Cardiac biomarkers are important for assessing individuals with suspected acute myocardial infarction (AMI). European and American guidelines recommend incorporating high-sensitivity cardiac troponin (hs-cTn) assays into rapid rule-in or rule-out protocols for initially assessing patients who potentially have AMI [
1,
2]. Creatine kinase-myocardial band (CK-MB) testing has traditionally been performed in emergency settings and is routinely tested alongside cTn, but lacks tissue specificity, being elevated in various non-cardiac conditions, such as muscle injury, renal insufficiency, and trauma [
3]. Despite reports of limited clinical utility and cost-effectiveness [
4-
6], limited data are available regarding the clinical value of CK-MB testing in conjunction with hs-cTn assays, particularly in urban tertiary centers located in regions with high prevalences of chronic kidney disease, heart failure, or ischemic heart disease. In addition, an insufficient number of studies have been conducted to examine the efficacy of CK-MB in emergency departments (EDs) using both hs-cTnT and hs-cTnI assays [
3,
7]. In this study, we investigated the frequency of hs-cTn and CK-MB measurements in tertiary hospital EDs to assess their practical value in clinical decision-making for diagnosing AMI in real-world clinical settings.
We analyzed 23,771 concurrent referrals for CK-MB and hs-cTn (hs-cTnT or hs-cTnI) from January 2022 to December 2022, involving 17,185 patients in the ED of Seoul St. Mary’s Hospital, a 1300-bed tertiary care hospital in Korea. Records requesting only CK-MB or hs-cTn alone were excluded from the analysis. The study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (approval number KC20TNSI0585). The need for informed consent was waived owing to the retrospective study design of medical record analysis. Statistical analysis was performed using PRISM version 10.0.3 for Windows (GraphPad, San Diego, CA, USA) and MedCalc Statistical Software version 20.114 (MedCalc Software Ltd., Ostend, Belgium). The hs-cTnT levels were analyzed using the Elecsys Troponin T hs electrochemiluminescence immunoassay (Roche Diagnostics, Basel, Switzerland) with a 99th percentile reference limit of 0.014 ng/mL. hs-cTnI levels were measured using the Atellica IM High-Sensitivity Troponin I chemiluminescent immunoassay (Siemens Healthineers, Munich, Germany) with a 99th percentile reference limit of 0.0452 ng/mL. The CK-MB was measured using the Atellica IM Creatine Kinase-MB reagent (Siemens Healthineers) with a 99th percentile reference limit for CK-MB of 5 ng/mL. At our hospital, physicians can choose whether to request cTn, CK-MB, or both tests.
Of 23,771 samples, 23,208 (97.6%) were requested for hs-cTnT and CK-MB testing; 484 (2.0%) were requested for hs-cTnI and CK-MB testing; and 79 were requested for hsTnT, hsTnI, and CK-MB testing. We observed 65.6% agreement between the hs-cTn and CK-MB assays, where 1,253 samples (5.3%) were positive for both, 14,330 samples (60.3%) were negative for both, 203 samples (0.9%) were positive for CK-MB despite being negative for hs-cTn, and 7,985 samples (33.6%) were positive for hs-cTn but negative for CK-MB. When we analyzed the initial test results from all 17,185 patients (mean age: 60.8±19.7 yrs; 47.9% male), excluding follow-up results, 567 (3.3%) tested positive for both hs-cTn and CK-MB, whereas 11,682 patients (68.0%) tested negative for both, indicating a 71.3% agreement between the two assays. Additionally, 4,779 patients (27.8%) exhibited positive hs-cTn results but negative CK-MB results, and 157 patients (0.9%) showed negative cTn results but positive CK-MB results (
Table 1).
Among all 17,185 patients, 131 were diagnosed as having AMI, with 68 cases classified as non-ST-segment elevation myocardial infarction (NSTEMI). More patients who were positive for both hs-cTn and CK-MB were diagnosed as having AMI than patients who were positive for hs-TnI alone (57 of 567 patients [10.1%] versus 63 of 4,779 patients [1.3%], P<0.05). Of 157 patients who were negative for hs-cTn and positive for CK-MB, none were diagnosed as having AMI. Among eleven patients who tested negative for both hs-cTn and CK-MB, seven were diagnosed as having ST-segment elevation myocardial infarction (STEMI), and subsequent percutaneous coronary intervention occurred. When analyzing hs-cTn and CK-MB results across different types of AMI (STEMI or NSTEMI) and unstable angina, we found that 44.4% (28/63) of patients with STEMI, 51.5% (35/68) of patients with NSTEMI, and 48.7% (19/39) of patients with unstable angina were positive for hs-cTn but negative for CK-MB. To assess the additional utility of including CK-MB in the 0/1 hr algorithm versus detecting hs-cTn alone, we examined the 1 hr follow-up results in four patients with NSTEMI who initially tested negative for both hs-cTn and CK-MB. Among the four patients, all had positive follow-up hs-cTn test results, and only one patient was positive for CK-MB, suggesting no additional benefit of CK-MB follow-up. The primary common causes contributing to CK-MB positivity alone were neuromuscular disease (N=25), infection/inflammation (N=18), cardiovascular disease (N=17), trauma (N=14), malignancy (N=11), non-specific chest pain (N=10), psychiatric conditions (N=9), and other factors (N=53).
When comparing hs-cTnT and hs-cTnI results for all 20 patients with data for both markers, six patients (30.0%) had discordant results (hs-cTnT positive and hs-cTnI negative). These six patients were diagnosed as having non-AMI, and only one patient tested positive for CK-MB. Notably, one non-AMI patient tested positive for all three tests. This patient had underlying medical conditions (including diabetes mellitus, chronic kidney disease, and stroke) and presented signs of rhabdomyolysis and acute kidney injury upon arrival at the ED. The patient showed no abnormal findings on the electrocardiogram or echocardiograph, indicating that the elevation of hs-cTnT, hs-cTnI, and CK-MB could have been due to rhabdomyolysis and renal failure [
8].
Diagnosing AMI in patients with renal disease is challenging owing to consistently elevated cTn levels. We investigated the test results in 420 patients with renal disease, examining hs-cTnT and CK-MB data for 413 patients and hs-cTnI and CK-MB for seven patients. Of them, 51 patients (12.1%) were positive for both hs-cTn and CK-MB, 322 patients (76.7%) were positive for hs-cTn alone, and one patient was positive for CK-MB alone. Previous data consistently confirmed that cTn provides more accurate assessments, being less influenced by marker-release fluctuations [
9]. However, in this study, none of the patients with renal disease were diagnosed with AMI; therefore, we could not assess the diagnostic value of hs-cTn and CK-MB in patients with renal disease.
Although cTn is a specific biomarker of cardiac injury, it also identifies myocardial injury irrespective of its cause, leading to potential overuse [
6,
10]. Wilson,
et al. [
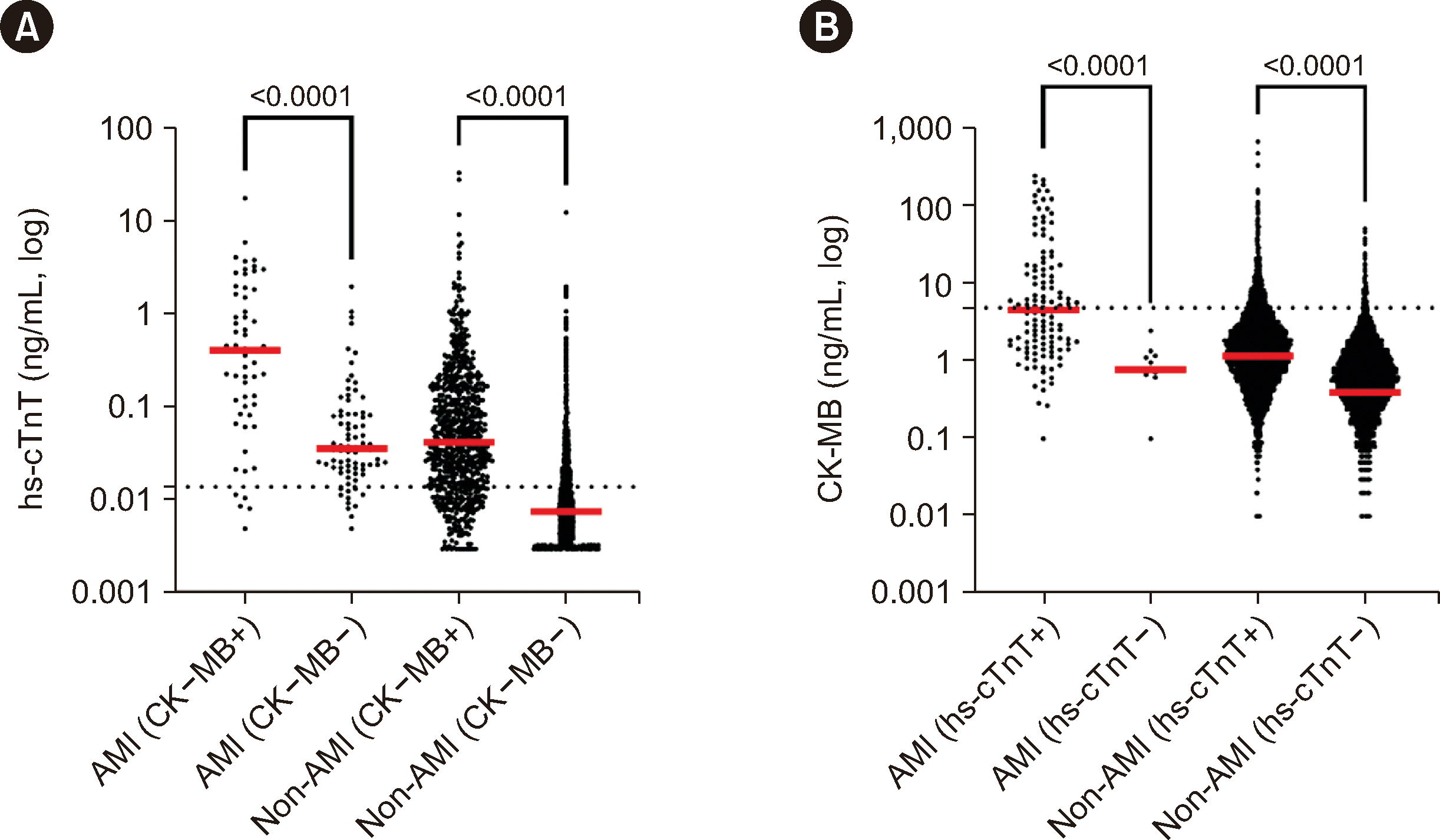
10] reported elevated cTn levels in hospitalized patients with non-cardiac conditions, with AMI being detected in only 3.5% of all cases. Most cTn orders were for a single test rather than serial testing. We found that 72.3% of tests were for single measurements and that AMI was identified in 2.3% of cTn-positive cases. However, the incidence of AMI increased to 10.2% when both cTn and CK-MB were positive (
Fig. 1). Nevertheless, additional CK-MB testing did not enhance AMI detection compared with using hs-cTn alone. These findings are consistent with prior studies, confirming CK-MB’s limited utility in diagnosing acute coronary syndrome (ACS) [
3-
7]. Similarly, US and European guidelines consider cardiac troponins to be the most sensitive and specific marker of cardiomyocyte injury, rather than CK or CK-MB testing for AMI diagnosis [
1,
2]. The European guidelines suggest that hs-cTnT and hs-cTnI assays offer comparable diagnostic accuracy for early AMI diagnosis, with higher prognostic accuracy noted for hs-cTnT [
1]. cTnT is reportedly released at a slower rate than cTnI owing to tighter binding to tropomyosin [
11]. cTnT is also more significantly affected by renal dysfunction and by diabetes. However, both troponin proteins serve as key biomarkers in AMI diagnosis and are used interchangeably in clinical practice.
An hs-cTn assay might not be universally available across all institutions. Additionally, a growing trend in EDs is the adoption of point-of-care tests (POCTs) for cTn owing to their advantages in rapidly obtaining results. However, most current POCTs are not highly sensitive [
12]. Recent research suggests that combining CK-MB with a single POCT cTn assay increases the diagnostic sensitivity for AMI compared with using the POCT cTn assay alone [
13]. Thus, in challenging conditions where high-sensitivity cTn testing is difficult, CK-MB testing (particularly in conjunction with POCT cTn assays) may aid in diagnosing AMI. However, our study has the following limitations. The retrospective design introduces selection bias and the single-center setting limits generalizability. Further studies are warranted to assess long-term outcomes and cost-effectiveness to validate findings and guide healthcare decisions.
In conclusion, careful considerations should be made to avoid indiscriminate CK-MB testing for diagnosing AMI in an ED setting. Strategic and appropriate utilization of CK-MB tests is crucial for reducing unnecessary testing costs and avoiding disruptions in patient care.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download