This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To determine the correlation between dentoskeletal parameters related to craniofacial morphology and the upper airway (UA) volume.
Methods
Cone-beam computed tomography images of 106 randomly selected orthodontic patients were analyzed using NemoFab Ortho software. The dentoskeletal variables assessed were anterior facial height (AFH), posterior facial height (PFH), PFH/AFH ratio, hyoid position, maxillary width (MW), and palatal depth. The UA volume (evaluation in anatomical regions and as a whole) was also assessed using the same software. We also evaluated potential differences in UA variables between age and sex groups. The correlation between the dentoskeletal parameters and UA volume was calculated using the Pearson correlation coefficient (R). Analysis of variance and Student’s t test were performed to assess differences between age and sex for UA variables. Statistical analyses were performed using SPSS software (version 26 for Windows).
Results
This study found that PFH, AFH, and MW were the dentoskeletal parameters most strongly correlated with UA volume. However, the ANB angle did not show any significant correlation with UA volume. Additionally, differences in UA volumes were observed between age groups. Sex differences were found in both the “8–12” and “≥ 16” age groups for oropharyngeal and pharyngeal volumes.
Conclusions
In conclusion, our findings indicate a significant correlation between UA volume and dentoskeletal parameters, particularly those related to facial height and MW.
Keywords: Airway, Three-dimensional cephalometrics, Growth and development, Three-dimensional diagnosis and treatment planning
INTRODUCTION
Respiratory function and upper airway (UA) morphology are highly relevant for orthodontic diagnosis and treatment planning, because altered breathing function can influence facial growth and morphology.
1 The impact of UA obstruction on craniofacial development and dental patterns has been well documented.
1-3 Studies suggest that reduced pharyngeal airway volume might lead to certain altered craniofacial characteristics in the vertical, transverse, and coronal dimensions.
4,5 Soft tissue appears to be the link between these respiratory patterns and craniofacial development.
6 Moss’ Functional Matrix Theory proposes that soft tissue, rather than bone or cartilage, is the primary determinant of facial growth.
7 Thus, strong pressure on the dentition is essential for normal mandibular and maxillary growth. Therefore, strong pressure in the hard palate is essential for the normal development of the maxilla. In an altered breathing pattern, the tongue usually assumes a lower and anterior position, exerting a force against the anterior teeth, potentially leading to maxillary atrophy and an open bite. The maxilla becomes narrower, the mandible rotates counterclockwise, and the facial growth pattern becomes more vertical.
8
The craniofacial morphology and the UA have been assessed and studied using a two-dimensional approach in many studies. This approach has limitations in representing the depth of anatomic structures. Three-dimensional (3D) radiographic imaging can provide spatial information regarding these structures. Cone-beam computed tomography (CBCT) is a 3D radiological imaging technique that is commonly used in UA volume assessment studies for several reasons, including a lower radiation dose than conventional computed tomography.
9-11
Although many studies have assessed airway space and its correlation with craniofacial morphology, there is still much controversy among authors. Therefore, the aim of our study was to analyze the correlation of dentoskeletal variables related to craniofacial morphology and the UA volume to contribute to further clarifying this issue.
MATERIALS AND METHODS
This retrospective observational study was approved by the Ethics Committee of the Faculty of Dental Medicine, University of Porto (Project number: 21/2022). As we used preexisting imaging data, all the written informed consent was obtained from all participants and it was preserved in the patients’ files. Preexisting imaging data from orthodontic patients’ clinical file archives were used, avoiding additional radiation exposure for any participants. The present investigation did not generate any physical or emotional discomfort in the participants.
A comprehensive literature search was conducted using PubMed, Medline, the Cochrane Database of Systematic Reviews, Embase, and Web of Sciences. Only formally published articles were included; informally published materials like conference proceedings or dissertations were excluded.
The sample included the clinical records of 106 patients aged ≥ 8 years who sought orthodontic treatment in a private clinic and had available CBCT scans. Patients were excluded if they were non-Caucasian, had craniofacial anomalies, had previous UA surgery, or had previous orthodontic treatment.
The sample was characterized by sex (female and male) and age groups (8–12 years, 13–15 years, and ≥ 16 years). To account for the potential influence of lymphoid tissue hypergrowth in younger individuals, correlations were analyzed independently for each age group. However, results for the entire sample are also presented.
All CBCT volumes were obtained using the Planmeca® ProMax® 3D Mid unit (Planmeca Oy, Helsinki, Finland). The clinical protocol for image collection involved instructing the patients to position the tongue against the palate and avoid swallowing during image acquisition, with the Frankfurt horizontal plane parallel to the ground. The resulting slices were reconstructed with a thickness of 0.4 mm and saved in Digital Imaging and Communications in Medicine format. The NemoFab Ortho® software (Nemotec, Madrid, Spain) was used to import the images and perform volume orientation prior to landmark identification and volume measurements.
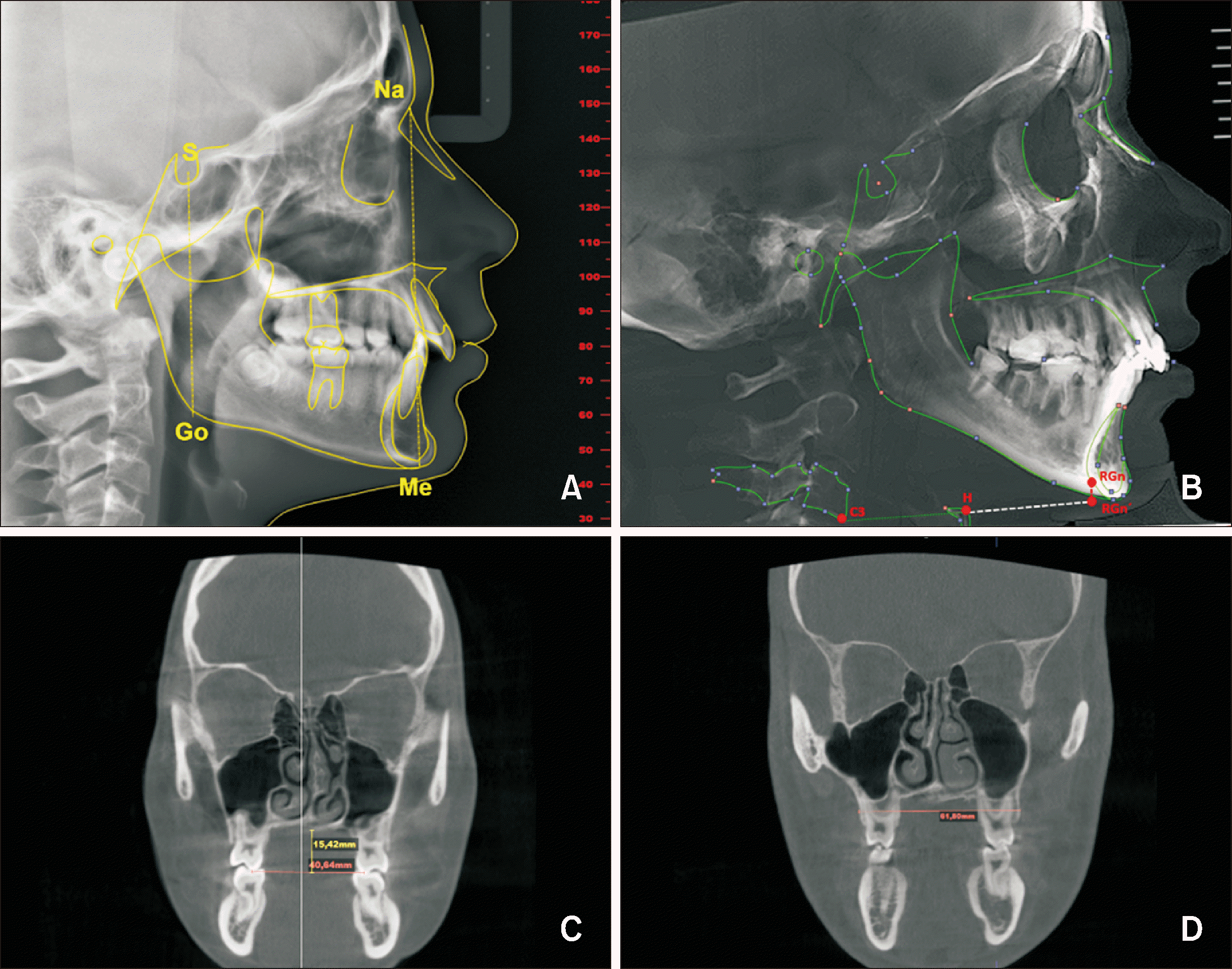
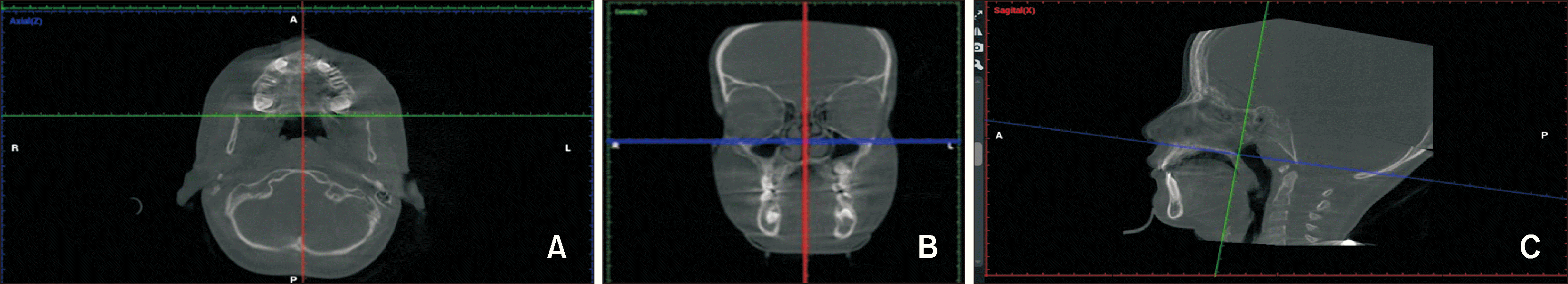
The following steps were performed using the NemoFab Ortho
® software to prepare the images. In the axial frame, the volume was reoriented to center the patient's midline and correct any axial rotations (
Figure 1A). In the coronal frame, the volume was reoriented to level the two Or points with the software’s horizontal reference line (
Figure 1B). In the sagittal frame, the volume was reoriented to place the left Or and Po points on the same horizontal reference line (
Figure 1C). Finally, a slice in the midsagittal plane was selected for cephalometric tracing
.
Using the listed cephalometric landmarks (
Tables 1 and
2), the following dentoskeletal variables were assessed: ANB angle, posterior facial height (PFH), anterior facial height (AFH), PFH/AFH ratio, hyoid position (HP), palatal depth (PD),
12 and maxillary width (MW)
13 (
Figure 2).
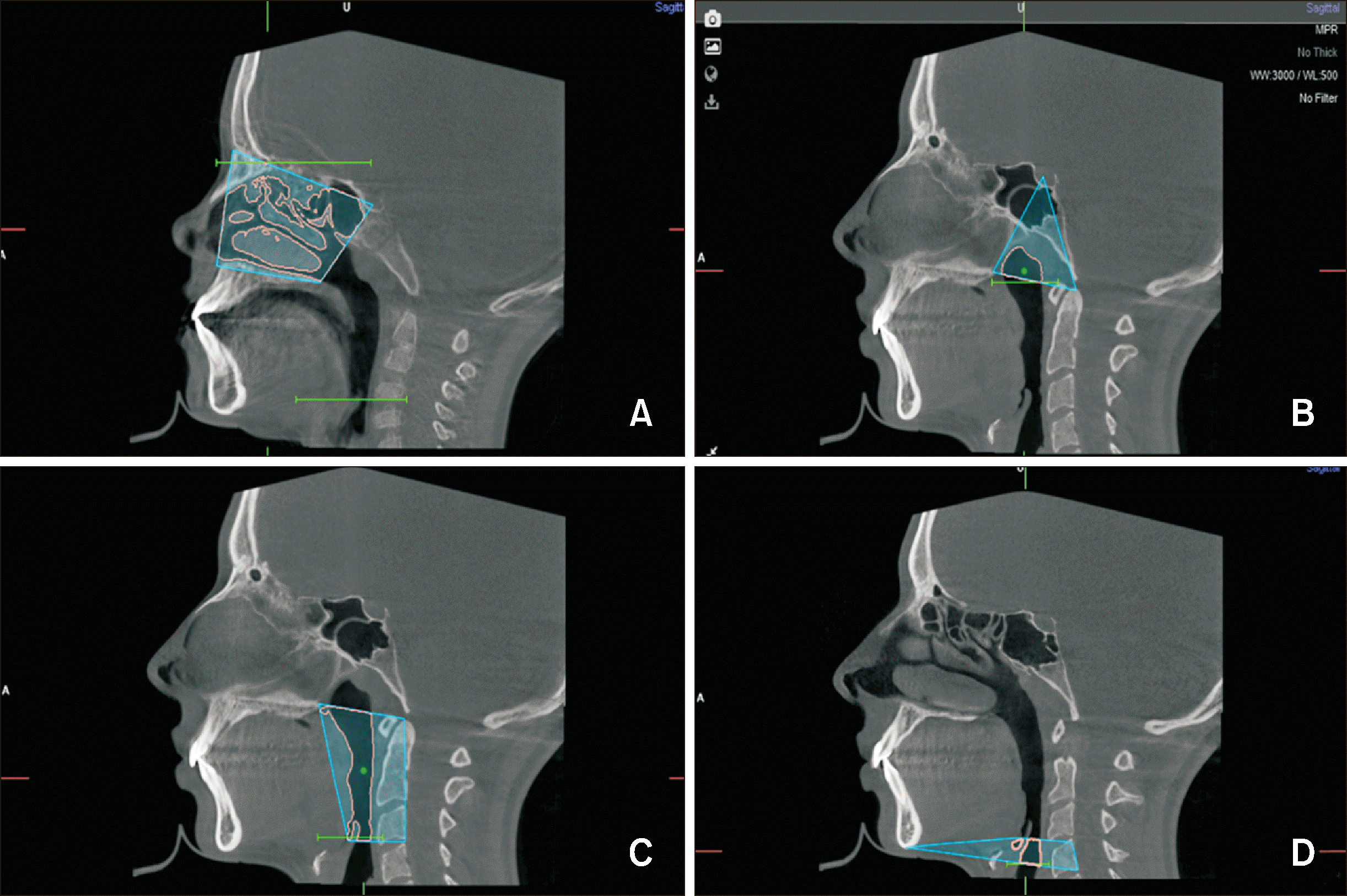
Four regions were considered for UA analysis according to Lotfi et al.:
14 nasal cavity, nasopharynx, oropharynx, and laryngopharynx. Landmarks proposed by the authors were used (
Table 3 and
Figure 3).
To differentiate soft tissue from the airway lumen, the software’s threshold sensitivity value (Hounsfield units [HU]) was manually adjusted. NemoFab Ortho® software was then used to calculate the lumen volume (in cubic centimeters, cm3) for each section. Finally, the total pharyngeal volume was calculated by summing the nasopharynx, oropharynx, and laryngopharynx (lumen) volumes.
Statistical analyses were performed using SPSS software (version 26 for Windows; IBM Corp., Armonk, NY, USA). Student’s
t test and intraclass correlation coefficients were used to evaluate the error in continuous measurements. Intraobserver error for linear and volumetric measurements was assessed on 30 randomly repeated measurements from the total sample, following the method proposed by Walter et al
.15 Pearson’s correlation coefficient (R) was used to determine the correlation between dentoskeletal variables and UA volume. Correlation strength was categorized as follows: weak (|R| < 0.25), moderate (0.25 ≤ |R| < 0.50), strong (0.50 ≤ |R| < 0.75), and very strong (|R| ≥ 0.75).
Analysis of variance (ANOVA) was used to compare UA variable values between age groups. If ANOVA indicated significance (P < 0.05), Tukey’s honestly significance difference multiple comparison test was used to compare means between specific groups. The Student’s t test was used to compare UA variable values between sexes. A significance level of 5% was considered for all statistical tests.
RESULTS
The intraclass correlation coefficient exceeded 0.97 for all variables, indicating excellent correlation between the first and second measurements. This demonstrates the high reliability of the results and confirms the absence of significant measurement errors.
The study sample comprised 106 patients (62 females and 44 males) with a mean age of 20.0 years (
Table 4). The age distribution was as follows: 40 patients were (8–12 years old), 17 patients (13–15 years old), and 49 patients (16 years or older).
Table 5 presents the results of the correlation analysis between dentoskeletal variables and UA volumes.
The ANB angle did not show any significant correlation with UA volume, neither for the total sample nor for any individual age group.
Within the “8–12” age group, a moderately positive correlation was found between nasal cavity volume and MW (R = 0.429, P = 0.006). Additionally, this age group showed a significant negative correlation between HP and laryngopharyngeal volume (R = –0.378, P = 0.019). No other significant correlations were observed between dentoskeletal variables and UA volumes in this age group.
Within the “13–15” age group, strong positive correlations were observed between nasopharyngeal volume and PFH (R = 0.520, P = 0.033), PFH/AFH ratio (R = 0.549, P = 0.023), and MW (R = 0.552, P = 0.021). No other significant correlations were observed between dentoskeletal variables and UA volumes in this age group.
Lastly, in the “≥ 16” age group, moderately significant positive correlations were found between nasal cavity volume and PFH (R = 0.290, P = 0.043), AFH (R = 0.345, P = 0.015), and MW (R = 0.317, P = 0.027). MW also showed a moderately significant positive correlation with nasopharyngeal volume (R = 0.347, P = 0.015). Oropharyngeal volume was moderately correlated with PFH (R = 0.373, P = 0.008) and the PFH/AFH ratio (R = 0.330, P = 0.021). The HP showed a moderately significant negative correlation with laryngopharyngeal volume (R = –0.441, P = 0.002). Furthermore, a moderately significant positive correlation was found between PFH and pharyngeal volume (R = 0.342, P = 0.016) in this age group.
The total sample analysis revealed significant moderate correlations between PFH and AFH with all UA volumes, except for the correlation between PFH and laryngopharynx, which was weak but significant (R = 0.194, P = 0.049). PD and MW were also significantly correlated with all UA volumes (P < 0.05). Furthermore, the HP presented a significant negative correlation with laryngopharyngeal volume (R = –0.370, P < 0.001) in the total sample.
Table 6 presents the correlations between age and UA volume. In the “8–12” age group, significant positive correlations were found between age and nasal cavity (R = 0.629,
P < 0.001), nasopharynx (R = 0.424,
P = 0.006), oropharynx (R = 0.402,
P = 0.010) and pharyngeal volume (R = 0.451,
P = 0.003). No significant correlations were observed between age and upper air volumes in the “13–15” age group. Interestingly, the “≥ 16” age group showed a significant negative correlation between oropharyngeal volume and age (R = –0.335,
P = 0.019). ANOVA analysis revealed significant differences (
P < 0.05) in mean UA volumes between the age groups.
Table 7 presents the sex differences in UA volumes. In the “8–12” age group, females had significantly larger oropharynx (
P = 0.023) and pharynx (
P = 0.033) volumes compared to males. No significant sex differences were observed in UA volumes for the “13–15” age group. However, in the “≥ 16” age group, males exhibited significantly larger oropharynx (
P = 0.019) and pharynx (
P = 0.018) volumes compared to females. Overall, the total sample analysis did not reveal any significant sex differences in UA volume.
DISCUSSION
The UA plays a critical role in orthodontics by influencing breathing, a major environmental factor determining craniofacial development. This study investigated potential correlations between UA volume and dentoskeletal variables related to craniofacial morphology. Our findings reveal that PFH, AFH, and MW exhibited stronger correlations with UA volume compared to the ANB angle, which showed no significant association. Additionally, UA volume differed between age groups. Notably, sex differences in oropharyngeal and pharyngeal volumes were observed in both “8–12” and “≥ 16” age groups.
CBCT has revolutionized airway space analysis, becoming an essential tool for orthodontic diagnosis and treatment planning. The availability of CBCT scans in patients’ clinical records as part of standard orthodontic protocols allowed us to leverage these existing data for all measurements in this study.
Our study found no significant correlation between the ANB angle and UA volume, neither in the total sample nor within age groups. This contrasts with Tseng et al.
16 who reported a significant negative correlation between ANB angle and total pharyngeal airway volume. However, their analysis excluded the nasopharynx, potentially explaining the discrepancy.
AFH and PFH exhibited moderately positive significant correlations with nasal cavity volume in both the total sample and the “≥ 16” age group. While previous studies by Gulsen et al.
17 and Nehra and Sharma
18 have shown a correlation between nose length and facial height, we found no prior research directly referencing correlations with nasal cavity volume. Individuals with longer faces or noses may have a larger nasal cavity volume, but this does not necessarily translate to better respiratory function.
Both PFH and AFH showed moderate correlations with other UA volumes in the total sample. Notably, the “13–15” age group displayed a strong significant correlation between PFH and nasopharyngeal volume. This finding appears to contradict Grauer et al.,
19 who reported no differences in airway volume related to vertical facial proportions. This discrepancy may be due to methodological differences. Our study used PFH and AFH to assess vertical proportions, while Grauer et al.
19 used a facial index based on bony bizygomatic width and nasion-menton distance. Furthermore, the methods used for airway analysis differed between the studies. The PFH/AFH ratio also presented a significant strong correlation with nasopharyngeal volume in the “13–15” age group.
The hyoid bone position showed a moderately negative correlation with laryngopharynx volume across the total sample and within both the “8–12” and “≥ 16” age groups. This suggests that a greater vertical distance between the hyoid bone and reference points is associated with a smaller laryngopharyngeal volume.
Previous studies have primarily focused on the anteroposterior position of the hyoid bone. They reported that a more posterior HP reduces the pharyngeal airway space, particularly in the laryngopharyngeal region,
20-22 while a more anterior position increases the airway space.
20 This aligns with the concept that Class III malocclusion patients might have a larger airway space due to the more anterior HP. This is supported by many studies in the field.
21,23-26 However, some studies observed minimal changes in airway volume after mandibular advancement surgery in Class II patients despite significant hyoid advancement.
27 These discrepancies might be due to variations in HP assessment methods or imaging modalities.
Our findings regarding the negative correlation between vertical HP and laryngopharyngeal volume are of particular interest, considering the limited research on vertical hyoid assessment and its relation to UA volume.
PD exhibited a significant moderate positive correlation with all UA volumes in the total sample, with the strongest correlation observed for total pharyngeal volume. However, no significant correlations were found between PD and UA volume within any specific age group.
Similar to PD, MW displayed significant moderate positive correlations with all UA volumes in the total sample. Additionally, it showed moderate correlations with nasal cavity volume in both the “8–12” and “≥ 16” age groups. Interestingly, the strongest correlation observed in this study was between MW and nasopharyngeal volume in the “13–15” age group.
Our findings regarding PD differ from those of Dastan et al.,
12 who reported no significant association between PD and UA volume. However, our results align with Kusumaningrum et al.,
28 who observed a correlation between smaller PD and airway obstruction in children. Our results support those of Miranda-Viana et al.
29 who found a significant relationship between MW and total UA volume. Furthermore, multiple studies reported increased UA volume after maxillary expansion procedures, suggesting a positive correlation between these variables.
30-32
For the “8–12” age group, our results showed significant positive correlations with nasal cavity, nasopharynx, oropharynx, and pharyngeal volumes. This indicates that these volumes increase with age, which is further confirmed by ANOVA analysis between age groups. These findings corroborate studies by Loftus et al.
33 and Ganjaei et al.,
34 who also reported a positive correlation between age and UA volumes, although their studies used different age ranges. Interestingly, our study found a significant negative correlation with oropharyngeal volume in the “≥ 16” age group.
Our analysis revealed no significant sex differences in UA volumes between the sexes in the total sample. This is not in line with a study by Saati et al.
35 who found a greater pharyngeal volume in males. However, our study did find significant sex differences in oropharyngeal and pharyngeal volumes for both the “8–12” and “≥ 16” age groups.
One limitation of this study is the manual adjustment of HU values to differentiate soft tissue from the airway space. Since a standardized value is not yet established, comparisons with other studies that may have used different HU thresholds are challenging. Additionally, the lack of a universal method for UA space assessment across studies can contribute to result variations. Head position and respiratory movements during imaging acquisition can also influence results. To mitigate these limitations, we ensured consistent image acquisition protocol by a single operator. Furthermore, interclass correlation coefficients were employed to verify the absence of measurement errors.
CONCLUSIONS
This study identified significant correlations between UA volume, analyzed in sections and as a whole, with dentoskeletal parameters, particularly those related to facial vertical proportions and maxillary transverse dimensions. Age also exhibited a correlation with UA volume. Notably, sex differences in UA volume were observed within specific age groups but not in the total sample.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download