Abstract
Background
Pharyngeal infection is more difficult to diagnose and treat than genital or rectal infection and can act as a reservoir for gonococcal infection. We determined the prevalence of pharyngeal gonorrhea in Korean men with urethritis and analyzed the molecular characteristics and antimicrobial susceptibility of the isolates.
Methods
Seventy-two male patients with symptoms of urethritis who visited a urology clinic in Wonju, Korea, between September 2016 and March 2018 were included. Urethral and pharyngeal gonococcal cultures, antimicrobial susceptibility testing, Neisseria gonorrhoeae multi-antigen sequence typing (NG-MAST), and multiplex real-time PCR (mRT-PCR) were performed.
Results
Among the 72 patients, 59 tested positive for gonococcus by mRT-PCR. Of these 59 patients, 18 (30.5%) tested positive in both the pharynx and urethra, whereas 41 tested positive only in the urethra. NG-MAST was feasible in 16 out of 18 patients and revealed that 14 patients had the same sequence types in both urethral and pharyngeal specimens, whereas two patients exhibited different sequence types between the urethra and pharynx. Of the 72 patients, 33 tested culture-positive. All patients tested positive only in urethral specimens, except for one patient who tested positive in both. All culture-positive specimens also tested positive by mRT-PCR. All isolates were susceptible to azithromycin and spectinomycin, but resistance rates to ceftriaxone and cefixime were 2.9% and 14.7%, respectively.
Gonorrhea, the second most common bacterial sexually transmitted disease, is caused by Neisseria gonorrhoeae. In 2020, the number of newly infected patients reached 82.4 million worldwide, with high numbers in the Western Pacific region (23.2 million) and South-East Asia region (21.2 million) [1]. Globally, the incidence and prevalence of gonorrhea have been steadily increasing over the past few decades but have declined since 2016. However, in South-East Asia, they are still on the rise, and there is growing concern about the recent global increase in drug-resistant N. gonorrhoeae [2].
Most infections are limited to the primary site, but if not properly treated, complications such as pelvic inflammation, infertility, and ectopic pregnancy in women and epididymitis and orchitis in men can occur in addition to simple urethritis. Moreover, gonorrhea can promote the spread of human immunodeficiency virus. In 0.5%–3% of patients with gonorrhea, disseminated infection occurs [3]. Gonococcal infection is asymptomatic in 10% of men and most women, and preventive vaccines are lacking; therefore, proper diagnosis and treatment may be key to reducing gonococcal infection and complications [2].
Pharyngeal infection is more difficult to diagnose and treat than genital or rectal infection and can act as a reservoir for gonococcal infection because symptoms are rare [4]. In particular, gonococcal infection can be transmitted through oropharyngeal practices such as rimming, kissing, and using saliva as a lubricant for anal sex [5]. Furthermore, the pharynx is prone to treatment failure because of its anatomical characteristics, which contribute to a reduced concentration of antimicrobial agents compared with other regions. Moreover, commensal Neisseria species can readily exchange genetic material in the pharynx, which can lead to resistance to cephalosporin. Therefore, appropriate treatment of pharyngeal infections is crucial to prevent the emergence and spread of multidrug-resistant N. gonorrhoeae [6].
The pharyngeal gonococcal infection rate in women with urethritis is as low as 2%–5% [7]; however, up to 20% [7] or as high as 25%–31% [8, 9] of women with cervical gonorrhea are co-infected in the pharynx. An Australian study reported that the incidence of urogenital and pharyngeal gonorrhea more than doubled and tripled between 2009 and 2015, respectively [10]. We investigated the prevalence of pharyngeal gonorrhea in Korean men with urethritis and analyzed the molecular characteristics and antimicrobial susceptibility of the isolates.
Seventy-two male patients with symptoms of urethritis, such as dysuria or urethral discharge, who visited Lee’s Urology Clinic in Wonju, Korea, between September 2016 and March 2018 were enrolled in this study. Pharyngeal and urethral specimens were collected in pairs from all patients. This study was approved by the Institutional Review Board for Human Research, Yonsei University College of Medicine, Severance Hospital (IRB 4-2016-0359).
The specimens were immediately transported to Wonju Severance Christian Hospital, Wonju, Korea, located 5 mins (1.6 km) away. Transgrow medium used for specimen transportation was prepared with V-C-N-T inhibitor (Becton Dickinson) containing hemoglobin, IsoVitaleX (Becton Dickinson), and vancomycin, colistin, nystatin, and trimethoprim in GC agar base (Becton Dickinson). The medium was dispensed into screw-cap tubes, allowed to solidify in a horizontal position, and incubated in CO2-enriched air. BD CultureSwab MaxV (+) (Becton Dickinson, Cockeysville, MD, USA) was used for genetic detection of gonococci, Chlamydia trachomatis, and Mycoplasma genitalium, and stored at –70°C.
For gonococcal isolation, primary cultures were established using modified Thayer–Martin medium (Becton Dickinson) at Wonju Severance Christian Hospital. Species were identified using the Vitek Neisseria-Haemophilus Identification (NHI) system (bioMérieux, Marcy-l’Étoile, France) and matrix-associated laser desorption ionization-time of flight mass spectrometry (MicroFlex LT, Bruker Daltonics GmbH, Bremen, Germany). All isolates were stored at –70°C in 20% skimmed milk (Difco, Detroit, MI, USA) before testing. Major sexually transmitted pathogens (C. trachomatis, M. genitalium, and N. gonorrhoeae) were detected using multiplex real-time PCR (mRT-PCR) (Real-Q Hexa-STI kit; BioSewoom, Seoul, Korea).
We studied the molecular epidemiology of the isolates obtained through N. gonorrhoeae culture or mRT-PCR-positive specimens. The allele numbers and sequence types of the por and tbpB genes were determined using N. gonorrhoeae multi-antigen sequence typing (NG-MAST) [11] and the PubMLST database (https://pubmlst.org/bigsdb?db=pubmlst_neisseria_seqdef&page=sequenceQuery, updated on August 2023). Phylogenetic analysis of each gene was performed using the neighbor-joining method in Molecular Evolutionary Genetics Analysis (MEGA v.11) [12].
Antimicrobial susceptibility was tested using the agar dilution method according to CLSI guidelines [13]. Briefly, ~104 colony-forming units of each isolate were added to 1% IsoVitaleX in GC II agar plates using a Steer’s replicator (Craft Machine, Chester, PA, USA), and the plates were incubated in a 5% CO2-enriched atmosphere at 35°C for 24 hrs. European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints for penicillin, ceftriaxone, cefixime, spectinomycin, tetracycline, ciprofloxacin, and azithromycin, and the Calibrated Dichotomous Sensitivity (CDS) breakpoint for gentamicin were used to determine minimum inhibitory concentrations (MICs). We used the Etest (bioMérieux) for ceftriaxone to confirm the MIC distribution for three breakpoints and the cefinase disk test for penicillinase-producing N. gonorrhoeae (PPNG).
Among the 72 patients with suspected urethritis, 59 patients tested positive for gonococcus by mRT-PCR, 13 tested C. trachomatis-positive, and five tested M. genitalium-positive. Among the 59 patients who tested gonorrhea-positive, 18 (30.5%) tested positive both in the pharynx and urethra, whereas 41 tested positive only in the urethra (Table 1). Of the 72 patients, 33 tested culture-positive. All patients tested culture-positive only in urethral specimens, except for one patient who tested positive in both. All culture-positive specimens tested positive by mRT-PCR.
We compared the NG-MAST sequence types of the strains and/or specimens in 16 out of 18 patients who were identified as having coinfection of the pharynx and urethra. The most common sequence type was ST14678 (por8529, tbpB455) (Table 2). In 14 patients, the pharynx and urethra harbored the same sequence types. However, two patients (Nos. 3 and 4) had distinct sequence types in both sites, and patient 4 was the sole patient who tested culture-positive in both sites. In this patient, not only were the sequence types in the two specimens different, but the antibiotic susceptibility results of the cultures also diverged, confirming these were distinct strains. Phylogenetic analysis showed that the strains were not genetically related (Fig. 1).
Antimicrobial susceptibility was investigated for 34 isolates (33 from the urethra and one from the pharynx) from 33 patients (Table 3). None of the N. gonorrhoeae isolates was resistant to spectinomycin; the MIC range, MIC50 value, and MIC90 value were 8–64, 16, and 32 mg/L, respectively. The ceftriaxone and cefixime resistance rates were 2.9% and 14.7%, respectively. The MIC90 values (range) of ceftriaxone and cefixime were 0.12 mg/L (≤0.008–0.25 mg/L) and 0.5 mg/L (≤0.008–0.5 mg/L), respectively. The cefixime and ceftriaxone MIC distributions for isolates detected in both urine and the pharynx and in urine alone showed similar patterns (Supplemental Data Table S1). All isolates were susceptible to azithromycin. The MIC value for azithromycin was 0.06–0.5 mg/L. All isolates were resistant (55.9%) or intermediate resistant (44.1%) to penicillin G. The proportion of PPNG was 29.4% (10/34). Most isolates were resistant to tetracycline (91.2%), with 12/34 (35.3%) showing high-level resistance. All isolates were resistant to ciprofloxacin, and none were resistant to gentamicin. Five cefixime-resistant isolates (14.7%) were also resistant to tetracycline and ciprofloxacin and had relatively high ceftriaxone MICs (0.16–0.47 mg/L).
Among the 59 patients with mRT-PCR-confirmed gonorrhea infection, 33 received ceftriaxone (1 g intramuscularly (IM)), 17 received spectinomycin (2 g IM), four received ribostamycin (1 g IM), and four had unclear medical records. Additionally, one patient did not receive an injection and took only 100 mg of doxycycline orally for 14 days. Twelve (36.4%) of the patients who received ceftriaxone, four (23.6%) of those who received spectinomycin, and the patients who received doxycycline revisited the clinic because their symptoms did not resolve. In contrast, none of the patients who received ribostamycin returned to the clinic. Excluding the patients with unclear medical records, 17 out of 55 patients (30.9%) revisited the clinic after treatment. Only one patient who showed resistance to ceftriaxone did not revisit. There was no significant correlation between antibiotic susceptibility and return to the clinic.
Considering that the sexual behavior of young generations has recently changed and quasi-sexual intercourse is prevalent, attention should be paid to pharyngeal gonorrhea as a dissemination source. Although some patients complain of a sore throat and cervical lymphadenopathy [14], symptoms of pharyngeal gonorrhea generally do not appear in both men and women. Therefore, pharyngeal gonorrhea is more difficult to treat and control than genital or anorectal infection [15] and can be silently transmitted through saliva and throat inoculation [5].
The pharyngeal gonorrhea rates differ among studies and population groups. In a study by Javanbakht, et al. [16], among young people who had oral sex, 27% had gonococcal infection, 28% had pharyngeal gonorrhea alone, and 27% had both urogenital and pharyngeal infections [16]. Therefore, the authors emphasized the importance of screening for pharyngeal gonorrhea. Chan, et al. [17] reported that the prevalence of pharyngeal gonorrhea was 2.1% in heterosexual women, 2.2% in heterosexual men, and 4.6% in men who had sex with men. In a study of heterosexual men in Japan, the prevalence of pharyngeal gonorrhea was 11.9% among patients with urethritis [9]. Moreover, of the patients with gonorrhea caused by urethral infection, 25.0% had a concomitant pharyngeal infection [9].
In our study, the pharyngeal gonorrhea rate in gonococcal urethritis in the general population in Korea was 31%, which is higher than that in heterosexual men in Japan [9]. According to data provided by the Korean National Health Insurance Service, as of 2016, among 450,000 patients with total urethritis, 15,218 patients were diagnosed as having gonococcal urethritis, but only six patients were diagnosed as having pharyngeal gonorrhea [18]. These findings suggest that many cases of pharyngeal gonorrhea may go unnoticed or unreported because of the lack of confirmation in patients with gonococcal urethritis, and screening for pharyngeal gonorrhea should be implemented in cases where gonorrhea is suspected, even in the general population.
Besides the exemption of pharyngeal sampling, the low recovery rate of pharyngeal gonococcal cultures is a reason for the underestimation of pharyngeal gonorrhea. The laboratory method of choice for gonorrhea is culture; however, it is subject to specimen collection techniques and transport conditions. Moreover, in men, pharyngeal specimens have substantially lower sensitivity than urethral discharge specimens. Although not yet regulatory approved, the nucleic acid amplification test (NAAT) is two- to five-fold more sensitive than culture in detecting pharyngeal gonorrhea, and it is gradually replacing culture [19]. In our study, N. gonorrhoeae was detected using mRT-PCR in 59 patients. However, only 34 gonococcal strains were isolated from cultures, only one of which was isolated from the pharynx. This is similar to previous findings [20]. As RT-PCR is preferred over culture for diagnosing pharyngeal gonorrhea, false positives owing to residual DNA in the body must be considered when determining a cure. As for the follow-up period, Hananta, et al. [21] reported that NAAT yielded a gonococcal persistence of 4.6% after one week of treatment with ceftriaxone 500 mg, which decreased to 1.0% after two weeks; therefore, they recommended testing-of-cure after two weeks of treatment.
Another problem with pharyngeal gonorrhea is that it is more difficult to treat than genital gonorrhea. Currently, there are no antimicrobial agents with a cure rate of >90% in pharyngeal gonorrhea [22]. In the pharynx, antibiotics do not maintain an adequate concentration for a sufficient time [6]. Particularly, spectinomycin has a low therapeutic effect on pharyngeal gonorrhea, and the treatment efficacy in sex workers or homosexual people, in whom pharyngeal gonorrhea is common, is limited [23]. Because of the low permeability of antimicrobial agents in the pharyngeal region, the treatment failure rate of pharyngeal gonorrhea is close to 60%, and the possibility of treatment failure for asymptomatic pharyngeal gonorrhea is high [24]. In Korea, spectinomycin is still available; however, additional antibiotics such as azithromycin should be concomitantly used for pharyngeal gonorrhea when spectinomycin is used to treat genital gonorrhea because of the 31% prevalence of pharyngeal gonorrhea in gonococcal urethritis. The test-of-cure for pharyngeal infections should be strengthened, and guidelines for follow-up to ensure proper antimicrobial treatment and for test-of-cure are urgently needed. The same issues should be considered when gentamicin is used for gonococcal treatment.
In one case report, ceftriaxone was not effective in treating pharyngeal gonorrhea despite the isolate being susceptible to ceftriaxone, which suggests that pharmacokinetic factors play a major role in treatment failure [25]. In the present study, ceftriaxone susceptibility, according to the EUCAST breakpoint, was 97%. However, the MICs of nine out of 11 strains (82%) from patients with pharyngeal gonorrhea exceeded 0.016 mg/L, which is in line with the MIC of ceftriaxone in a rare treatment-failure case of pharyngeal gonorrhea [26]. Therefore, even when MIC test results suggest susceptibility to ceftriaxone, the possibility of treatment failure must be considered. Moreover, in two of our cases, NG-MAST sequence types differed between pharyngeal and urethral specimens, indicating that independent infections can occur at different sites and the possibility of treatment failure remains even when one isolate tests susceptible.
One limitation of our study was that the treatment effect in our patients was determined based on the presence of clinical follow-up in the urologic clinic, where no revisit was necessary if symptoms improved. However, some patients may have visited another hospital when symptoms persisted. Therefore, assessing the effectiveness of a treatment based solely on revisits is unreasonable. Second, the molecular test used in our study was not certified by the authority for use in the diagnosis of pharyngeal gonorrhea. However, referring to the NG-MAST results, the molecular test results appeared to be sufficiently reliable. Finally, the prevalence of gonococcal infection in patients with urethritis symptoms reportedly is approximately 30% [27]. However, because we focused on oropharyngeal gonococcal co-infections, we only included patients with specific urethritis symptoms (urethral pain, dysuria, and purulent urethral discharge) suggestive of gonococcal infection at the time of specimen collection, resulting in a relatively high gonococcal infection rate.
In conclusion, the prevalence of pharyngeal gonorrhea in Korean men with gonococcal urethritis in the general population is high at 30.5%, and pharyngeal screening is more widely required even in heterosexual men or women with urogenital gonorrhea. Confirmed cases of pharyngeal gonorrhea are preferably treated with ceftriaxone, currently the best option, followed by NAAT confirmation two weeks into the treatment.
Notes
AUTHOR CONTRIBUTIONS
Roh KH, Liu C, Seo YH, Lee H, Lee S, Uh Y, and Lee K contributed to the study concept and design. Seo YH, Lee S, Uh Y, and Lee H were responsible for specimen collection, susceptibility testing, and data generation. Roh KH and Liu C wrote the manuscript draft. Roh KH, Lee H, and Lee K contributed to the data analysis. All authors were responsible for result interpretation and manuscript revision. All authors have read and approved the final manuscript.
Appendix
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.3343/alm.2024.0025
References
1. WHO. 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Geneva: Available from: https://www.who.int/publications/i/item/9789240027077. Updated on May 2024.
2. Unemo M, Golparian D, Eyre DW. 2019; Antimicrobial resistance in Neisseria gonorrhoeae and treatment of gonorrhea. Methods Mol Biol. 1997:37–58. DOI: 10.1007/978-1-4939-9496-0_3. PMID: 31119616.
3. Bleich AT, Sheffield JS, Wendel GD Jr., Sigman A, Cunningham FG. 2012; Disseminated gonococcal infection in women. Obstet Gynecol. 119:597–602. DOI: 10.1097/AOG.0b013e318244eda9. PMID: 22353959.
4. Fairley CK, Hocking JS, Zhang L, Chow EP. 2017; Frequent transmission of gonorrhea in men who have sex with men. Emerg Infect Dis. 23:102–4. DOI: 10.3201/eid2301.161205. PMID: 27983487. PMCID: PMC5176237. PMID: 9053814eef8c43b481d30983cc68c4d3.
5. Chow EP, Tabrizi SN, Phillips S, Lee D, Bradshaw CS, Chen MY, et al. 2016; Neisseria gonorrhoeae bacterial DNA load in the pharynges and saliva of men who have sex with men. J Clin Microbiol. 54:2485–90. DOI: 10.1128/JCM.01186-16. PMID: 27413195. PMCID: PMC5035428.
6. Lewis DA. 2015; Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains? Sex Transm Infect. 91:234–7. DOI: 10.1136/sextrans-2014-051731. PMID: 25911525.
7. Giannini CM, Kim HK, Mortensen J, Mortensen J, Marsolo K, Huppert J. 2010; Culture of non-genital sites increases the detection of gonorrhea in women. J Pediatr Adolesc Gynecol. 23:246–52. DOI: 10.1016/j.jpag.2010.02.003. PMID: 20434928.
8. Jenkins WD, Nessa LL, Clark T. 2014; Cross-sectional study of pharyngeal and genital chlamydia and gonorrhoea infections in emergency department patients. Sex Transm Infect. 90:246–9. DOI: 10.1136/sextrans-2013-051358. PMID: 24366777.
9. Wada K, Uehara S, Mitsuhata R, Kariyama R, Nose H, Sako S, et al. 2012; Prevalence of pharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae among heterosexual men in Japan. J Infect Chemother. 18:729–33. DOI: 10.1007/s10156-012-0410-y. PMID: 22491994.
10. Callander D, McManus H, Guy R, Hellard M, O'Connor CC, Fairley CK, et al. 2018; Rising chlamydia and gonorrhoea incidence and associated risk factors among female sex workers in Australia: a retrospective cohort study. Sex Transm Dis. 45:199–206. DOI: 10.1097/OLQ.0000000000000714. PMID: 29420449.
11. Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. 2004; Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis. 189:1497–505. DOI: 10.1086/383047. PMID: 15073688.
12. Kumar S, Stecher G, Tamura K. 2016; MEGA7: Molecular evolutionary genetics analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 33:1870–4. DOI: 10.1093/molbev/msw054. PMID: 27004904. PMCID: PMC8210823.
13. CLSI. 2023. Performance standards for antimicrobial susceptibility testing. 33rd ed. Clinical and Laboratory Standards Institute;Wayne, PA: CLSI M100. DOI: 10.1016/s0196-4399(01)88009-0.
14. Newman LM, Moran JS, Workowski KA. 2007; Update on the management of gonorrhea in adults in the United States. Clin Infect Dis. 44(S3):S84–101. DOI: 10.1086/511422. PMID: 17342672.
15. Shim BS. 2011; Current concepts in bacterial sexually transmitted diseases. Korean J Urol. 52:589–97. DOI: 10.4111/kju.2011.52.9.589. PMID: 22025952. PMCID: PMC3198230.
16. Javanbakht M, Westmoreland D, Gorbach P. 2018; Factors associated with pharyngeal gonorrhea in young people: implications for prevention. Sex Transm Dis. 45:588–93. DOI: 10.1097/OLQ.0000000000000822. PMID: 29485543. PMCID: PMC6086760.
17. Chan PA, Robinette A, Montgomery M, Almonte A, Cu-Uvin S, Lonks JR, et al. 2016; Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Infect Dis Obstet Gynecol. 2016:5758387. DOI: 10.1155/2016/5758387. PMID: 27366021. PMCID: PMC4913006. PMID: 0b0d82fcb18b442297b000d1fdbcb9df.
18. Health Insurance Review & Assessment Service. Medical statistics information. https://opendata.hira.or.kr/op/opc/olap4thDsInfoTab1.do. Updated on May 2024.
19. Cornelisse VJ, Chow EP, Huffam S, Fairley CK, Bissessor M, De Petra V, et al. 2017; Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis. 44:114–7. DOI: 10.1097/OLQ.0000000000000553. PMID: 27984552.
20. Oree G, Naicker M, Maise HC, Tinarwo P, Ramsuran V, Abbai NS. 2021; Comparison of methods for the detection of Neisseria gonorrhoeae from South African women attending antenatal care. Int J STD AIDS. 32:396–402. DOI: 10.1177/0956462420971439. PMID: 33570465.
21. Hananta IPY, De Vries HJC, van Dam AP, van Rooijen MS, Soebono H, Schim van der Loeff MF. 2017; Persistence after treatment of pharyngeal gonococcal infections in patients of the STI clinic, Amsterdam, the Netherlands, 2012-2015: a retrospective cohort study. Sex Transm Infect. 93:467–71. DOI: 10.1136/sextrans-2017-053147. PMID: 28822976. PMCID: PMC5739854.
22. Kong FYS, Hocking JS. 2022; Treating pharyngeal gonorrhoea continues to remain a challenge. Lancet Infect Dis. 22:573–4. DOI: 10.1016/S1473-3099(21)00649-6. PMID: 35065062.
23. Lee H, Lee K, Chong Y. 2016; New treatment options for infections caused by increasingly antimicrobial-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 14:243–56. DOI: 10.1586/14787210.2016.1134315. PMID: 26690658.
24. Moran JS. 1995; Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex Transm Dis. 22:39–47. DOI: 10.1097/00007435-199501000-00007. PMID: 7709324.
25. Unemo M, Golparian D, Potočnik M, Jeverica S. 2012; Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill. 17:DOI: 10.2807/ese.17.25.20200-en. PMID: 22748003.
26. Tapsall J, Read P, Carmody C, Bourne C, Ray S, Limnios A, et al. 2009; Two cases of failed ceftriaxone treatment in pharyngeal gonorrhoea verified by molecular microbiological methods. J Med Microbiol. 58:683–7. DOI: 10.1099/jmm.0.007641-0. PMID: 19369534.
27. Ito S, Hanaoka N, Shimuta K, Seike K, Tsuchiya T, Yasuda M, et al. 2016; Male non-gonococcal urethritis: from microbiological etiologies to demographic and clinical features. Int J Urol. 23:325–31. DOI: 10.1111/iju.13044. PMID: 26845624.
Fig. 1
Phylogenetic analysis of the por (A) and tbpB (B) genes using the maximum likelihood method (MEGA v.11). Dots represent different strains isolated from pharyngeal and urethral specimens. In panel A, the dots correspond to the por genes from patient 3 in Table 2, and in panel B, they correspond to the tbpB genes from patient 4.
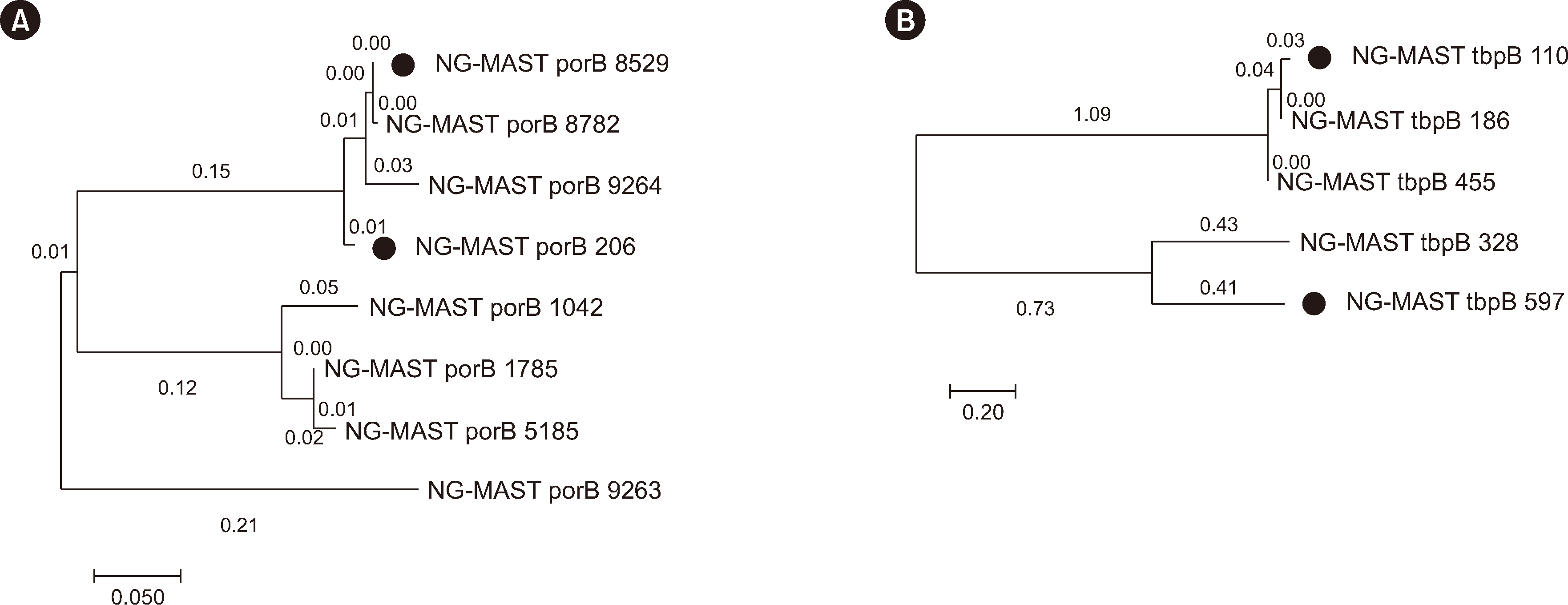
Table 1
Numbers of patients who carried N. gonorrhoeae, C. trachomatis, and M. genitalium according to mRT-PCR results
Table 2
Comparison of Neisseria gonorrhoeae multi-antigen sequence typing (NG-MAST) sequence types in pharyngeal and urethral specimens in patients with pharyngeal gonorrhea
Table 3
Antimicrobial susceptibility of N. gonorrhoeae (N=34)
| Antibiotic | MIC (mg/L) | Antimicrobial susceptibility (%)‡ | |||||
|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | S | I | R | ||
| Penicillin* | 0.12–>128 | 2 | 64 | 0 | 44.1 | 55.9 | |
| Ceftriaxone | ≤0.008–0.25 | 0.03 | 0.12 | 97.1 | 2.9 | ||
| Cefixime | ≤0.008–0.5 | 0.06 | 0.5 | 85.3 | 14.7 | ||
| Spectinomycin | 8–64 | 16 | 32 | 100 | 0 | 0 | |
| Gentamicin† | 2–16 | 4 | 8 | 52.9 | 47.1 | 0 | |
| Tetracycline* | 0.5–32 | 2 | 32 | 0 | 8.8 | 91.2 | |
| Ciprofloxacin | 1–32 | 8 | 16 | 0 | 0 | 100 | |
| Azithromycin | 0.06–0.5 | 0.25 | 0.5 | 100 | 0 | 0 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download