary antibodies. Positively stained cells were counted in ten random fields. The study induced the differentiation of hWJ-MSCs using growth factors (FGF2, EGF, and PDGF-AA). The differentiated cells exhibited changes in morphology and expressed PDGFR α or A2B5 markers. The researchers evaluated remyelination after the transplantation of OPCs. Sections from the corpus callosum were sampled for LFB staining, which revealed demyelination in the corpus callosum of the vehicle-transplanted control animals. However, the severity of demyelination was attenuated by the treatment with hWJ-MSC-derived OPCs. EAE mice that received OPC transplantation showed a significant attenuation of the CS compared to the vehicle-transplanted control animals. This indicates a beneficial effect of hWJ-MSC-derived OPC transplantation in reduction of severity of CS associated with EAE. Liu et al. [9] found that transplantation of hUCMSCs in EAE mice improved behavioral functions and reduced histopathological deficits in the spinal cord. The EAE-induced behavioral impairments were attenuated in animals that received hUCMSCs, as evidenced by a significant reduction in disease severity at days 22 and 50 post-transplantation. This functional recovery was associated with the amelioration of EAE-induced histopathological deficits, including decreased perivascular immune cell infiltrations, demyelination, and axonal injury in spinal cord of hUCMSC-treated EAE mice. The study provides scientific evidence supporting the clinical application of hUCMSC transplantation in patients with MS. The long-term recovery of behavioral functions and improvement of histopathological symptoms observed in hUCMSC-treated EAE mice suggest the potential therapeutic effects of hUCMSCs in MS. The study suggests that hUCMSC transplantation may involve multiple regenerative pathways, including immunomodulation and remyelination, which contribute to the beneficial outcomes of hUCMSC therapy. The key signaling protein, LINGO-1, plays a pivotal role in mediating both immunomodulatory and myelin-inducing mechanisms, offering new avenues for optimizing the therapeutic effects of hUCMSC transplantation in MS and other disorders characterized by immune and myelin dysfunctions. Overall, this study provides promising evidence that hUCMSC transplantation could be a potential therapy for MS, improving behavioral functions and reducing histopathological deficits in a mouse model of MS. The findings offer insights into the regenerative pathways involved in hUCMSC therapy and suggest potential targets for further optimizing its therapeutic effects.
Evaluation of cellular differentiation, including adipogenesis and osteogenesis, is crucial for the accurate identification of SCs isolated from umbilical cord tissue. This is because the SCs have the potential to differentiate into various cell types, and their differentiation patterns can be influenced by various factors, including the environment and the presence of specific growth factors. Thus, assessing their differentiation capacity is essential for understanding their potential therapeutic applications. Adipogenesis, the process by which the SCs differentiate into adipocytes, is a critical aspect of SC biology. Adipocytes play a crucial role in energy storage and metabolism, and their dysfunction has been implicated in various diseases, including obesity and insulin resistance. The ability of SCs to differentiate into adipocytes can be used to study the mechanisms of adipocyte development and to develop new therapeutic strategies for metabolic disorders. Osteogenesis, the process by which the SCs differentiate into osteoblasts, is also an important aspect of SC biology. Osteoblasts are responsible for bone formation and maintenance, and their dysfunction can lead to various bone disorders, including osteoporosis. The ability of SCs to differentiate into osteoblasts can be used to study the mechanisms of bone development and to develop new therapeutic strategies for bone disorders. The importance of evaluating cellular differentiation in SCs isolated from umbilical cord tissue cannot be overstated.
Regarding the limitations of the present systematic review and meta-analysis study, the small number of eligible selected studies was an important limitation. For this purpose and to eliminate possible scientific defects, studies were first collected based on strict inclusion/exclusion criteria, then, they were statistically evaluated in a pilot study regarding the publication bias and heterogeneity values. These analyses showed that although the number of studies is small, the same number can be valid for reports. On the other hand, the statistical analysis of missed articles was found in 1,604 representing high valid outcome of this study. Also, it is recommended for other original experimental investigations to consider the exact protocol of hUCMSCs extraction, differentiation, and supplementation. In some studies, these important protocols were missed leading to a scientific failure for future experiments. Thus, an incomplete explanation of protocols is a major limitation of the study. In this era, for future studies, it is recommended to explain the exact protocol of hUCMSCs extraction, and the supplementations used for cell differentiation are necessary to be reported.
This systematic review and meta-analysis study investigated the probable role of cellular transplantation of hUCMSCs in alleviation of CSs and restoration of motor defects in EAE animals. Since the results of meta-analysis are represented, this procedure can be effective in animal studies. For further studies, it is suggested to note the exact concentration of cells and optimization with exogenous materials.
Notes
References
1. Pérez-Jeldres T, Alvarez-Lobos M, Rivera-Nieves J. 2021; Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs. 81:985–1002. DOI: 10.1007/s40265-021-01528-8. PMID: 33983615. PMCID: PMC8116828.
2. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, Wallin M, Helme A, Angood Napier C, Rijke N, Baneke P. 2020; Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 26:1816–21. DOI: 10.1177/1352458520970841. PMID: 33174475. PMCID: PMC7720355.
3. Constantinescu CS, Farooqi N, O'Brien K, Gran B. 2011; Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 164:1079–106. DOI: 10.1111/j.1476-5381.2011.01302.x. PMID: 21371012. PMCID: PMC3229753.
4. Yamanaka S. 2020; Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 27:523–31. DOI: 10.1016/j.stem.2020.09.014. PMID: 33007237.
5. Brignier AC, Gewirtz AM. 2010; Embryonic and adult stem cell therapy. J Allergy Clin Immunol. 125(2 Suppl 2):S336–44. DOI: 10.1016/j.jaci.2009.09.032. PMID: 20061008.
6. Alatyyat SM, Alasmari HM, Aleid OA, Abdel-Maksoud MS, Elsherbiny N. 2020; Umbilical cord stem cells: background, processing and applications. Tissue Cell. 65:101351. DOI: 10.1016/j.tice.2020.101351. PMID: 32746993.
7. Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. 2010; Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells. 2:34–8. DOI: 10.4252/wjsc.v2.i2.34. PMID: 21607114. PMCID: PMC3097922.
8. Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. 2019; Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 233:116733. DOI: 10.1016/j.lfs.2019.116733. PMID: 31394127.
9. Liu R, Zhang Z, Lu Z, Borlongan C, Pan J, Chen J, Qian L, Liu Z, Zhu L, Zhang J, Xu Y. 2013; Human umbilical cord stem cells ameliorate experimental autoimmune encephalomyelitis by regulating immunoinflammation and remyelination. Stem Cells Dev. 22:1053–62. DOI: 10.1089/scd.2012.0463. PMID: 23140594.
10. Gordon D, Pavlovska G, Uney JB, Wraith DC, Scolding NJ. 2010; Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 69:1087–95. DOI: 10.1097/NEN.0b013e3181f97392. PMID: 20940628.
11. Liu S, Wang J, Han R, Meng M, Wang W, Zhao Y, Yang F, Yang L, Gao H, Zhao Y, Yang L, Wang R, Tang W, Li Y, Duan S, Wang J, He Z, Li L, Hou Z. 2019; Therapeutic effect of transplanted umbilical cord mesenchymal stem cells in a cynomolgus monkey model of multiple sclerosis. Am J Transl Res. 11:2516–31. PMID: 31105859. PMCID: PMC6511768.
12. Subirana M, Solá I, Garcia JM, Gich I, Urrútia G. 2005; A nursing qualitative systematic review required MEDLINE and CINAHL for study identification. J Clin Epidemiol. 58:20–5. DOI: 10.1016/j.jclinepi.2004.06.001. PMID: 15649667.
13. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. 2020; The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 40:1769–77. DOI: 10.1177/0271678X20943823. PMID: 32663096. PMCID: PMC7430098.
14. Abdolmaleki A, Kondori BJ, Raei M, Ghaleh HEG. 2023; Cell therapy procedure using anti-inflammatory macrophage M2 can potentially reduce Clinical Score in animals with Experimental Autoimmune Encephalomyelitis: a preclinical systematic review and meta-analysis study. Fundam Clin Pharmacol. 37:215–25. DOI: 10.1111/fcp.12844. PMID: 36300567.
15. Ahmadvand Koohsari S, Absalan A, Azadi D. 2021; Human umbilical cord mesenchymal stem cell-derived extracellular vesicles attenuate experimental autoimmune encephalomyelitis via regulating pro and anti-inflammatory cytokines. Sci Rep. 11:11658. DOI: 10.1038/s41598-021-91291-3. PMID: 34079033. PMCID: PMC8172573.
16. Zhou X, Liu X, Liu L, Han C, Xie Z, Liu X, Xu Y, Li F, Bi J, Zheng C. 2020; Transplantation of IFN-γ primed hUCMSCs significantly improved outcomes of experimental autoimmune encephalomyelitis in a mouse model. Neurochem Res. 45:1510–7. DOI: 10.1007/s11064-020-03009-y. PMID: 32172400.
17. Torkaman M, Ghollasi M, Mohammadnia-Afrouzi M, Salimi A, Amari A. 2017; The effect of transplanted human Wharton's jelly mesenchymal stem cells treated with IFN-γ on experimental autoimmune encephalomyelitis mice. Cell Immunol. 311:1–12. DOI: 10.1016/j.cellimm.2016.09.012. PMID: 27697286.
18. Donders R, Vanheusden M, Bogie JF, Ravanidis S, Thewissen K, Stinissen P, Gyselaers W, Hendriks JJ, Hellings N. 2015; Human Wharton's jelly-derived stem cells display immunomodulatory properties and transiently improve rat experimental autoimmune encephalomyelitis. Cell Transplant. 24:2077–98. DOI: 10.3727/096368914X685104. PMID: 25310756.
19. Mikaeili Agah E, Parivar K, Joghataei MT. 2014; Therapeutic effect of transplanted human Wharton's jelly stem cell-derived oligodendrocyte progenitor cells (hWJ-MSC-derived OPCs) in an animal model of multiple sclerosis. Mol Neurobiol. 49:625–32. DOI: 10.1007/s12035-013-8543-2.
20. Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X. 2017; Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep. 7:4323. DOI: 10.1038/s41598-017-04559-y. PMID: 28659587. PMCID: PMC5489510.
21. Amari A, Ebtekar M, Moazzeni SM, Soleimani M, Amirabad LM, Tahoori MT, Massumi M. 2015; Investigation of immunomodulatory properties of human Wharton's Jelly-derived mesenchymal stem cells after lentiviral transduction. Cell Immunol. 293:59–66. DOI: 10.1016/j.cellimm.2014.12.003. PMID: 25569483.
22. Zhong XY, Zhang B, Asadollahi R, Low SH, Holzgreve W. 2010; Umbilical cord blood stem cells: what to expect. Ann N Y Acad Sci. 1205:17–22. DOI: 10.1111/j.1749-6632.2010.05659.x. PMID: 20840248.
23. Gasiūnienė M, Valatkaitė E, Navakauskienė R. 2020; Long-term cultivation of human amniotic fluid stem cells: the impact on proliferative capacity and differentiation potential. J Cell Biochem. 121:3491–501. DOI: 10.1002/jcb.29623. PMID: 31898359.
24. Wang L, Wang J, Zhou X, Sun J, Zhu B, Duan C, Chen P, Guo X, Zhang T, Guo H. 2020; A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front Bioeng Biotechnol. 8:564731. DOI: 10.3389/fbioe.2020.564731. PMID: 33042966. PMCID: PMC7521201.
25. Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, Razmkhah M. 2020; Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther. 11:492. DOI: 10.1186/s13287-020-02001-1. PMID: 33225992. PMCID: PMC7681994.
26. Latifpour M, Shakiba Y, Amidi F, Mazaheri Z, Sobhani A. 2014; Differentiation of human umbilical cord matrix-derived mesenchymal stem cells into germ-like cells. Avicenna J Med Biotechnol. 6:218–27. PMID: 25414784. PMCID: PMC4224661.
27. Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Zhu D, Bayard F, Han ZC. 2010; Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 135:448–58. DOI: 10.1016/j.clim.2010.01.015. PMID: 20207200.
28. Yun JW, Ahn JH, Kwon E, Kim SH, Kim H, Jang JJ, Kim WH, Kim JH, Han SY, Kim JT, Kim JH, Kim W, Ku SY, Do BR, Kang BC. 2016; Human umbilical cord-derived mesenchymal stem cells in acute liver injury: hepatoprotective efficacy, subchronic toxicity, tumorigenicity, and biodistribution. Regul Toxicol Pharmacol. 81:437–47. DOI: 10.1016/j.yrtph.2016.09.029. PMID: 27693706.
29. Na L, Wang S, Liu T, Zhang L. 2020; Ultrashort wave combined with human umbilical cord mesenchymal stem cell (HUC-MSC) transplantation inhibits NLRP3 inflammasome and improves spinal cord injury via MK2/TTP signalling pathway. Biomed Res Int. 2020:3021750. DOI: 10.1155/2020/3021750. PMID: 33376718. PMCID: PMC7738785.
30. Feng YW, Wu C, Liang FY, Lin T, Li WQ, Jing YH, Dai P, Yu HX, Lan Y, Pei Z, Xu GQ. 2020; hUCMSCs mitigate LPS-induced trained immunity in ischemic stroke. Front Immunol. 11:1746. DOI: 10.3389/fimmu.2020.01746. PMID: 33013828. PMCID: PMC7516337.
31. Jiang Z, Wang J, Sun G, Feng M. 2022; BDNF-modified human umbilical cord mesenchymal stem cells-derived dopaminergic-like neurons improve rotation behavior of Parkinson's disease rats through neuroprotection and anti-neuroinflammation. Mol Cell Neurosci. 123:103784. DOI: 10.1016/j.mcn.2022.103784. PMID: 36228967.
32. Ahmed SO, El Fakih R, Elhaddad A, Hamidieh AA, Altbakhi A, Chaudhry QU, Bazarbachi A, Adil S, Al-Khabori M, Ben Othman T, Gaziev J, Khalaf M, Alshammeri S, Alotaibi S, Alshahrani M, Bekadja MA, Ibrahim A, Al-Wahadneh AM, Altarshi M, Alsaeed A, Madani A, Abboud M, Abujazar H, Bakr M, Abosoudah I, El Cheikh J, Almasari A, Alfraih F, Baldomero H, Elsolh H, Niederwieser D, Chaudhri N, Aljurf M. 2023; Strategic priorities for hematopoietic stem cell transplantation in the EMRO region. Hematol Oncol Stem Cell Ther. 16:162–9. DOI: 10.1016/j.hemonc.2021.09.006. PMID: 34688625.
33. Sterner RC, Sterner RM. 2021; CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11:69. DOI: 10.1038/s41408-021-00459-7. PMID: 33824268. PMCID: PMC8024391.
34. Rohaan MW, Borch TH, van den Berg JH, Met Ö, Kessels R, Geukes Foppen MH, Stoltenborg Granhøj J, Nuijen B, Nijenhuis C, Jedema I, van Zon M, Scheij S, Beijnen JH, Hansen M, Voermans C, Noringriis IM, Monberg TJ, Holmstroem RB, Wever LDV, van Dijk M, Grijpink-Ongering LG, Valkenet LHM, Torres Acosta A, Karger M, Borgers JSW, Ten Ham RMT, Retèl VP, van Harten WH, Lalezari F, van Tinteren H, van der Veldt AAM, Hospers GAP, Stevense-den Boer MAM, Suijkerbuijk KPM, Aarts MJB, Piersma D, van den Eertwegh AJM, de Groot JB, Vreugdenhil G, Kapiteijn E, Boers-Sonderen MJ, Fiets WE, van den Berkmortel FWPJ, Ellebaek E, Hölmich LR, van Akkooi ACJ, van Houdt WJ, Wouters MWJM, van Thienen JV, Blank CU, Meerveld-Eggink A, Klobuch S, Wilgenhof S, Schumacher TN, Donia M, Svane IM, Haanen JBAG. 2022; Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N Engl J Med. 387:2113–25. DOI: 10.1056/NEJMoa2210233. PMID: 36477031.
35. Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, Heke M, Nguyen LT. 2022; Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 7:272. DOI: 10.1038/s41392-022-01134-4. PMID: 35933430. PMCID: PMC9357075.
36. Ma D, Xu K, Zhang G, Liu Y, Gao J, Tian M, Wei C, Li J, Zhang L. 2019; Immunomodulatory effect of human umbilical cord mesenchymal stem cells on T lymphocytes in rheumatoid arthritis. Int Immunopharmacol. 74:105687. DOI: 10.1016/j.intimp.2019.105687. PMID: 31295689.
37. Remya NS, Nair PD. 2016; Mechanoresponsiveness of human umbilical cord mesenchymal stem cells in in vitro chondrogenesis- a comparative study with growth factor induction. J Biomed Mater Res A. 104:2554–66. DOI: 10.1002/jbm.a.35792. PMID: 27227673.
38. Gao X, He GH, Zhang XT, Chen S. 2021; Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway. Int J Ophthalmol. 14:1683–9. DOI: 10.18240/ijo.2021.11.06. PMID: 34804857. PMCID: PMC8569562.
39. Liao Z, Yang X, Wang W, Deng W, Zhang Y, Song A, Ni B, Zhao H, Zhang S, Li Z. 2021; hucMSCs transplantation promotes locomotor function recovery, reduces apoptosis and inhibits demyelination after SCI in rats. Neuropeptides. 86:102125. DOI: 10.1016/j.npep.2021.102125. PMID: 33486279.
Fig. 1
PRISMA flow diagram (2020) representing the process of eligible paper selection. WoS, Web of Science; SID, scientific information database; ISC, Islamic World Science Citation Center.
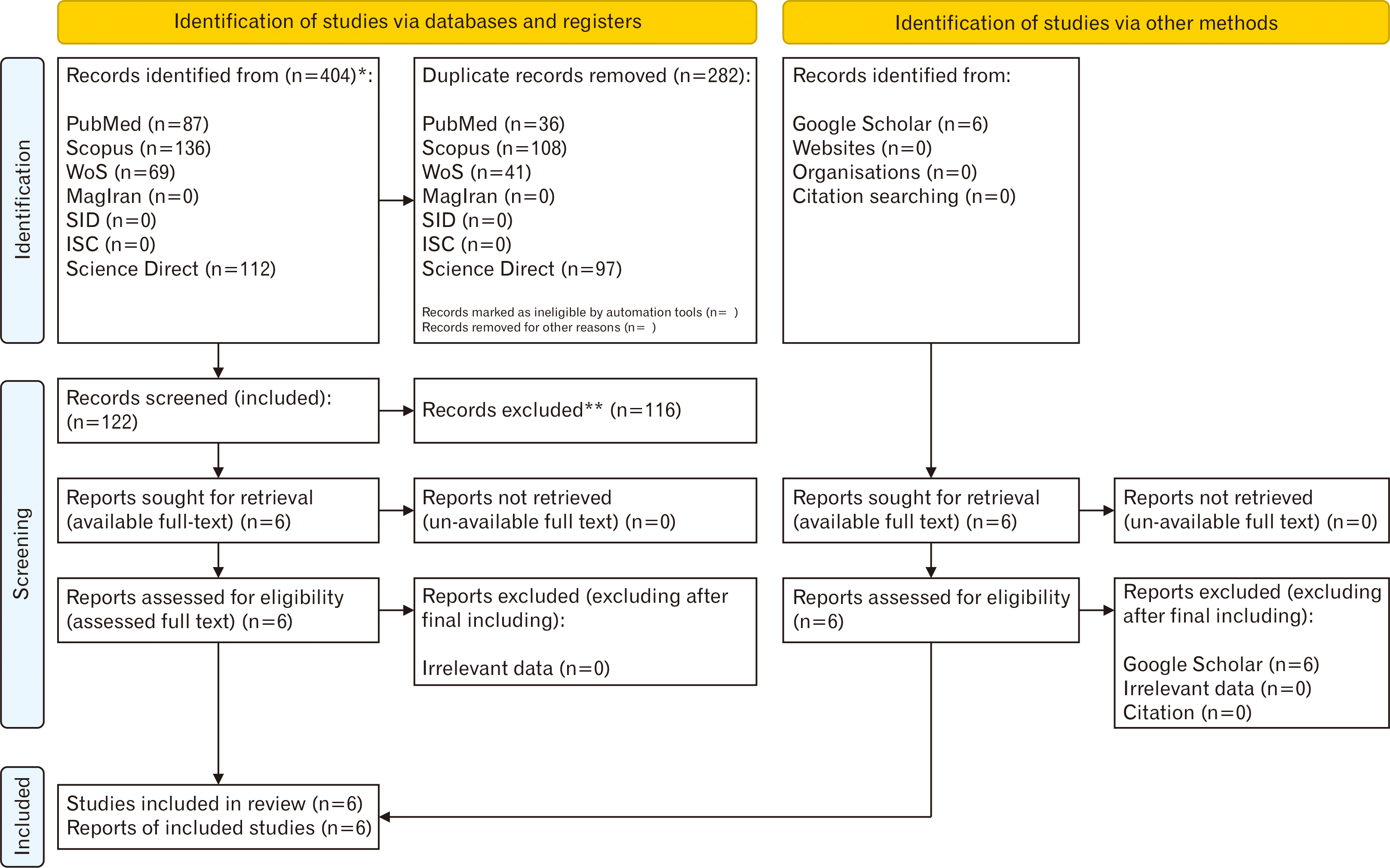
Fig. 2
Quality study representing the risk of bias of included papers according to the ARRIVE checklist. Yes, items mentioned in the text according to the ARRIVE; No, not mentioned items in the text according to the ARRIVE; N/A, not applicable items according to the ARRIVE; ARRIVE, Animal Research: Reporting of In Vivo Experiments.
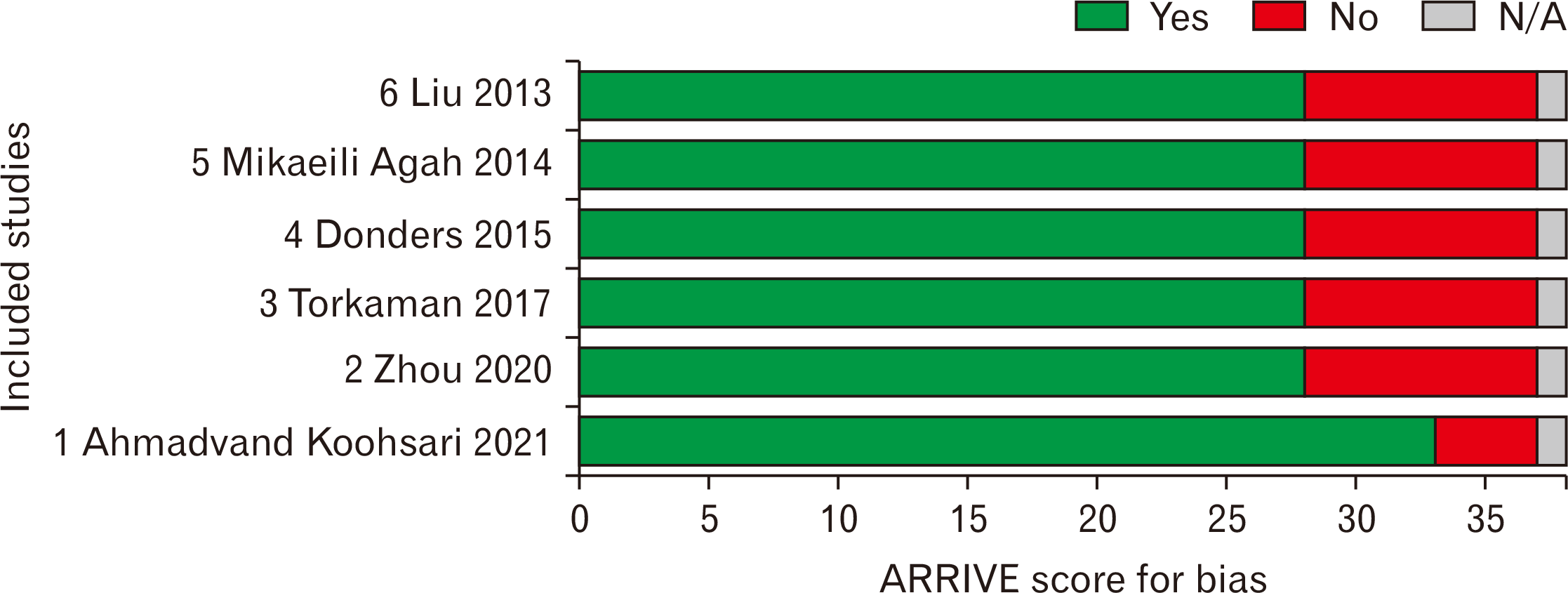
Fig. 3
High-resolution forest plot table representing difference in means and 95% CI in included animal studies with EAE treated by hUCMSCs (random effect model). CI, confidence interval; EAE, experimental autoimmune encephalomyelitis; hUCMSCs, human umbilical cord mesenchymal stem cells.
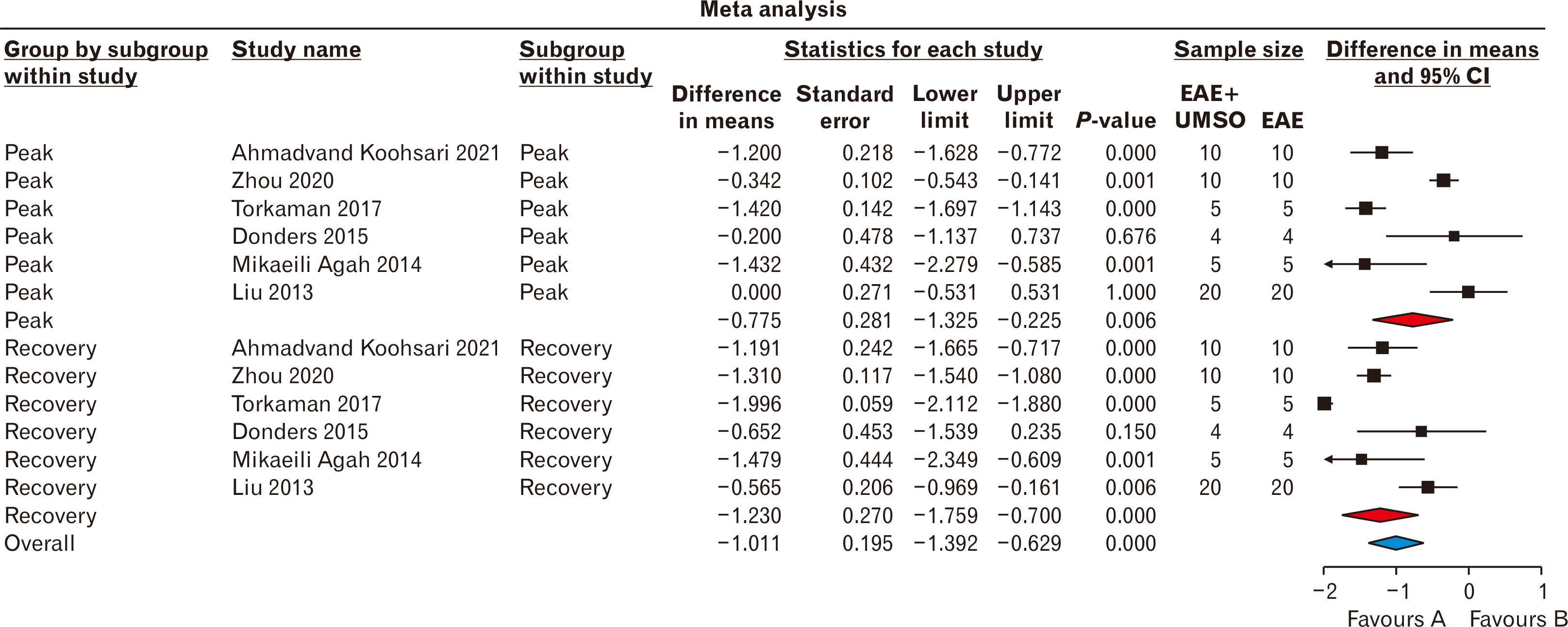
Fig. 5
Sensitivity assessment using one-study removed in a random-effect model of clinical score value. CI, confidence interval.
Table 1
Main keywords and designed searching strategies
Table 2
Static characteristics table representing extracted data from included articles
| Ahmadvand Koohsari et al. [15] (2021) | Zhou et al. [16] (2020) | Torkaman et al. [17] (2017) | Donders et al. [18] (2015) | Mikaeili Agah et al. [19] (2014) | Liu et al. [9] (2013) | |
|---|---|---|---|---|---|---|
| Country | Iran | China | Iran | Belgium | Iran | China |
| Experimental MS model | EAE using MOG35-55 | EAE using MOG35-55 | EAE using MOG35-55 | EAE using MOG35-55 | EAE using MOG35-55 | EAE using MOG35-55 |
| Animal strains | C57/BL6 mice | C57/BL6 mice | C57/BL6 mice | DA rats | C57/BL6 mice | C57/BL6 mice |
| Animal sex | Female | Female | Female | Female | Female | Female |
| Animal weight (g) | NR | NR | NR | NR | NR | NR |
| Animal age (wk) | 6–8 | 6–8 | 6–8 | 8 | 6–8 | 6–7 |
| Sample size (N/group) | 10 | 10 | 5 | 4 | 5 | 20 |
| Source of umbilical cord | Human | Human | Human | Human | Human | Human |
| Citation source of hUCMSCs isolation and culture procedures | Bai et al. [20] | NR | Amari et al. [21] | NR | NR | NR |
| hUCMSCs surface markers & immunophenotyping | CD45-, CD73+, CD105+ | CD11b, CD19, CD34, CD45, CD73, CD90, CD105, HLA-DR | HLA-I, HLA-DR, CD34, CD73, CD45, CD117, CD90, CD105, CD31, CD44, CD80, CD86, CD40, IgG1 | HLA-DR-PE, CD45-FITC, CD34-PE, CD14-PE, CD19-PE, CD105-PE, CD90-FITC, CD40-FITC, CD73-PE, CD80-PE, CD86-FITC | CD90, CD105, CD45, CD31 | CD3-APC, CD4-FITC, CD8-PE |
| hUCMSCs differentiation | Adipogenesis (using dexamethasone and insulin) & osteogenesis (using dexamethasone and ascorbic acid) | Not mentioned | Adipogenesis (using dexamethasone and indomethacin) | Not mentioned | Neurogenesis (using insulin transferrin selenium, fibronectin, FGF2, EGF, poly-l-ornithine, and PDGF) | Not mentioned |
| Main applied component | Extracellular vesicles derived from hUCMSCs | hUCMSCs | hUCMSCs | Co-culturing of WJ-MSCs and peripheral blood mononuclear cells | Differentiation of human Wharton’s jelly stem cells into OPCs | hUCMSCs |
| Route of material application | IV (tail vein) | IV (tail vein) | IV (tail vein) | IV (tail vein) | Intracerebroventricular injection | IV (tail vein) |
| Day of treatment post-immunization | 9 | 14 | 3 & 11 | 28 | 7 | 15 |
| Level of material application | 50 μg of extracellular vesicles derived from hUCMSCs | 107 cells/ml | 106 cells/ml | 2×106 cells/ml | 5×105 cells/7.5 μl OPC medium | 2×107 |
| Optimized (pre-treatment) with | None | IFN-γ (20 ng/ml & 50 ng/ml) | IFN-γ (500 U/ml) | IFN-γ, IL-1β, TNF-α (20 ng/ml) | ||
| Quality of study (ARRIVE score) | High (89) | High (75) | High (75) | High (75) | High (75) | High (75) |
MS, multiple sclerosis; EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; NR, not reported; hUCMSCs, human umbilical cord mesenchymal stem cells; HLA, human leukocyte antigen; IV, intravenous; OPC, oligodendrocyte progenitor cell; ARRIVE, Animal Research: Reporting of In Vivo Experiments.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download