Abstract
Pseudothrombocytopenia caused by platelet clumping (PC) can lead to unnecessary platelet transfusions or underdiagnosis of hematologic neoplasms. To overcome these limitations, we assessed the capacity of the Sysmex DI-60 digital morphology analyzer (Sysmex, Kobe, Japan) for detecting PC and determining an accurate platelet count in the presence of PC. For this purpose, 135 samples with or without PC (groups Y and N, respectively) were processed by an examiner (a hematologic specialist) using both the Sysmex XN-9000 and DI-60 analyzers. Although the platelet aggregate (PA) and giant platelet (GP) counts reported by the DI-60 and the examiner exhibited strong correlations, they proved inadequate as effective indicators for screening samples containing PC. Between the PA and GP counts and four platelet indices (the platelet distribution width [PDW], mean platelet volume [MPV], platelet large cell ratio [P_LCR], and plateletcrit [PCT]) reported by the XN-9000, we observed statistically significant correlations (both overall and with group Y), but they were relatively weak. The platelet counts determined using the DI-60 and light microscopy in group Y showed substantial variations. Although the performance of the DI-60 was reliable for detecting PA and GP in smear images, such fixed areas are not representative of whole samples. Further, in the presence of PC, the resulting platelet counts determined using the DI-60 were not sufficiently accurate to be accepted as the final count.
Pseudothrombocytopenia (PTCP) is a phenomenon where a platelet count obtained with an automated hematology analyzer is artificially low because of the presence of platelet clumps (PCs), resulting in an abnormal histogram and inaccurate platelet enumeration [1, 2]. PTCP can appear simultaneously under various conditions, such as autoimmune disease, pregnancy, viral infection, and treatment with certain drugs. PTCP can also occur in healthy individuals [1, 3, 4]. In healthy patients, the failure to identify a low platelet count owing to PTCP can lead to unnecessary platelet transfusions, bone marrow biopsies, or underdiagnosis of hematologic neoplasms. In clinical laboratories, detecting PTCP and obtaining accurate platelet counts is important for patient care. However, minimal investigation has been conducted on the potential utility of digital microscopes, which represent emerging tools in modern hematology laboratories, in treating samples with PCs [5-8]. In this study, we evaluated the DI-60 digital microscope (Sysmex, Kobe, Japan) for its ability to detect PCs and report accurate platelet counts in the presence of PCs. This study was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital (approval number 2022-04-013).
In our laboratory (Department of Laboratory Medicine at Soonchunhyang University Cheonan Hospital, Cheonan, Korea), we performed a blood smear for every sample flagged by an SP-10 analyzer (Sysmex, Kobe, Japan) and examined by a medical technician. From June 5 through June 25, 2021, examiner A (a skilled medical technician with six years of experience) collected all initial complete blood count (CBC) results, including four platelet indices (the platelet distribution width [PDW], mean platelet volume [MPV], platelet large cell ratio [P_LCR], and plateletcrit [PCT]), reported by an XN-9000 analyzer (Sysmex, Kobe, Japan) and blood smears made from EDTA-anticoagulated whole blood with a “PLT_clump?” flag found during daily examinations. Samples with incomplete CBC data or a missing smear were excluded. All collected smears were then reviewed again by examiner B (a hematologic specialist with three years of experience) and classified according to the consensus among both examiners. At 200× magnification, the sample was categorized into group Y when there were more than two platelet aggregates (PAs), each comprising at least three platelets on the smear (the criterion for PA used in our other study, which is currently in press). The sample was determined to have PCs and was thus assigned to group Y.
Conversely, in the absence of PCs or microscopic fibrin clots (amorphous basophilic materials usually located at the ends of smears), the sample was assigned to group N. Through this process, 135 CBC results and blood smears, comprising group N (N=40, 29.6%) and group Y (N=95, 70.3%), were included. For the detection of PC, the DI-60 analyzer captured images of platelets, giant platelets (GPs; platelets of the same size or larger than a normal red cell) [9], and PAs in predetermined regions of blood smears. Subsequently, the analyzer provided these images along with the PA and GP counts to a human examiner. The examiner then counted the platelets and re-classified the PA and GP counts to report the results. That is, the reported platelet count by DI-60 actually reflects the number manually counted by an examiner from digital microscopic images on a computer monitor.
To assess the capacity of the DI-60 for PC detection, all smears from both groups were processed using the DI-60. Next, examiner B counted the platelets in every smear from group Y using the Fonio method [10] under a standard light microscope. Subsequently, we compared the numbers of PAs and GPs in groups N and Y counted using the DI-60 along with those counted by the examiner via t-test and interclass-correlation coefficient (ICC) analysis. Areas under the receiver operating characteristic curves (AuROC) and the associated 95% confidence intervals (CIs) were determined to assess the PC-detecting function of the DI-60 and optimal cut-off values for the PA and GP counts. We also compared the PA and GP counts reported by the DI-60 with the four platelet indices (PDW, MPV, P_LCR, and PCT) initially reported by the XN-9000 via Pearson’s correlation analysis. Finally, the counts found in group Y using the DI-60 and by performing light microscopy were compared via t-test and ICC analysis to determine whether digital microscopy was better suited for analyzing clumped samples.
IBM SPSS version 26.0 (SPSS Inc., Armonk, NY, USA) was used for all statistical analyses. The results were considered statistically significant at P<0.05.
The DI-60 reported PA and GP counts for groups N and Y that strongly correlated with those counted by examiner B. In group N, the ICCs for the PA and GP counts were 0.996 (95% CI=0.994–0.997) and 0.992 (95% CI=0.984–0.996), respectively. Correspondingly, in group Y, the ICCs were 0.987 (95% CI=0.981–0.992) for PA counts and 0.699 (95% CI=0.424–0.842) for GP counts (Table 1). The screening performances of the PA and GP counts of PC-positive samples were analyzed using the DI-60 (Fig. 1). Sensitivity and specificity, with a cut-off of 3.5 PAs, were determined to be 71.6% and 52.5%, respectively (Fig. 1A). Similarly, sensitivity and specificity, with a cut-off of 66.50 GPs, were determined to be 48.4% and 65.0%, respectively (Fig. 1B).
By analyzing the PA and GP counts reported by the DI-60 and the four platelet indices (PDW, MPV, P_LCR, and PCT) initially reported by the XN-9000, overall statistically significant positive correlations were identified. However, these correlations were not strong (Table 2). When the correlations were analyzed separately for each group, group N showed a weak and statistically insignificant correlation, whereas group Y revealed a moderate and statistically significant correlation (Table 2). In group N, the platelets counted using digital images provided by the DI-60 aligned with the number reported by the XN-9000, which is traditionally considered the final count (ICC=0.975, 95% CI=0.952–0.987; P=0.662). Conversely, in group Y, the platelet count obtained using DI-60 did not agree with the final count manually measured using a conventional microscope (ICC= 0.873, 95% CI=0.097–0.959; P<0.001).
Digital microscopy is a new tool for modern hematology. Its performance and utility have been vigorously evaluated [5-8, 11]. Platelet counts reported by the DI-60 and by XN-series analyzers reportedly correlated well with each other, although the DI-60 can underestimate platelet counts in samples with marked thrombocytosis [12]. However, no research has been conducted to examine PC detection with the DI-60, with the closest work being three studies [5-7] performed to examine PC with CellaVision, which uses the same software as the DI-60 but with different hardware. The results from these studies revealed sensitivity values ranging from 40.4%–82.8% in terms of PC detection.
Because the final DI-60-derived PA and GP counts aligned with those recorded by both the DI-60 and an examiner, we analyzed PA and GP counts in various aspects: First, the PA and GP counts determined by the DI-60 and a human examiner were significantly correlated with each other (Table 1), indicating the reliability of the DI-60 in PA and GP detection and classification when analyzing smear images. However, the PA and GP counts did not serve as good indicators for screening samples containing PCs (Fig. 1). Although this finding may initially seem contradictory, we believe it reflects the fact that the DI-60 captures pictures of a fixed area of each smear, while PCs tend to form in the marginal areas of smears. This possibility aligns with previous reasoning applied to the variable sensitivity of CellaVision [8, 10, 11]. When analyzing the PA and GP counts reported by the DI-60 and the four platelet indices (PDW, MPV, P_LCR, and PCT) initially reported by the XN-9000, correlations were observed (both overall and in group Y). However, these correlations were relatively weak in all cases (Table 2). These data might indicate that the fixed area examined by the DI-60 represents the whole sample to some extent. However, it does not provide a sufficient representation, as discussed earlier. Lastly, the platelet count of the DI-60 was reliable in the absence of PCs. In contrast, in the presence of PCs, the platelet count determined using the DI-60 could not be accepted as the final count because the two counts differed significantly from each other, thereby requiring a manual count. This finding might be attributable to the various sizes and uneven distributions of PCs.
This study has some limitations that should be noted. First, a relatively small number of samples were included in this study. Second, we did not use a charged program provided by Sysmex to analyze platelets. Finally, we used the Fonio method rather than counting with a fluorescence flow cytometer, which is a reference method for enumerating platelets [13, 14].
In conclusion, digital microscopes (which have recently attracted substantial attention in a wide range of research areas) are limited in their ability to handle samples containing PCs; thus, caution should be exercised when using them for PC detection and platelet counting. When PCs are present, a final confirmation of the accurate platelet count is still required from an experienced examiner.
Notes
AUTHOR CONTRIBUTIONS
Woo S performed the research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; Kim B designed the research, analyzed and interpreted data; Huh NH analyzed and interpreted data; Kim MS and Yoon YA performed the research; Choi YJ analyzed and interpreted data.
References
1. Lardinois B, Favresse J, Chatelain B, Lippi G, Mullier F. 2021; Pseudothrombocytopenia-a review on causes, occurrence and clinical implications. J Clin Med. 10:594. DOI: 10.3390/jcm10040594. PMID: 33557431. PMCID: PMC7915523. PMID: d53aef395a6d4be6951f486001d28eb1.
2. Baccini V, Geneviève F, Jacqmin H, Chatelain B, Girard S, Wuilleme S, et al. 2020; Platelet counting: ugly traps and good advice. Proposals from the French-speaking Cellular Hematology Group (GFHC). J Clin Med. 9:808. DOI: 10.3390/jcm9030808. PMID: 32188124. PMCID: PMC7141345. PMID: 704f5f47e8944dcf89f9bf3558e2f964.
3. Bizzaro N. 1995; EDTA-dependent pseudothrombocytopenia: a clinical and epidemiological study of 112 cases, with 10-year follow-up. Am J Hematol. 50:103–9. DOI: 10.1002/ajh.2830500206. PMID: 7572988.
4. Choe WH, Cho YU, Chae JD, Kim SH. 2013; Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 35:70–6. DOI: 10.1111/j.1751-553X.2012.01466.x. PMID: 22958573.
5. Kratz A, Lee SH, Zini G, Riedl JA, Hur M, Machin S, et al. 2019; Digital morphology analyzers in hematology: ICSH review and recommendations. Int J Lab Hematol. 41:437–47. DOI: 10.1111/ijlh.13042. PMID: 31046197.
6. Billard M, Lainey E, Armoogum P, Alberti C, Fenneteau O, Da Costa L. 2010; Evaluation of the CellaVision DM automated microscope in pediatrics. Int J Lab Hematol. 32:530–8. DOI: 10.1111/j.1751-553X.2009.01219.x. PMID: 20132350.
7. Gulati G, Uppal G, Florea AD, Gong J. 2014; Detection of platelet clumps on peripheral blood smears by CellaVision DM96 system and microscopic review. Lab Med. 45:368–71. DOI: 10.1309/LM604RQVKVLRFXOR. PMID: 25316670.
8. Gao Y, Mansoor A, Wood B, Nelson H, Higa D, Naugler C. 2013; Platelet count estimation using the CellaVision DM96 system. J Pathol Inform. 4:16. DOI: 10.4103/2153-3539.114207. PMID: 23858391. PMCID: PMC3709429. PMID: d0df0893d0284640bc3763bf02e0f263.
9. Gulati G, Caro J. 2014. Blood cells: morphology and clinical relevance. 2nd ed. American Society for Clinical Pathology;Chicago: p. 94.
10. Mannuß S. 2020; Influence of different methods and anticoagulants on platelet parameter measurement. J Lab Med. 44:255–72. DOI: 10.1515/labmed-2020-0037. PMID: d188b7b31a0844bb8b0c934e06005564.
11. Nam M, Yoon S, Hur M, Lee GH, Kim H, Park M, et al. 2022; Digital Morphology Analyzer Sysmex DI-60 vs. Manual Counting for White Blood Cell Differentials in Leukopenic Samples: A Comparative Assessment of Risk and Turnaround Time. Ann Lab Med. 42:398–405. DOI: 10.3343/alm.2022.42.4.398. PMID: 35177560. PMCID: PMC8859564.
12. Kim HN, Hur M, Kim H, Kim SW, Moon HW, Yun YM. 2017; Performance of automated digital cell imaging analyzer Sysmex DI-60. Clin Chem Lab Med. 56:94–102. DOI: 10.1515/cclm-2017-0132. PMID: 28672770.
13. Briggs C, Harrison P, Machin SJ. 2007; Continuing developments with the automated platelet count. Int J Lab Hematol. 29:77–91. DOI: 10.1111/j.1751-553X.2007.00909.x. PMID: 17474881.
14. ICSH. 2001; Platelet counting by the RBC/platelet ratio method: A reference method. Am J Clin Pathol. 115:460–4. DOI: 10.1309/W612-MYEP-FA7U-8UYA. PMID: 11246942.
Fig. 1
Comparison of the areas under the receiver operating characteristic curves (AuROCs) for the number of platelet aggregates (PAs) (solid line) and the number of giant platelets (GPs) (dotted line) determined by the DI-60 when screening samples containing platelet clumping (PC). For PA counts, the AuROC was 0.636 (95% CI=0.535–0.737). For cut-off value of 3.5 of PA counts, the sensitivity was 0.716 (95% CI=0.614–0.804), and the specificity was 0.525 (95% CI=0.361–0.685). For GP counts, the AuROC was 0.538 (95% CI=0.431–0.646). For cut-off value of 66.5 of GP counts, the sensitivity was 0.484 (95% CI: 0.380–0.589), and the specificity was 0.650 (95% CI: 0.483–0.794).
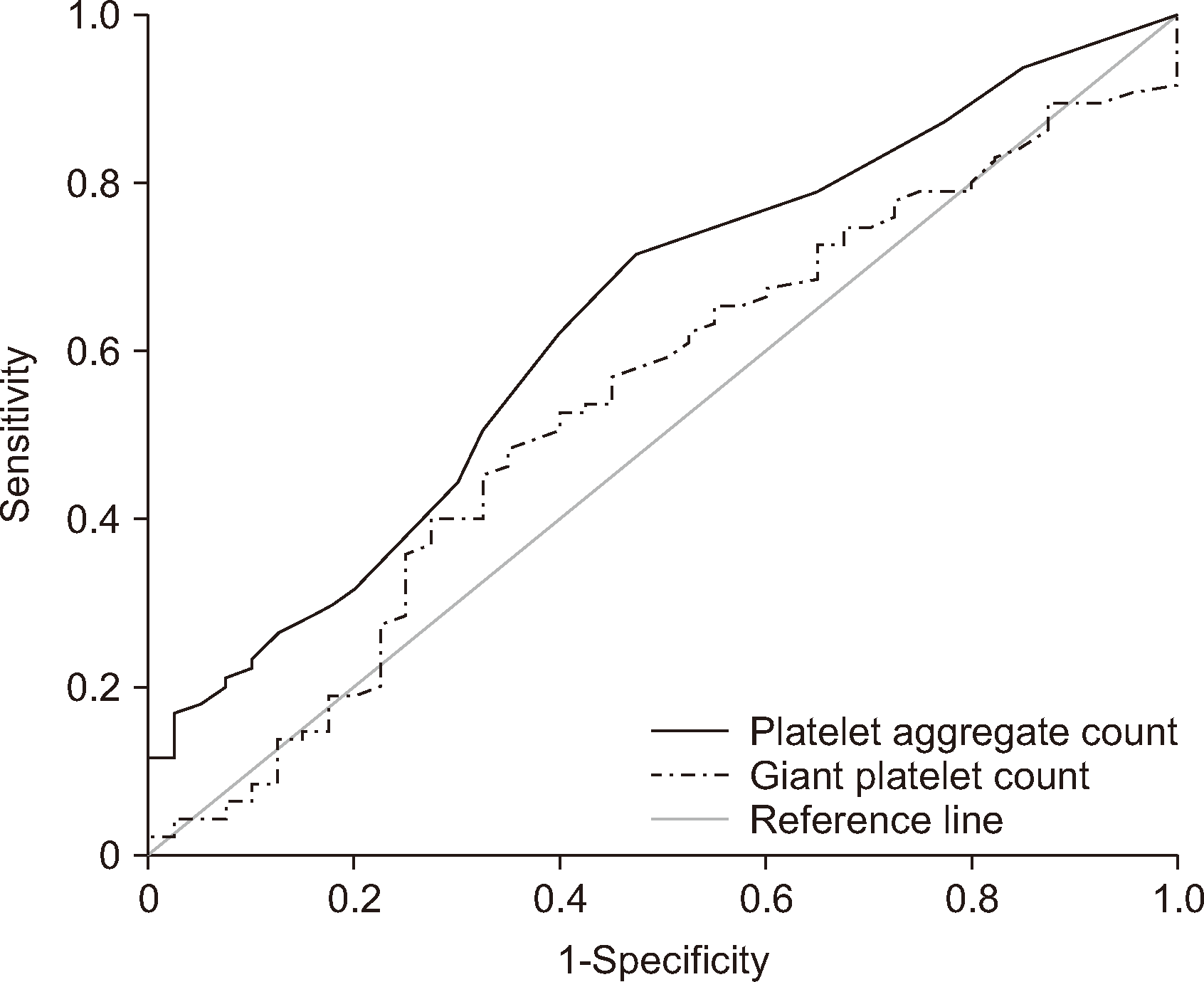
Table 1
Correlations between platelet aggregate and giant platelet counts of group N (samples with over two platelet aggregates consisting of at least three platelets on smears at 200× magnification) and group Y (samples without platelet clumps and microscopic fibrin clots on smears at 200× magnification)
Table 2
Correlations between the platelet aggregate and giant platelet counts reported by the DI-60 and four PLT indices reported by the XN-9000
| Group | Flag | Platelet aggregate count | Giant platelet count |
|---|---|---|---|
| Groups N and Y combined | PDW (fL) | 0.359‡ | 0.238† |
| MPV (fL) | 0.378‡ | 0.296† | |
| P_LCR | 0.347‡ | 0.246† | |
| PCT | 0.180* | 0.395‡ | |
| Group N | PDW (fL) | −0.251 | 0.090 |
| MPV (fL) | −0.178 | 0.154 | |
| P_LCR | −0.208 | 0.102 | |
| PCT | 0.310 | 0.642‡ | |
| Group Y | PDW (fL) | 0.406‡ | 0.272† |
| MPV (fL) | 0.423‡ | 0.331† | |
| P_LCR | 0.391‡ | 0.280† | |
| PCT | 0.116 | 0.292† |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download