This article has been
cited by other articles in ScienceCentral.
Dear Editor,
Periodontitis is an inflammatory disease with a high global prevalence and is characterized by microbially associated host-mediated inflammation that results in lost periodontal attachment [
1]. Several cultivable periodontal pathogens are used as markers of chronic periodontitis [
2], but a consensus has not been reached regarding the definitive periodontal pathogens. In this study, we assessed the role of the salivary microbiome as a potential biomarker of periodontitis based on comparisons with a periodontitis-resistant control group in the Korean population. We included 13 patients with stage IV periodontitis [
3] and 19 periodontitis-free control patients recruited at Chonnam National University Dental Hospital, Gwangju, Korea, from May 2022 to December 2022. We excluded patients taking medications that can affect periodontitis progression within 3 months of the start of this investigation. No statistically significant differences were observed in terms of factors known to influence the oral microbiome between the two groups, such as age, smoking habits, or medication usage (
Table 1). This study was carried out in accordance with all the relevant institutional guidelines. Ethical approval was obtained from the Chonnam National University Hospital (CNUDH-2023-015), and written informed consent was obtained from all participants.
Each participant provided an unstimulated saliva sample using the OMNIgene ORAL OM-505 collection device (DNAGenotek, Ontario, Canada), according to a previously reported protocol [
4]. Genomic DNA was extracted using a GeneAll Exgene Blood SV Mini Kit from GeneAll (Seoul, Korea) according to the manufacturer’s instructions. The V3–V4 regions of bacterial 16S rRNA genes were amplified and sequenced by Macrogen (Seoul, Korea) using the MiSeq platform. Sequences were trimmed, merged, and then clustered into operational taxonomic units using CLC Genomics Workbench v. 10.1.1 and CLC Microbial Genomics Module v. 2.5 (Qiagen, Hilden, Germany), as previously reported [
5]. The Mann–Whitney test and the χ
2 test were used to test for phenotypic differences in the microbiomes using the GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA).
P<0.05 was considered to reflect a statistically significant difference.
The overall diversity of the salivary microbiome in patients with periodontitis was lower than that of the controls, and the overall composition of the salivary microbiome seemed to differ between both groups. However, this difference was not statistically significant (data not shown). When we compared the bacterial compositions of the salivary microbiomes of both groups at the species level, the periodontitis group showed a predominance of
Selenomonas and
Rothia species. In contrast, the control group showed a predominance of
Prevotella species (
Fig. 1A). Linear discriminant analysis revealed several taxa that correlated highly with the periodontitis or control group (
Fig. 1B). A volcano plot confirmed that
Fretibacterium spp. were associated with patients in the periodontitis group (
Fig. 1C). Furthermore, we compared the ratio of
Fretibacterium species to
Fusobacterium periodonticum (Freti/FPO ratio) between both groups. The periodontitis group showed a significantly higher Freti/FPO ratio than the control group (median, Freti/FPO ratio, 20.16 vs. 0.28, periodontitis group vs. control group,
P=0.0054;
Fig. 1D). Controversy remains as to whether dysbiosis is related to disease severity of periodontitis [
6,
7].
Our findings suggest that dysbiosis is commonly observed in patients with periodontitis, but the degree of dysbiosis could not be quantified absolutely. However, the periodontitis group harbored different salivary microbial community compositions, consistent with previous findings [
7]. Among several taxa specifically associated with the periodontitis group in this study, we focused on
Fretibacterium species. This focus was supported by previous findings showing significant positive correlations between the levels of
Fretibacterium sp. HOT 360 and periodontal parameters [
8] and peri-implantitis (
F. fastidiosum) [
9]. We suggest the detection and quantification of
Fretibacterium species as a saliva-based diagnostic bacterial biomarker for periodontitis screening. When considering potential protective markers, we observed several commensal taxa, including
Haemophilus species and
F. periodonticum [
10]. Previously, those taxa were inversely associated with
S. mutans, the key pathogen causing dental caries [
11]. Hence, we speculate that the abundances of
Fretibacterium species and
F. periodonticum might be inversely correlated. Finally, we observed the potential of a high Freti/FPO ratio as a biomarker of periodontitis.
Our study has some limitations. The V3–V4 region of the 16S rRNA gene is limited in its ability to resolve fine-level taxonomic differences. As an observational study, causality was not tested. Larger studies of other populations, incorporating shotgun metagenomic sequencing, quantification of absolute microbial loads, and site-specific measures of oral bacterial communities, are warranted. The broader implications of this study may include the potential identification of salivary microbial biomarkers, particularly Fretibacterium species, and the Freti/FPO ratio, for periodontitis screening, offering insights into diagnostic strategies and paving the way for further research on oral microbiome-related interventions.
ACKNOWLEDGEMENTS
We truly appreciate Sung Hoon Kim for assistance in sample collection and preparation.
References
1. Kassebaum NJ, Bernab E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. 2014; Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 93:1045–53. DOI:
10.1177/0022034514552491. PMID:
25261053. PMCID:
PMC4293771.
2. Riep B, Edesi-Neuss L, Claessen F, Skarabis H, Ehmke B, Flemmig TF, et al. 2009; Are putative periodontal pathogens reliable diagnostic markers? J Clin Microbiol. 47:1705–11. DOI:
10.1128/JCM.01387-08. PMID:
19386852. PMCID:
PMC2691128.
3. Tonetti MS, Greenwell H, Kornman KS. 2018; Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 89(S1):S159–72. DOI:
10.1002/JPER.18-0006. PMID:
29926952.
4. Vogtmann E, Chen J, Kibriya MG, Amir A, Shi J, Chen Y, et al. 2019; Comparison of oral collection methods for studies of microbiota. Cancer Epidemiol Biomarkers Prev. 28:137–43. DOI:
10.1158/1055-9965.EPI-18-0312. PMID:
30262598. PMCID:
PMC6324947.
7. Lu H, He L, Xu J, Song W, Feng X, Zhao Y, et al. 2020; Well-maintained patients with a history of periodontitis still harbor a more dysbiotic microbiome than health. J Periodontol. 91:1584–94. DOI:
10.1002/JPER.19-0498. PMID:
32490546.
9. Belibasakis GN, Mir-Mari J, Sahrmann P, Sanz-Martin I, Schmidlin PR, Jung RE. 2016; Clinical association of Spirochaetes and Synergistetes with peri-implantitis. Clin Oral Implants Res. 27:656–61. DOI:
10.1111/clr.12690. PMID:
26354174.
10. Kim M, Yun SY, Lee Y, Lee H, Yong D, Lee K. 2022; Clinical differences in patients infected with
Fusobacterium and antimicrobial susceptibility of
Fusobacterium isolates recovered at a tertiary-care hospital in Korea. Ann Lab Med. 42(2):188–95. DOI:
10.3343/alm.2022.42.2.188. PMID:
34635612. PMCID:
PMC8548237.
Fig. 1
Salivary microbiome analysis for patients in the periodontitis and control groups. (A) The periodontitis group showed a predominance of Selenomonas and Rothia species, whereas the control group showed a predominance of Prevotella species. (B) Linear discriminant analysis (LDA) scores for differentially abundant taxa in the salivary microbiome among patients in the periodontitis group (purple) and the control group (blue). The length denotes the effect size for a given taxon. Among the taxa shown, we observed P=0.05 via Kruskal–Wallis testing and an LDA score of ≥4.0. (C) Volcano plot showing several bacterial taxa specifically associated with patients in the periodontitis or control group. (D) Ratio of Fretibacterium species to Fusobacterium periodonticum (Freti/FPO ratio) between the periodontitis and control groups. The periodontitis group showed a significantly higher Freti/FPO ratio than the control group did (median, Freti/FPO ratio, 20.16 vs. 0.28, periodontitis group vs. control group, P=0.0054).
Abbreviations: Ambig, ambiguous; Uncultu, unculturable.
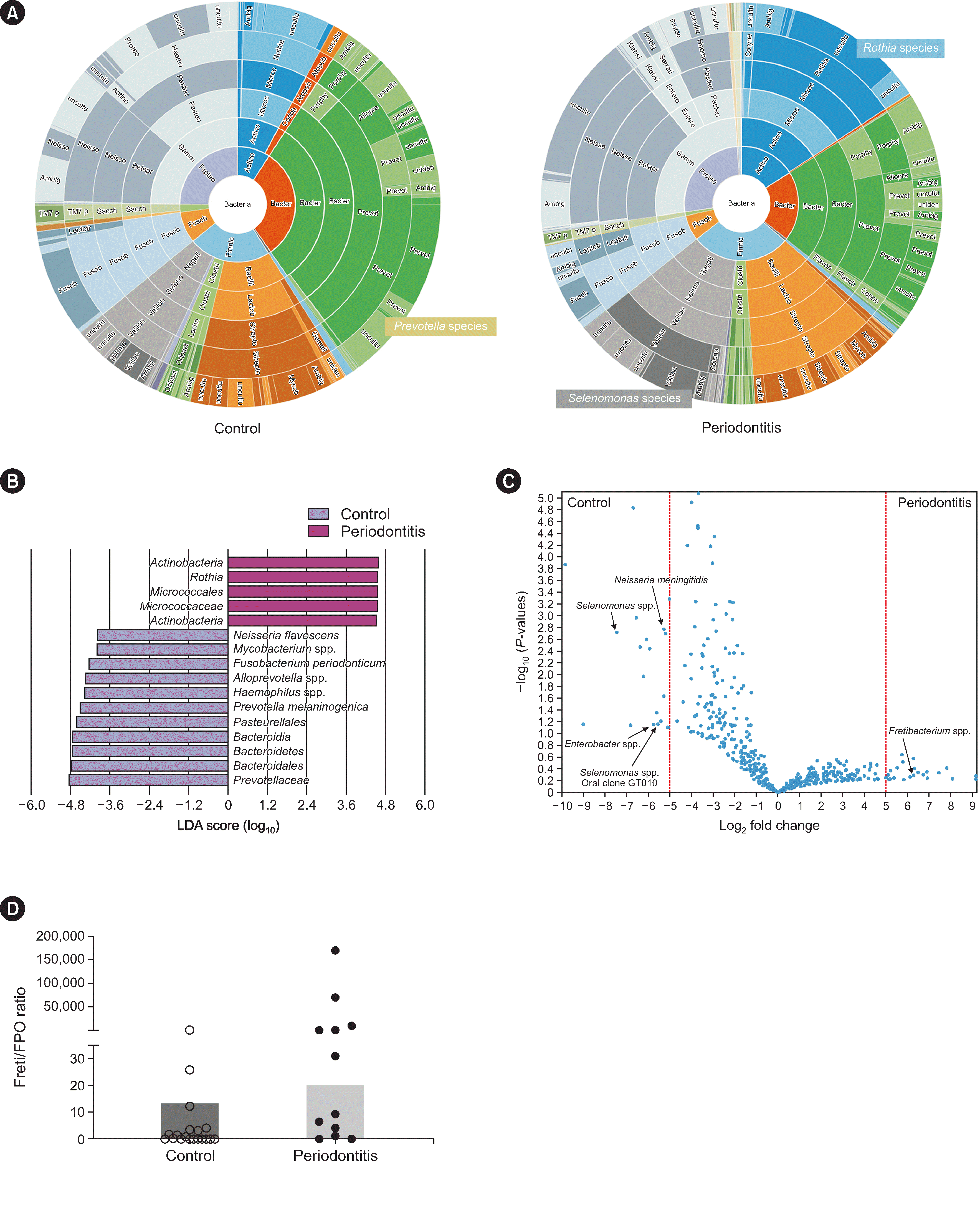
Table 1
Clinical characteristics of participants in the study cohort
|
Characteristics |
Periodontitis group (N=13) |
Periodontitis-free control group (N=19) |
|
Sex (female/male), N |
7/6 |
9/10 |
|
Age, yrs, mean±SD |
64.3±8.2 |
30.9±4.4 |
|
Smoking habit, N of cases |
|
|
|
Non-smoker/ex-smoker/current smoker |
12/1/0 |
17/2/0 |
|
N of missing teeth, mean±SD |
16.5±8.2 |
0 |
|
N of periodontal pockets >4 mm, mean±SD |
4.8±2.4 |
0 |
|
N of periodontal pockets >6 mm, mean±SD |
3.1±1.6 |
0 |
|
Periodontitis classification, N |
|
|
Stage I/II/III/IV |
0/0/0/13 |
0/0/0/0 |
|
Underlying disease, N (%) |
|
|
Any cancer or tumor type |
3 (23.1) |
0 (0.0) |
|
Hypertension |
3 (23.1) |
0 (0.0) |
|
Any cardiovascular disease |
2 (15.4) |
0 (0.0) |
|
Diabetes mellitus |
1 (7.7) |
0 (0.0) |





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download