INTRODUCTION
Reference intervals (RIs) and clinical decision limits (CDLs) constitute vital information from laboratories that support the interpretation of laboratory results [
1]. They are critical for health assessments, disease diagnosis, treatment monitoring, and prognostic judgments. An RI is commonly defined as 95% of the range of a certain indicator in a healthy population [
2]. Owing to variants in population and measurement methods, RIs can differ across different regions and laboratories [
3]. CDLs refer to specific thresholds, where values above or below the threshold are associated with a significantly higher risk of adverse clinical outcomes or are used to help diagnose the presence of a specific disease. CDLs are established based on comparisons with gold-standard diagnostic results or clinical outcomes in patients. When laboratory results exceed the CDL threshold, they can support clinical decision-making, such as diagnosis or treatment. CDLs vary for different purposes.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common human enzymopathy, affecting over 500 million people worldwide [
4,
5]. Quantitative detection of G6PD is a commonly used screening method for assessing G6PD deficiency. Improving the interpretation of quantitative G6PD-detection results requires a predetermined definition of normal (100%) G6PD activity. In 2013, Domingo,
et al. [
6] introduced a standardized method for calculating normal G6PD activity, involving two steps: (1) calculating the initial median (M
0) value of the male population and (2) recalculating the median for the male population with values of more than 10% of the M
0, designated as the adjusted male median (AMM). In 2018, the WHO acknowledged the aforementioned method for calculating normal G6PD activity [
7]. In 2022, the WHO proposed a new definition and calculation method for normal G6PD activity: (1) male individuals with abnormal G6PD expression are excluded by genetic testing (typically targeting prevalent
G6PD variants in a specific geographic region, such as the 18 common
G6PD variants in China), and (2) the median G6PD activity in the remaining male population is calculated and referred to as the normal male median (NMM) [
5].
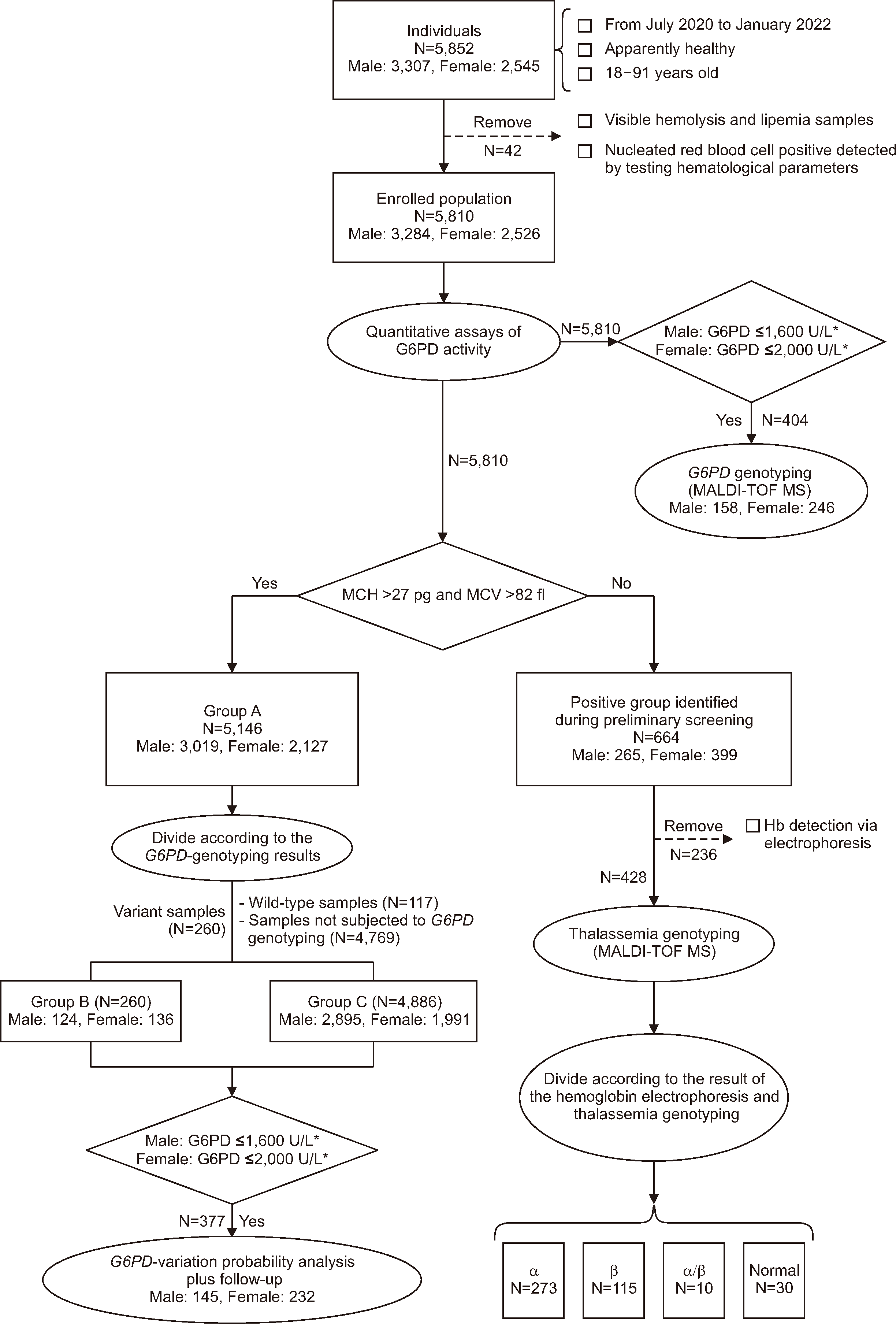
Currently, for quantitative G6PD detection, laboratories typically utilize the G6PD-activity RI provided by test manufacturers (1,300–3,600 U/L). RI values are not specific to any region and fail to combine AMM, NMM, and CDL data to facilitate clinical decision-making. We established reliable and region-specific RIs for G6PD activity to improve the assessment of G6PD activity levels in a local population. We calculated the AMM and NMM values for G6PD activity to define normal G6PD activity in individuals from Guangzhou, China and better interpret the quantitative G6PD results. We also used appropriate G6PD-activity CDLs to provide a reference for the probability of G6PD variants and hemolysis-risk assessment based on G6PD activity.
DISCUSSION
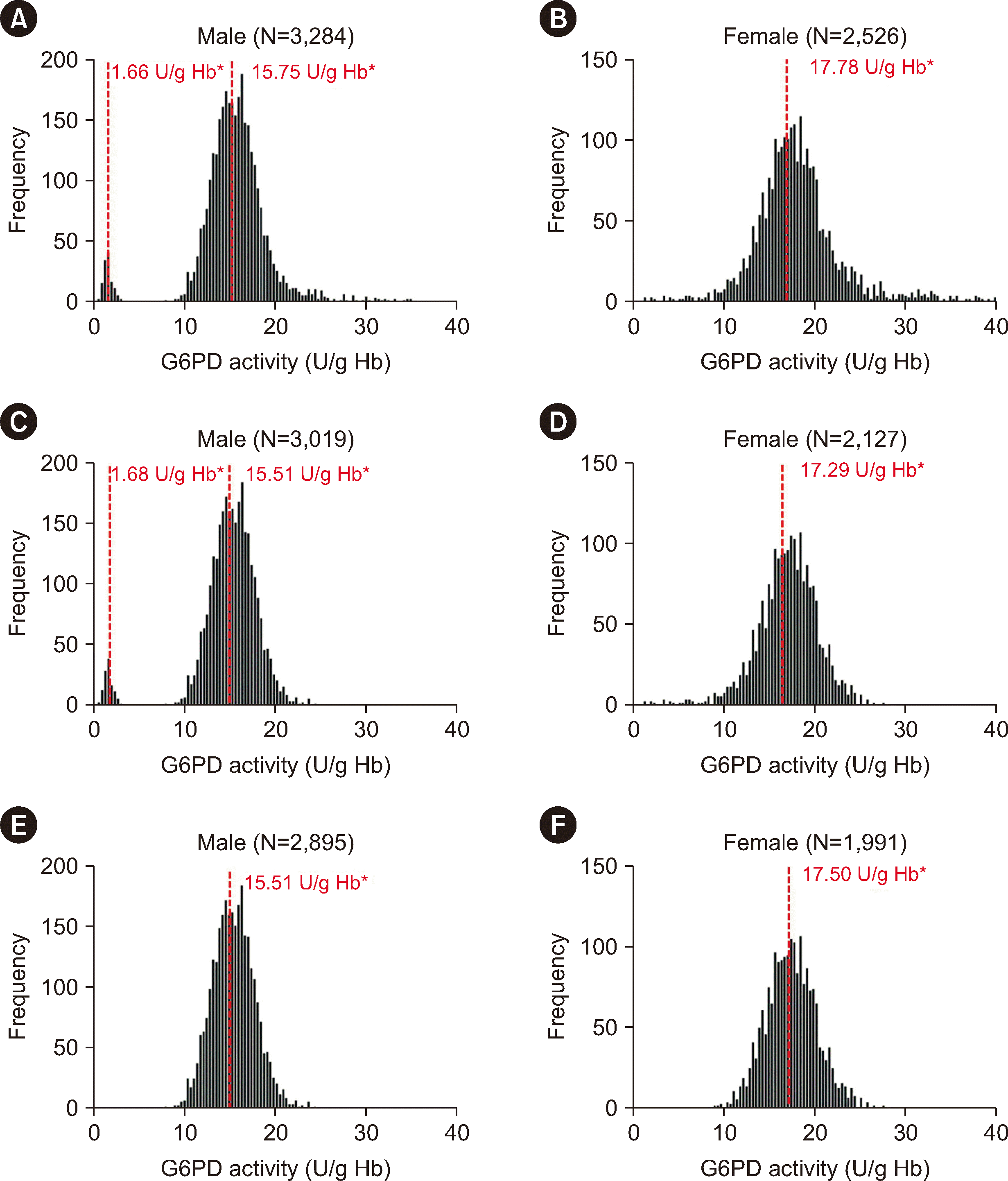
G6PD deficiency is a common inherited hematological disorder in southern China [
11]. Several reports have shown G6PD-activity distributions for different populations [
6,
12]. Consistent with previous reports, in this study, the G6PD activity in the enrolled population showed a bimodal distribution in male and a unimodal distribution in female, both before and after removing samples through positive preliminary screening for thalassemia. The G6PD activities of male and female in the high range had a narrower distribution, and the overall distribution shifted to the left, suggesting that thalassemia may influence the measured G6PD activity. After excluding individuals with
G6PD variants, the G6PD activities of male and female showed a unimodal distribution in accordance with a normal distribution (NS).
Thalassemia is another common inherited hematological disorder in southern China [
13]. Notably, G6PD activity is higher in individuals with thalassemia [
14-
16]. Concordantly, our results showed that G6PD activity was higher in both male and female in the thalassemia group than in the non-thalassemia group. These findings may reflect the compensatory production of new red blood cells in individuals with thalassemia, resulting in a false increase in the measured G6PD-activity values. Quantitative detection of G6PD in individuals with thalassemia cannot truly reflect the G6PD activity level. To establish regional RIs for G6PD activity, individuals with thalassemia must be excluded.
The AMM and NMM values are based on different criteria for defining normal (100%) G6PD activity. Unlike previous studies, we compared the calculated AMM and NMM values between the two groups. The differences between AMM1 and AMM2 (NS) and between NMM1 and NMM2 (P<0.001) were statistically significant, indicating that thalassemia can increase the AMM and NMM values. No statistical difference was found between AMM and NMM values among both populations (P>0.05), indicating that the AMM and NMM results were very similar. Theoretically, the NMM is more accurate than the AMM, although it involves G6PD genotyping. Conducting G6PD genotyping for all samples is expensive, complex, and time-consuming, and it requires considerable human and material resources. We propose that AMM can be used to estimate NMM and that AMM2 (15.47 U/g Hb) and NMM2 (15.51 U/g Hb) can be used to define normal G6PD activity (100%) in adults in this region.
Excluding thalassemia-positive samples identified during screening, we established the RIs for G6PD activities in Groups A and C. G6PD activities between male and female differed significantly (P<0.001). No statistical difference in G6PD activity was found between the two groups (NS) among male. However, a statistical difference was observed between both groups among female (P=0.036). We believe that this difference was likely owing to the removal of female heterozygotes from Group C through G6PD genetic testing, which made the RIs more accurate. Therefore, we selected Group C as the reference population to establish the RIs for G6PD activity for male (11.20–20.04 U/g Hb, 72%–129% of normal G6PD activity) and female (12.29–23.16 U/g Hb, 79%–149% of normal G6PD activity) in the local adult population.
Currently, no region-specific RIs for G6PD activity have been established in China. Test manufacturers provide the RIs for G6PD activity in most regions. We established RIs for G6PD activities that differed from the RIs provided by manufacturers (1,300–3,600 U/L, 54%–149% of normal G6PD activity), with the lower limit of our RI being higher. Specifically, we standardized the RI using Hb levels and accounted for different factors, such as thalassemia and gender differences. Standardizing G6PD activity using Hb levels is recommended by both the WHO and ICSH [
7,
9]. However, the manufacturers did not provide standardized G6PD-activity RIs. In a prospective study of G6PD deficiency in 74,114 healthy adults from 21 provinces and cities in China, Ying,
et al. [
11] found that the mean G6PD-activity values in normal male and female were 15.49±2.67 U/g Hb and 18.01±3.37 U/g Hb, respectively. Consistently, in this study, the mean G6PD-activity values of male and female in Group C were 15.51±2.24 U/g Hb and 17.51±2.69 U/g Hb, respectively.
Increased G6PD-activity values were associated with decreased
G6PD-variant probabilities in both male and female; the decreasing trend in male was more evident, whereas that in female was more gradual. Heterozygous female showed a wide range of G6PD levels, consistent with previously published studies and guidelines [
7,
17-
19]. All male and female homozygotes had G6PD activities of less than 45% of the NMM, which is consistent with the 2022 WHO guidelines for G6PD deficiency [
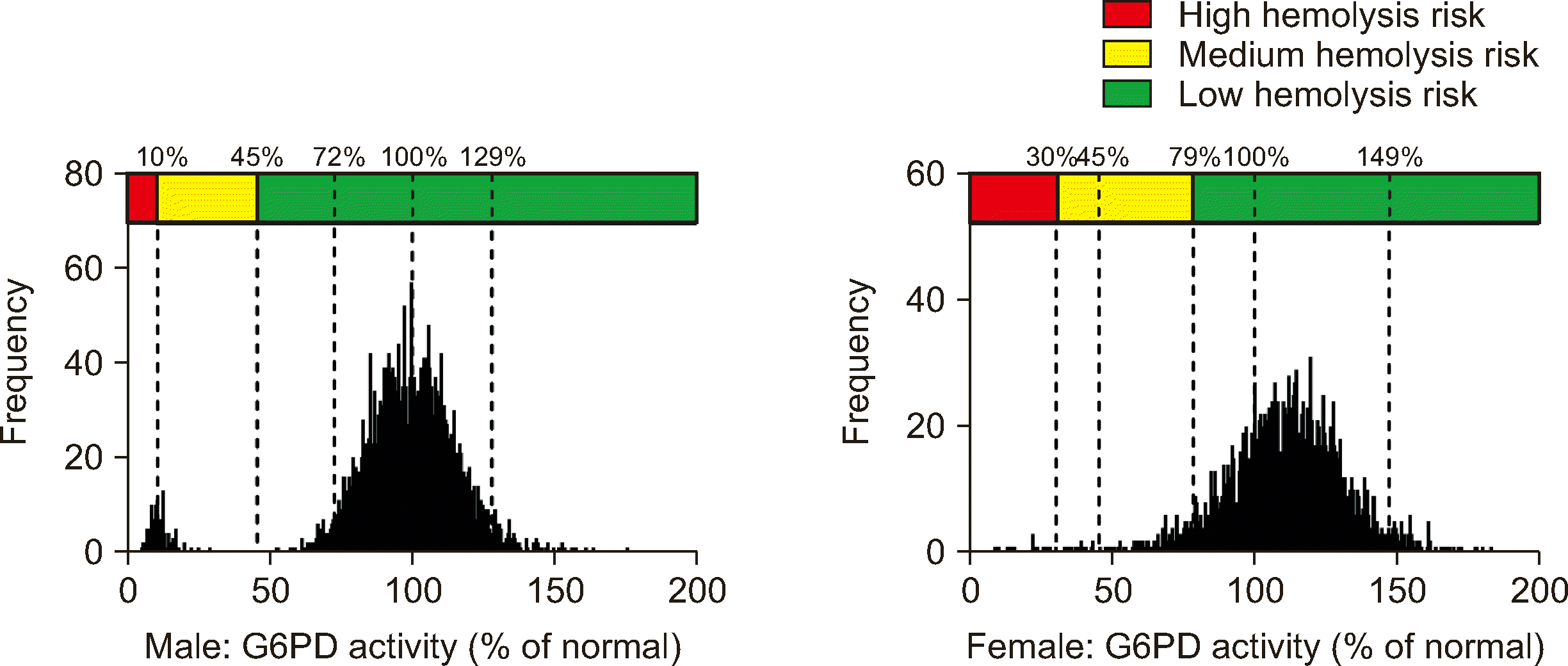
5]. In 2022, the WHO established a new classification scheme for G6PD variants using thresholds of 20%, 45%, 60%, and 150% NMM and indicated that no variants have been identified with median G6PD-activity values in male hemizygous and/or female homozygous individuals that fell between 45% and 60%. We found that a threshold of 45% of the NMM could serve as a CDL to estimate the probability of a
G6PD variant and indicate the necessity for G6PD testing in individuals. When the G6PD activity was greater than 45% NMM, the probability of a
G6PD variant was 0% for male and between 30% and 91.3% for female. Further
G6PD genetic testing is necessary for female but not for male in this region. The limitations of using 45% NMM to estimate the probability of a
G6PD variant should be noted. The results of studies conducted in China and other countries revealed some rare variants that are classified as class IV variants (class C, 60%–150% of the NMM), such as c.660C>G (G6PD São Paulo) [
20,
21], c.152C>T, c.290A>T, and c.1285A>G (G6PD Yucatan) [
22]. Estimating the
G6PD-variant probability using 45% NMM only applies to common
G6PD variants. We detected 18 common variants in
G6PD in human peripheral blood from Chinese individuals, as outlined in the materials and methods section.
Foods and drugs that trigger hemolysis in G6PD-deficient individuals include fava beans, antimalarial drugs, analgesics, antipyretics, and antibacterial agents [
9]. Although antimalarial drugs are rarely used in this region, edible fava beans and other oxidative drugs and some traditional Chinese medicines (including honeysuckle and bezoar) are often used. G6PD is expressed abundantly in the human body, and many people do not develop hemolytic symptoms even when the enzymatic activity of G6PD is below the normal RI. This indicates that the risk for hemolysis in individuals is not well assessed using the G6PD activity as an RI. We correlated G6PD-activity levels with the risk of acute hemolysis through follow-up analysis (
Supplemental Data Table S2,
Table 3), which provided a reference for establishing CDLs based on G6PD-activity levels to evaluate the risk for hemolysis in individuals with G6PD deficiency in the region in the future.
Our follow-up analysis revealed that most individuals did not know their status and had no symptoms or complications. Two compound heterozygous female and four homozygous female had no symptoms. Only seven individuals (cases 1–7) showed hemolysis symptoms; all seven individuals tested positive for a G6PD variant, and G6PD activity below the RI established in this study, and hemolysis did not re-occur after it was resolved. They paid special attention to their diet and medications after hemolysis occurred. These findings suggest that G6PD deficiency can greatly reduce the risk of hemolysis by preventing exposure to oxidative stress factors after diagnosis and that standardized life guidance is of great importance for individuals with G6PD deficiency. Cases 8–10 had no hemolytic symptoms, but their relatives showed symptoms of hemolysis, suggesting that relatives of individuals with G6PD deficiency should also pay attention to preventing G6PD deficiency. In cases where hemolysis symptoms occurred during the follow-up period, the triggers included fava beans, sulfonamides, analgesics, and antipyretics, and the symptoms included jaundice, blood in the urine, paleness, and rash. These findings suggest that more attention should be paid to these triggers and symptoms to prevent, diagnose, and manage G6PD deficiency in this region.
CDLs were previously established to better assess the risk of acute hemolysis in individuals with G6PD deficiency. In 2016, the WHO predicted the risk of acute hemolysis with primaquine treatment based on G6PD-activity levels (10%, 30%, and 80% of the AMM) in male and female [
10]. In 2019, a cut-off value of 70% was used to evaluate the risk for hemolysis in individuals who took tafenoquine [
23]. In 2020, Commons,
et al. [
24] recommended a threshold of 70% normal G6PD activity to evaluate tafenoquine use in terms of hemolysis risk. These CDLs are associated with antimalarial drug use. Antimalarial drugs were not a trigger in Guangzhou because malaria is not endemic to the area. No CDLs have been established for this region that can be used to assess the risk of hemolysis in individuals with G6PD deficiency.
We classified G6PD-activity levels into high, medium, and low hemolysis risk groups. This classification utilized CDLs of 10% and 45% of the NMM for male and 30% and 79% of the NMM for female. The basis for the specific classifications is shown in
Supplemental Data Text S3. People with high and medium hemolysis risk should establish G6PD profiles and indicate their G6PD-deficiency status. People at high risk for hemolysis should be prohibited from taking fava beans, sulfonamides, analgesics, and antipyretics, as well as oxidative drugs. People at medium risk for hemolysis should be cautious when taking these drugs, taking them only after assessment and guidance from professional doctors and undergoing close observation. When acute hemolysis occurs, patients should immediately stop taking suspicious food and drugs. Consumption of fava beans and the aforementioned drugs is generally considered safe for people with a low risk of hemolysis.
In summary, based on the complex genetic background of the high prevalence of G6PD deficiency and thalassemia in Guangzhou, our findings contribute to a more accurate evaluation of G6PD activity levels within the local population and provide valuable insights for clinical decision-making. Specifically, the identification of threshold values for G6PD variants and hemolysis risk enables improved prediction and management of the associated conditions, ultimately enhancing patient care and treatment outcomes. In the future, establishing hemolysis risk threshold values for each commonly consumed drug in this region will enhance clinical drug decision-making guidance.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download