Dear Editor,
The ABO blood group system, established by Karl Landsteiner over a century ago, continues to be the cornerstone of blood transfusion, prenatal serological testing, and bone marrow and organ transplantation. To date, more than 200 ABO alleles have been documented on the International Society of Blood Transfusion (ISBT) website (https://www.isbtweb.org/resource/001aboalleles.html, accessed on Jan 31, 2024). The majority of weak ABO phenotypes or subgroups frequently arise from sequence variants within coding exons, flanking introns, and hybrid formation between common alleles. Some weak ABO subgroups are attributable to mutations in regulatory regions, such as the CCAAT-binding factor/nuclear factor Y (CBF/NF-Y) binding site [1] (although controversial [2]), the proximal promoter [3-5], and the +5.8-kb site [6]. These subgroups are often misclassified as having common ABO genotypes. Long-read single-molecule real-time sequencing, developed by Pacific BioSciences (PacBio, Menlo Park, CA, USA), has immense potential in the quest for comprehensive haplotype sequence collections of blood group alleles [7]. Compared with Sanger and next-generation sequencing technologies, this third-generation sequencing technology, which has not been widely promoted in the field of blood grouping, can capture two ABO haplotypes containing the full-length and flanking regulatory regions of the ABO gene without ignoring mutations outside the coding region.
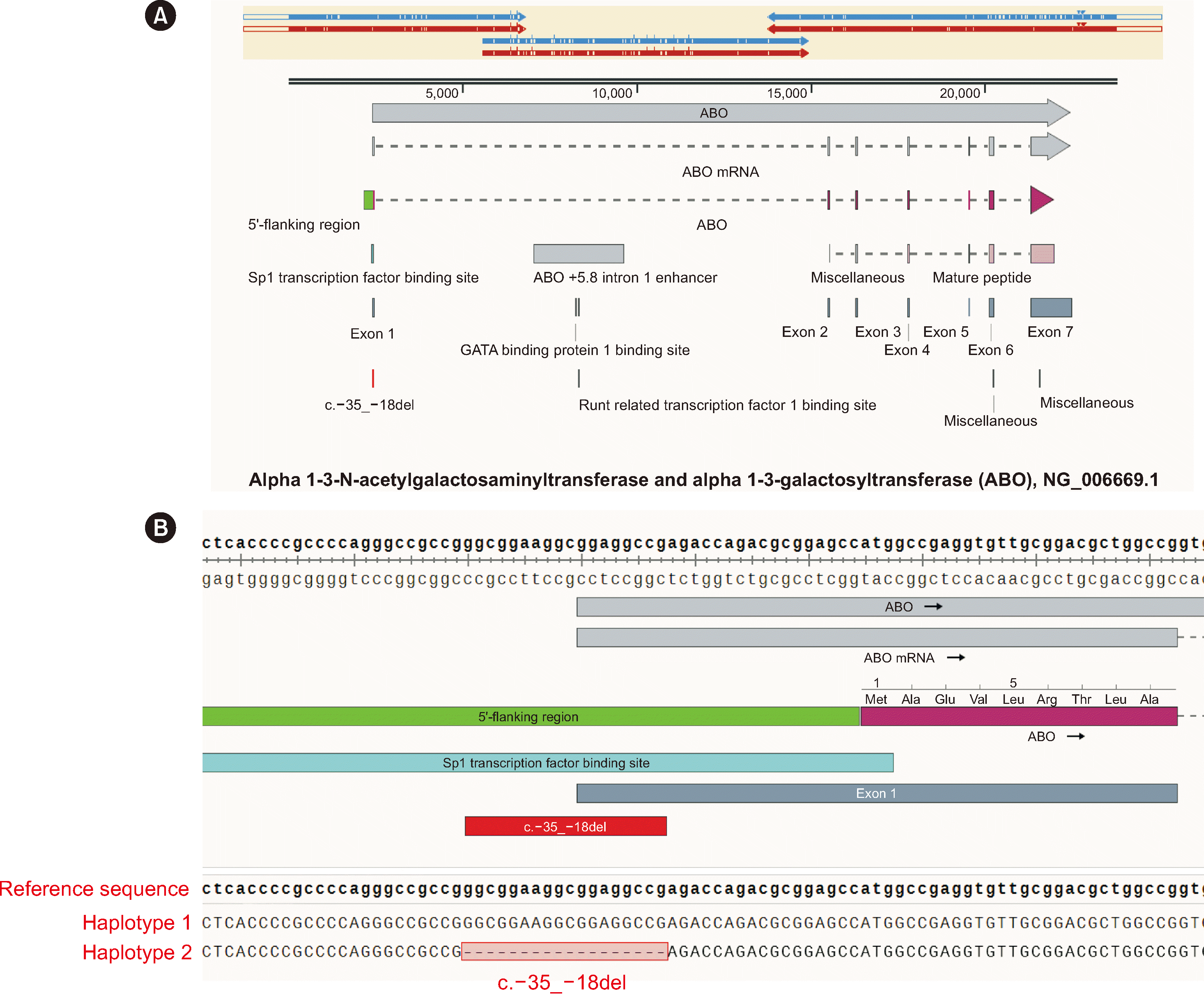
We describe two cases of patients harboring an ABO gene promoter mutation, NC_000009.12:g.133275211_133275228del i.e., c.–35_–18delGGCGGAAGGCGGAGGCCG, which resulted in weakened B antigen expression, identified using PacBio third-generation sequencing. Case 1 was that of a male patient who underwent maxillofacial surgery, and Case 2 was that of a male low-birthweight premature infant; both had not received blood transfusions. The research protocol was reviewed and approved by the Ethics Committee of the Dalian Blood Center, Dalian, China (approval No: LL-05001), and informed consent was obtained from the patient/guardian. ABO typing using the saline tube method [8] revealed weakened B antigen expression and mixed-field agglutinations in two probands displaying AB3- and B3-like phenotypes, respectively (Table 1). To further identify the reason for the inconclusive blood typing results, the entire ABO gene, including the flanking regulatory regions, was sequenced using PacBio technology, as previously described [8]. Based on the PacBio sequencing results, probands 1 and 2 were found to have the ABO*A1.02/ABO*B.01 and ABO*O.01.01/ABO*B.01 genotypes, respectively. Exons 1–7 and their exon–intron boundaries harbored no sequence variants that could account for the mixed-field phenotypes. However, both probands harbored the same mutation, c.–35_–18del (Fig. 1), in the proximal promoter region, which was cis-linked to the ABO*B.01 allele. Because mutations in CBF/NF-Y binding site and +5.8-kb site can also cause weak ABO subgroups, we searched for these regions; however, no mutations were detected compared with the normal ABO*B.01 allele.
The proximal promoter mutation c.–35_–18del was first reported by Cai, et al. [3] in six unrelated Chinese B3 or AB3 individuals. Dual luciferase assays confirmed that mutant promoter activity was reduced by more than 50% compared with that of the wild type [3]. A previous study has shown that the –117 to +31 region was essential for directing the expression of a reporter gene in red blood cells (RBCs), and the ABO promoter sequence from –22 to 14 serves as a binding site for the transcription factor Sp1 or Sp1-like protein(s) [9]. Further, the absence of the sequence from –35 to –18 may disrupt the binding of transcription factors, leading to mixed-field weak B antigen expression on RBCs. Subsequently, the same deletion was reported in 10 B3 or AB3 Japanese individuals [10]. The mutation c.–35_–18del is recorded in the NCBI dbSNP database (rs1588650511), with frequencies of 0.0006 in Koreans and 0.00006–0.00007 in the Japanese. Interestingly, this mutation has been hitherto described only in Asian populations and was observed in cis with the B allele, leading to weakened B antigen expression, and not in cis with A or O alleles, suggesting the existence of a population-specific distribution and linkage disequilibrium with the B allele.
In summary, third-generation PacBio sequencing was employed to sequence the entire ABO gene and flanking regulatory regions in two probands, revealing the mutation c.–35_–18del as the sole variant from the consensus ABO*B.01 sequence in the CBF/NF-Y binding site, proximal promoter, +5.8-kb site, exons 1–7, and exon–intron boundaries. This mutation leads to mixed-field weak B antigen expression on RBCs and may interfere with the binding of transcription factors to the promoter, thereby affecting transcriptional activity.
Notes
References
1. Kominato Y, Tsuchiya T, Hata N, Takizawa H, Yamamoto F. 1997; Transcription of human ABO histo-blood group genes is dependent upon binding of transcription factor CBF/NF-Y to minisatellite sequence. J Biol Chem. 272:25890–8. DOI: 10.1074/jbc.272.41.25890. PMID: 9325321.
2. Thuresson B, Hosseini-Maaf B, Hult AK, Hustinx H, Alan Chester M, Olsson ML. 2012; A novel B(weak) hybrid allele lacks three enhancer repeats but generates normal ABO transcript levels. Vox Sang. 102:55–64. DOI: 10.1111/j.1423-0410.2011.01497.x. PMID: 21592135.
3. Cai X, Jin S, Liu X, Fan L, Lu Q, Wang J, et al. 2013; Molecular genetic analysis of ABO blood group variations reveals 29 novel ABO subgroup alleles. Transfusion. 53(11S2):2910–6. DOI: 10.1111/trf.12168. PMID: 23521133.
4. Isa K, Yamamuro Y, Ogasawara K, Yabe R, Ogiyama Y, Ito S, et al. 2016; Presence of nucleotide substitutions in the ABO promoter in individuals with phenotypes A3 and B3. Vox Sang. 110:285–7. DOI: 10.1111/vox.12363. PMID: 26529276.
5. Kim TY, Yu H, Seo JY, Cho D. 2022; Molecular basis of weak A subgroups in the Korean population: identification of three novel subgroup-causing variants in the ABO regulatory regions. Transfusion. 62:286–91. DOI: 10.1111/trf.16730. PMID: 34786713.
6. Sano R, Nakajima T, Takahashi K, Kubo R, Kominato Y, Tsukada J, et al. 2012; Expression of ABO blood-group genes is dependent upon an erythroid cell-specific regulatory element that is deleted in persons with the B(m) phenotype. Blood. 119:5301–10. DOI: 10.1182/blood-2011-10-387167. PMID: 22408256.
7. Chen Z, He X. 2021; Application of third-generation sequencing in cancer research. Med Rev. 1:150–71. DOI: 10.1515/mr-2021-0013. PMID: 37724303. PMCID: PMC10388785. PMID: efd1600dbcdb42ac8e19e84e56e679b0.
8. Shao LN, Yang YC, Xia YX, Li CX, Zhou SH, Liang XH. 2024; Novel missense mutation c.797T>C (p.Met266Thr) gives rise to the rare B(A) phenotype in a Chinese family. Vox Sang. doi: 10.1111/vox.13591. Epub ahead of print. DOI: 10.1111/vox.13591. PMID: 38245843.
9. Hata Y, Kominato Y, Yamamoto FI, Takizawa H. 2002; Characterization of the human ABO gene promoter in erythroid cell lineage. Vox Sang. 82:39–46. DOI: 10.1046/j.0042-9007.2001.00134.x. PMID: 11856466.
10. Takahashi Y, Isa K, Sano R, Nakajima T, Kubo R, Takahashi K, et al. 2014; Presence of nucleotide substitutions in transcriptional regulatory elements such as the erythroid cell-specific enhancer-like element and the ABO promoter in individuals with phenotypes A3 and B3, respectively. Vox Sang. 107:171–80. DOI: 10.1111/vox.12136. PMID: 24602004.
Fig. 1
Genetic structure of the ABO gene. (A) Three overlapping segments of the long-range PCR amplification covering the entire ABO gene. These segments were circularly sequenced multiple times to directly obtain high-quality consensus sequences. The fragments overlapped by more than 1 kb. The vertical lines in the blue and red arrows indicate the mutation sites. Full-length gene haplotypes can be assembled based on the mutation sites in the overlapping regions. A key advantage of long-read sequencing over short-read sequencing is its ability to sequence very long DNA fragments as haplotypes. (B) Mutation c.–35_–18del in the probands. NG_006669.1 was used as the reference sequence. Sequence alignment and analysis were performed using the SnapGene v6.0.2 software, with the ISBT Names for ABO (ISBT 001) blood group alleles v1.1 171023 serving as the reference data source.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download