Dear Editor,
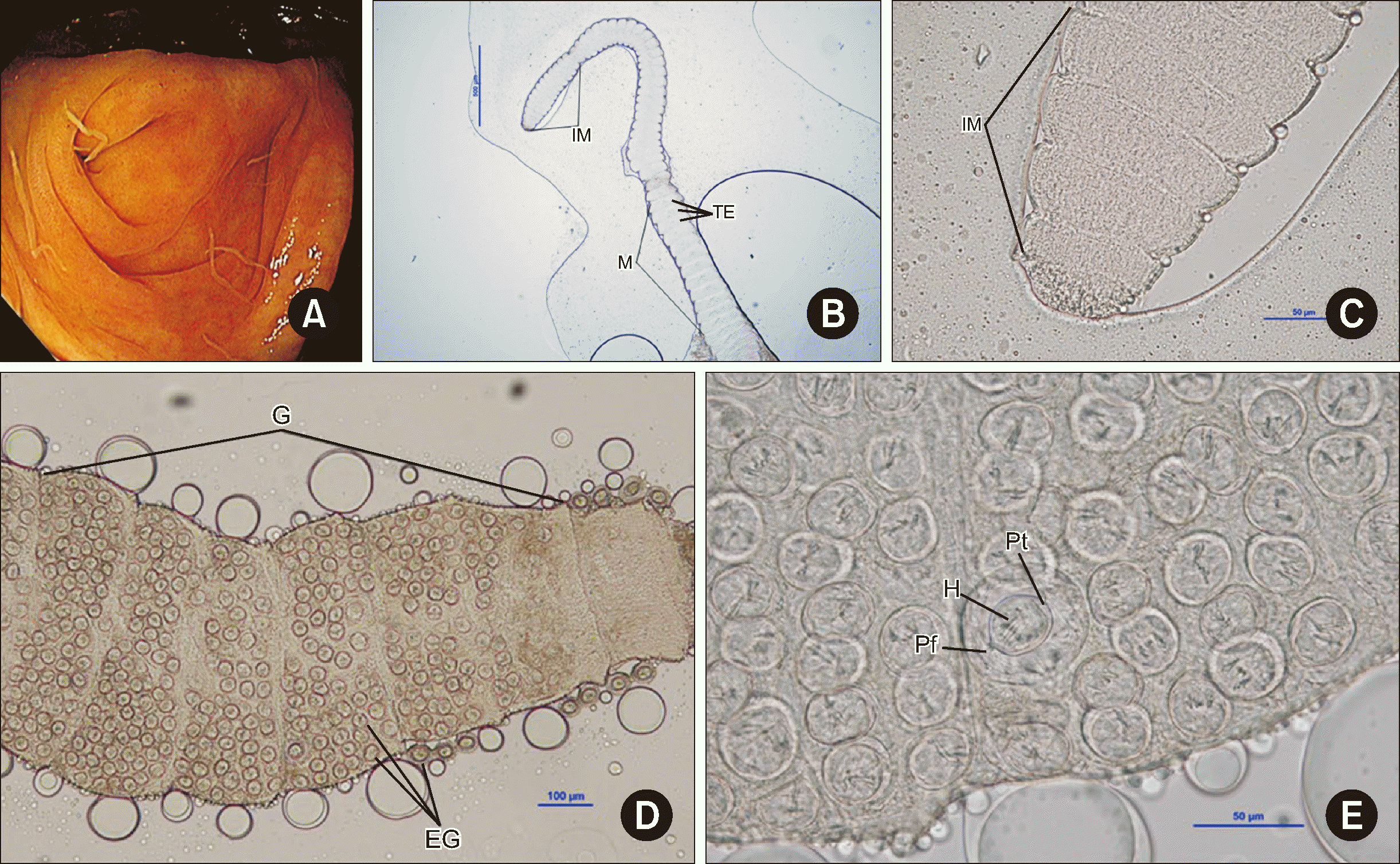
Human Hymenolepis nana infections have been scarcely reported in Korea recently. We describe an uncommon case in a medical tourist diagnosed during a screening colonoscopy. A 55-yr-old man from Kazakhstan with a medical history of hypertension, stroke, and hepatitis B visited the health screening clinic at Asan Medical Center, Korea, for a health checkup in March 2024. The man was suffering from nocturia due to benign prostate hyperplasia but did not present any gastrointestinal symptoms, such as abdominal pain or diarrhea. The patient reported a history of consuming undercooked beef, horse, and lamb. Colonoscopy revealed multiple whitish worms of approximately 2–4 cm in length; they were scattered on the mucosa across the terminal ileum and cecum (Fig. 1A). The worms were fixed in 10% formalin and transferred to the clinical microbiology laboratory for species identification. In the specimens, we did not observe scolices but only some strobilae (Fig. 1B–1E). Fig. 1B shows a segment of the parasite, revealing a gradual maturation from the anterior to the posterior extremity. The inferior portion showed discernible proglottids with testes, and the structure is markedly delineated by two grooves. Fig. 1C reveals that each proglottid has a rectangular configuration, with grooves conspicuously situated between successive proglottids. In Fig. 1D, gravid proglottids filled with eggs can be discerned. A detailed inspection unveiled mature eggs at the core, distinguished by hooks, polar thickening, and polar filaments similar to onion roots, culminating in the diagnosis of H. nana infection (Fig. 1E). The average size of the mature eggs was 59.6 μm, whereas that of the surrounding immature eggs ranged from 26.3 to 35.7 μm.
To identify the species molecularly, a partial sequence of the 18 small subunit ribosomal RNA gene was PCR-amplified from the segments using previously reported forward (5′-GTGAATCGCAGACTGCTTTG-3′) and reverse (5′-CTGAGGTCAG-GTCTTCCATAC-3′) primers [1, 2]. Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) per the manufacturer’s protocol. The ~550-bp PCR products were sent to Cosmogenetech (Seoul, Korea) for direct sequencing using the PCR primers. The sequence chromatograms were trimmed manually and assembled using the SeqMan software (DNASTAR, Madison, WI, USA). Basic Local Alignment Search Tool searches revealed that the assembled sequences showed 98.1%–99.7% identity with that of H. nana (GenBank Nos. LC389873, MN 096882), 92.5% identity with that of Hymenolepis diminuta (KR349973), and 78.7% identity with that of Hymenolepis microstoma (AB494478). Therefore, the worms were identified as H. nana, and the patient was administered 20 mg/kg praziquantel orally three times a day. This study was approved by the Institutional Review Board of Asan Medical Center (No. 2024-0425).
H. nana, the dwarf tapeworm, is the smallest tapeworm in humans worldwide, and infections most frequently occur in warm, dry regions of the developing world [3-5]. When humans ingest eggs, the oncospheres are liberated and penetrate the villi of the small intestine. After maturation, they return to the intestinal lumen by rupturing the villi. H. nana is capable of autoinfection, and although arthropods such as beetles and fleas are known to serve as intermediate hosts, its unusual life cycle enables its direct transmission, rendering intermediate hosts non-essential [6]. Clinical presentation varies depending on the amount of infecting worms. Light infections are usually asymptomatic, whereas heavy infections can induce a wide range of gastrointestinal symptoms and allergic responses [7]. Human infections with adult hymenolepidid tapeworms occur worldwide [3-5]; however, national surveys conducted in Korea during 1971–2004 demonstrated a low prevalence of H. nana, with rates <1%, and the most recent survey conducted in 2004 revealed an infection rate of nearly 0% [8, 9]. Although this parasite has become rare in Korea [10, 11], it is widespread in the southern regions of Kazakhstan and Uzbekistan, accounting for 18.9% of helminthiasis cases [5].
Our patient had a dietary history of undercooked beef, horse, and lamb, which are potential sources of infection in his country. Accurate diagnosis of H. nana infection via colonoscopy alone is challenging, particularly in the circumstances of low endemic regions. In such cases, the correct identification of the worm can be made by using a combination of morphological and molecular data in addition to colonoscopy findings. While H. nana infections are rare among Koreans, the rising populations of foreign workers and medical tourists necessitate the consideration of H. nana when parasites with the stated characteristics are identified during colonoscopy. This awareness is crucial given the changing demographics and its potential impact on the incidence of parasitic infections. Moreover, continuous education of clinical laboratory workers in Korea on this rare parasite is necessary.
ACKNOWLEDGEMENTS
We thank Woo Jin Tak, M.T., working at the clinical microbiology laboratory at Asan Medical Center, for the technical assistance.
Notes
AUTHOR CONTRIBUTIONS
Won EJ conceptualized the study; Won EJ, Park HW, and Park HJ contributed to the methodology; Park B, Won EJ, and Park HJ contributed to the investigation; Park B, Won EJ, and Park HW visualized the data; Won EJ acquired funding; Won EJ, Sung H, and Kim MN administered the project; Park HJ, Park HW, Sung H, and Kim MN supervised the study; Park B and Won EJ wrote the original draft; Park B, Won EJ, Park HJ, Park HW, Sung H, and Kim MN reviewed and edited the manuscript. All authors read and approved the final manuscript.
References
1. Alomashi GBA, Al-Shabbani AHA, Khayoon SQ. 2021; Molecular identification of Hymenolepis spp. in diarrheal patients using RFLP/PCR technique for 18SS ribosomal RNA gene. Gene Reports. 24:101294. DOI: 10.1016/j.genrep.2021.101294.
2. Kim JY, Yi M, Kim M, Yeom J, Yoo HD, Kim SM, et al. 2022; Diagnosis of Balamuthia mandrillaris encephalitis by thymine-adenine cloning using universal eukaryotic primers. Ann Lab Med. 42:196–202. DOI: 10.3343/alm.2022.42.2.196. PMID: 34635613. PMCID: PMC8548236.
3. Jacobsen KH, Ribeiro PS, Quist BK, Rydbeck BV. 2007; Prevalence of intestinal parasites in young Quichua children in the highlands of rural Ecuador. J Health Popul Nutr. 25:399–405.
4. Waloch M, Sobolewska A, Dzbeński TH. 2010; Evaluation of epidemiological situation of cestode infections in Poland in the years 1997-2006 on the basis of data from san-epid stations. Przegl Epidemiol. 64:533–6.
5. Abdiev TA, Karimova MT, Umarova PH. 2007; Situation due to helmintnic protozoa diseases in Uzbekistan. Vestnik Vracha. 1:75–6.
6. Brar SK, Singla N, Singla LD. 2021; Comparative comprehensive analysis on natural infections of Hymenolepis diminuta and Hymenolepis nana in commensal rodents. Helminthologia. 58:248–62. DOI: 10.2478/helm-2021-0027. PMID: 34934388. PMCID: PMC8647958. PMID: a40d815d74174bcabf60f6dda2ea5a86.
7. Marseglia GL, Marseglia A, Licari A, Castellazzi AM, Ciprandi G. 2007; Chronic urticaria caused by Hymenolepis nana in an adopted girl. Allergy. 62:821–2. DOI: 10.1111/j.1398-9995.2007.01362.x. PMID: 17573732.
8. Kim CH, Park CH, Kim HJ, Chun HB, Min HK, Koh TY, et al. 1971; Prevalence of intestinal parasites In Korea. Kisaengchunghak Chapchi. 9:25–38. DOI: 10.3347/kjp.1971.9.1.25. PMID: 12913622.
9. Kim TS, Cho SH, Huh S, Kong Y, Sohn WM, Hwang SS, et al. 2009; A nationwide survey on the prevalence of intestinal parasitic infections in the Republic of Korea, 2004. Korean J Parasitol. 47:37–47. DOI: 10.3347/kjp.2009.47.1.37. PMID: 19290090. PMCID: PMC2655332.
10. Kim BJ, Song KS, Kong HH, Cha HJ, Ock M. 2014; Heavy Hymenolepis nana infection possibly through organic foods: report of a case. Korean J Parasitol. 52:85–7. DOI: 10.3347/kjp.2014.52.1.85. PMID: 24623888. PMCID: PMC3949000.
11. Cho SC, Lee HL, Lee OY, Yoon BC, Choi HS, Hahm JS, et al. 2009; Hymenolepis nana infection of the colon in an adult male. Gastrointest Endosc. 70:784–5. DOI: 10.1016/j.gie.2009.05.024. PMID: 19577746.
Fig. 1
Morphological findings of Hymenolepis nana in a colonoscopy. (A) Colonoscopic view of H. nana infection in the cecum, revealing whitish worms approximately 2–4 cm in length. (B–E) Microscopic images of H. nana revealing proglottids ranging from immature (IM) to mature (M) with visible testes (TE), showing the progression of parasite maturation (magnification, 40×) (B), details of rectangular-shaped immature proglottids (IM) and intervening grooves, highlighting the parasite’s morphology (magnification, 400×) (C), gravid proglottids (G) filled with eggs (EG) (magnification, 100×) (D), and details of mature eggs, revealing diagnostic characteristics such as hooks (H), polar thickening (Pt), and onion-root-shaped polar filaments (Pf) (magnification, 400×) (E).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download