Abstract
The adenovirus detection rate is <10% throughout the year in South Korea; however, during the summer of 2023, it showed an unusual increase. We analyzed the adenovirus detection rate using data from the Korea Respiratory Integrated Surveillance System before and after coronavirus disease (COVID-19) collected from 2019 to week 36 of 2023. Before the COVID-19 outbreak in 2019, the mean detection rate was 8.2%, which decreased to 6.1% during the COVID-19 pandemic from 2020 to 2022. In 2023, the mean detection rate was 14.3% in week 36 and the highest in week 34, at 42.2%, and adenovirus was predominantly detected in the summer. The detection rate by age group showed substantially high activity among 0–12-yr-olds after the pandemic. This age group had a steady mean rate of 9.5% during the pandemic, without seasonality. In 2023, the detection rate surged in the 0–6-yr and 7–12-yr age groups, peaking at 61.6% and 57.1%, respectively. The dominant epidemic serotypes were HAdV-1 and HAdV-2 during and HAdV-3 after the pandemic. The multifaceted non-pharmaceutical interventions during the COVID-19 pandemic considerably impacted the prevalence of common respiratory viruses and complicated respiratory virus patterns after the pandemic. Constant surveillance is crucial for epidemic preparedness to monitor the possible surge of certain respiratory viruses.
Adenovirus infection, which is prevalent in infants and young children, primarily manifests as an upper respiratory disease. It causes conjunctivitis and gastroenteritis in addition to respiratory symptoms, such as high fever, cough, runny nose, and sore throat. Approximately 100 serotypes, some of which may have increased virulence, have been identified to date [1, 2]. Therefore, serotype analysis is of clinical and epidemiological importance.
The Korea Disease Control and Prevention Agency (KDCA) has conducted national surveillance since 2000 and was expanded to the Korea Respiratory Virus Integrated Surveillance System (K-RISS) in 2022. The K-RISS added severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a target pathogen, along with eight other human respiratory viruses (influenza virus, rhinovirus, adenovirus, bocavirus, respiratory syncytial virus, parainfluenza virus, coronavirus, and metapneumovirus). According to the surveillance data, the adenovirus detection rate tends to be <10% throughout the year in South Korea, with no seasonality [3]. Because of the unusual increase in the detection rate in the summer of 2023, we analyzed the annual patterns of adenovirus occurrence to analyze epidemic factors. This study was reviewed and approved by the institutional review board of the KDCA (Cheongju, Korea; approval Nos.: 2016-05-02-4C-A and 2022-02-05-C-A).
The detection rate was analyzed based on adenovirus gene detection results of 42,100 samples collected from the K-RISS from 2019 to week 36 of 2023. In addition, a serotype analysis was performed on 187 cases identified as adenovirus-positive between 2020 and 2023. After DNA extraction, the hexon gene, which encodes the adenovirus envelope protein, was partially amplified and sequenced to identify the subtype [4]. Sex and clinical symptoms were analyzed according to the subtype. The detection status analysis revealed a mean detection rate of 8.2% in 2019, before the COVID-19 pandemic, with the highest rate of 16.9% in week 34. During the COVID-19 pandemic (2020–2022), the mean detection rate was 6.1%, and the highest rate of 14.5% occurred in week 25. However, in week 36 of 2023, the mean adenovirus detection rate was higher, at 14.2% (Table 1). The mean detection rate rapidly increased after week 29 and peaked at 42.2% in week 33, which was >2.5 times higher than that in the corresponding period between 2019 and 2022 (Fig. 1A).
Analysis by age group revealed that the 0–12-yr age group was predominantly affected by the current COVID-19 pandemic (Fig. 1B and 1C). The detection rate was consistently the highest in the 0–6-yr age group in 2023. Specifically, the mean adenovirus detection rate in 2023 in this age group was 24.4%, which was significantly higher than the rates recorded between 2019 and 2022. Moreover, in week 34 of 2023, an unprecedented detection rate of 61.6% was observed. The detection rates in those aged 7–12 and 13–64 yrs were similar in 2019 and 2020–2022, whereas in 2023, the rate unusually increased to 57.1% among those aged 7–12 yrs (Fig. 1C and 1D). Among those aged 0–6 yrs, it increased as of week 29, whereas among those aged 7–12 yrs, it increased two weeks later, in week 31. In those aged ≥65 yrs, the detection rate trends did not differ between 2019 and 2023, and detection was sporadic or very low (Fig. 1E).
Analysis of the adenovirus serotypes occurring between 2020 and 2023 revealed the presence of 11 serotypes over the 4 yrs. Type 3 was prevalent at a high rate in 2023, whereas type 2 was prevalent in the preceding 3 yrs (2020–2022; Fig. 2). In 2020, the detection rates for types 2, 1, and 3 were 31.7%, 24.4%, and 22.2%, respectively, and types 4, 5, 8, and 54 were also identified. In 2021, types 2, 1, 34, and 37 were detected at rates of 70.8%, 20.8%, 4.2%, and 4.2%, respectively. In 2021, type 2 was detected the most, followed by types 1 and 6. In 2023, type 3, which was previously detected at a relatively low rate, had the highest rate of 48.0% and was predominantly detected in the second half of the year (Fig. 2).
Analysis of the detection rate by sex for type 3 adenovirus, which was predominantly identified in 2023, and other serotypes revealed that, among the patients infected with adenovirus type 3, 47.4% were male and 52.6% were female. In contrast, for serotypes other than type 3, 56.9% were male, and 43.1% were female. However, these differences were not significant (data not shown). Adenovirus type 3 commonly circulates in pediatric patients with respiratory diseases and causes severe acute respiratory illness in children [5-7]. Unfortunately, we could not examine symptom severity because the study data were obtained from outpatients. Respiratory symptoms in outpatients, such as fever, cough, nasal discharge, and sputum, did not significantly differ among the adenovirus types (Supplemental Data Fig. S1). The long-term COVID-19 pandemic has globally markedly influenced the epidemic patterns of other respiratory viruses, including influenza, as evidenced by case reports documenting epidemic patterns that deviate from historical trends [8-11].
During the COVID-19 pandemic, Korea was among the countries implementing the strictest non-pharmaceutical interventions (NPIs), such as prohibiting social gatherings, wearing masks, and quarantining infected persons. These multifaceted NPIs have influenced the prevalence of respiratory pathogens by lowering the probability of virus infection, resulting in a different epidemic pattern from that before COVID-19 [3, 12]. We found that the adenovirus detection rate unusually rapidly increased during the summer of 2023 despite the absence of seasonality until 2022 [3]. Type 3, which mainly circulated in South Korea during 2023, is a major serotype globally reported to cause respiratory symptoms [13-15]. This serotype has been detected in the past; therefore, the specific genotype was not determined [3, 16]. Our findings imply that the prevalence and virulence of type 3, initially detected at a lower rate than the other types during the pandemic, are increasing. This trend was particularly prominent in the 0–12-yr age group. The detection rates were significantly higher in younger age groups than in older age groups during the 2023 summer season (weeks 28 to 36); 43.3% in the 0–6-yr age group, 48.1% in the 4–6-yr age group, and 28.8% in the 7–12-yr age group versus 4.9% in the 13–64-yr age group and 1.2% in the ≥65-yr age group. The high detection rates in younger age groups can be attributed to several factors, including low immunity to adenovirus among infants and young children and the resumption of normal group activities in kindergartens and schools after lifting infection control measures such as NPIs. Additional analysis is required to ascertain whether the genetic information and transmission characteristics of type 3 differ from those of the types previously widespread in South Korea. Further, constant surveillance is required to detect emerging serotypes as potential health threats.
The epidemic patterns of adenovirus and seasonal respiratory viruses have changed because NPIs are implemented in response to COVID-19, suggesting the need for preparation for possible pandemics from other known respiratory viruses. In conclusion, continuous surveillance is required to better understand the long-term impact of the COVID-19 pandemic on the prevalence of common respiratory viruses. This may benefit public health practice for respiratory virus epidemics and inform responses to respiratory virus pandemics in the future.
ACKNOWLEDGEMENTS
We would like to thank 18 Public Health and Environment Research Institutes in South Korea for submitting data to the K-RISS.
Notes
AUTHOR CONTRIBUTIONS
Lee NJ, Woo SH, Rhee JE, and Lee JH contributed to specimen preparation and performed the experiments. Lee SW and Kim EJ conceived and planned the experiments. Lee NJ, Woo SH, and Rhee JE interpreted the results. Lee NJ took the lead in manuscript writing. Lee SW and Kim EJ supervised the study. All authors provided critical feedback that contributed to the research and final manuscript. All authors have read and approved the final manuscript.
Appendix
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.3343/alm.2023.0484
References
1. HAdV Working Group. http://hadvwg.gmu.edu. Updated on Mar 2024.
2. Loeb M, Kuchar E. Human Adenovirus Infections. https://empendium.com/mcmtextbook/chapter/B31.II.18.1.16. Updated on June, 2024.
3. Kim IH, Kang SG, Cha JO, Seo YJ, Kwak J, Lee NJ, et al. 2023; Changes in patterns of respiratory virus since the coronavirus disease 2019 pandemic (until April 2023). Public Health Wkly Rep. 16:626–31.
4. Lee WJ, Jung HD, Cheong HM, Kim K. 2015; Molecular epidemiology of a post-influenza pandemic outbreak of acute respiratory infections in Korea caused by human adenovirus type 3. J Med Virol. 87:10–7. DOI: 10.1002/jmv.23984. PMID: 24889391. PMCID: PMC7167096.
5. Duan Y, Xu B, Li C, Bao Y, An S, Zhou Y, et al. 2021; Molecular characteristics of human adenovirus type 3 circulating in parts of China during 2014-2018. Front Microbiol. 12:688661. DOI: 10.3389/fmicb.2021.688661. PMID: 34267738. PMCID: PMC8276179. PMID: da6578faa7cb4cfaa1ad2dc92fee14cc.
6. Lai CY, Lee CJ, Lu CY, Lee PI, Shao PL, Wu ET, et al. 2013; Adenovirus serotype 3 and 7 infection with acute respiratory failure in children in Taiwan, 2010-2011. PLoS One. 8:e53614. DOI: 10.1371/journal.pone.0053614. PMID: 23326469. PMCID: PMC3542335. PMID: 19264ac8561749119906a0d88c231feb.
7. Wo Y, Lu QB, Huang DD, Li XK, Guo CT, Wang HY, et al. 2015; Epidemical features of HAdV-3 and HAdV-7 in pediatric pneumonia in Chongqing, China. Arch Virol. 160:633–8. DOI: 10.1007/s00705-014-2308-8. PMID: 25504360. PMCID: PMC7087000.
8. Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N. 2021; Resurgence of respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan. Emerg Infect Dis. 27:2969–70. DOI: 10.3201/eid2711.211565. PMID: 34388086. PMCID: PMC8544984. PMID: dfc60d5d1b534de680ff26a2f12a56e3.
9. Ben Moussa M, Buckrell S, Rahal A, Schmidt K, Lee L, Bastien N, et al. 2023; National influenza mid-season report, 2022-2023: a rapid and early epidemic onset. Can Commun Dis Rep. 49:10–4. DOI: 10.14745/ccdr.v49i01a03. PMID: 36815865. PMCID: PMC9902033.
10. Chow EJ, Uyeki TM, Chu HY. 2023; The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. 21:195–210. DOI: 10.1038/s41579-022-00807-9. PMID: 36253478. PMCID: PMC9574826.
11. Tesema L, Sullivan D, Pulido M, Traub E, Escobar J, Moore L, et al. 2022; Notes from the field: influenza A(H3N2) outbreak following a school event - Los Angeles, California, March 2022. MMWR Morb Mortal Wkly Rep. 71:745–6. DOI: 10.15585/mmwr.mm7122a4. PMID: 35653298. PMCID: PMC9169524.
12. Lee NJ, Woo SH, Lee JH, Rhee JE, Kim EJ. 2023; 2021-2022 influenza and respiratory viruses laboratory surveillance report in the Republic of Korea. Public Health Wkly Rep. 16:53–65.
13. Lin MR, Yang SL, Gong YN, Kuo CC, Chiu CH, Chen CJ, et al. 2017; Clinical and molecular features of adenovirus type 2, 3, and 7 infections in children in an outbreak in Taiwan, 2011. Clin Microbiol Infect. 23:110–6. DOI: 10.1016/j.cmi.2016.11.004. PMID: 27851998. PMCID: PMC7129580.
14. James L, Vernon MO, Jones RC, Stewart A, Lu X, Zollar LM, et al. 2007; Outbreak of human adenovirus type 3 infection in a pediatric long-term care facility-Illinois, 2005. Clin Infect Dis. 45:416–20. DOI: 10.1086/519938. PMID: 17638187.
15. Adhikary AK. 2017; Genomic diversity of human adenovirus type 3 isolated in Fukui, Japan over a 24-year period. J Med Microbiol. 66:1616–22. DOI: 10.1099/jmm.0.000625. PMID: 29068283.
16. Choi EH, Kim HS, Park KH, Lee HJ. 2006; Genetic Heterogeneity of the hexon gene of adenovirus type 3 over a 9-year period in Korea. J Med Virol. 78:379–83. DOI: 10.1002/jmv.20550. PMID: 16419117.
Fig. 1
Adenovirus detection rate by age group from 2019 to 2023. (A) All ages, (B) 0–6-yr age group, (C) 7–12-yr age group, (D) 13–64-yr age group, (E) ≥65-yr age group.
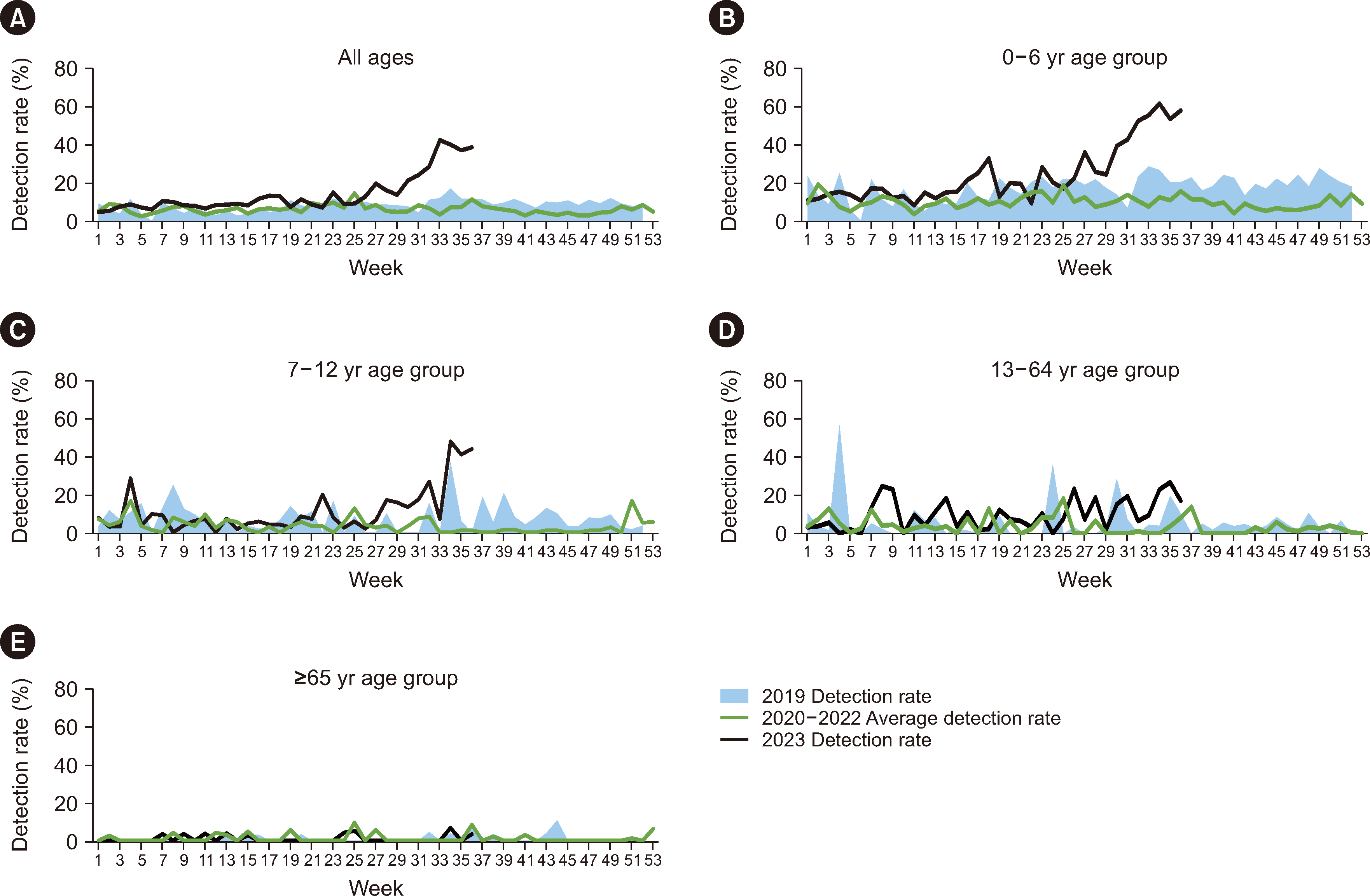
Fig. 2
Monthly distribution of adenovirus types since 2020 determined using data from the Korea Respiratory Virus Integrated Surveillance System.
Abbreviation: HAdV, human adenovirus.
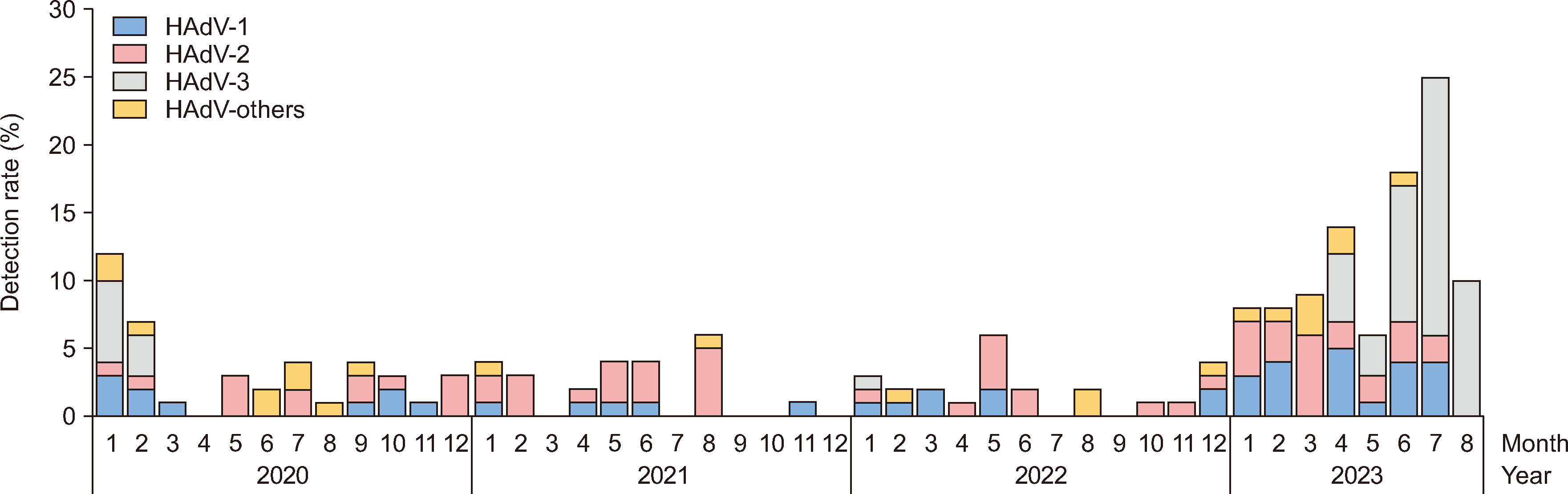




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download