Abstract
Background
EDTA-induced pseudothrombocytopenia (PTCP) during whole blood collection requires significant laboratory resources to obtain accurate results. We evaluated platelet-deaggregation function in EDTA-induced PTCP and platelet-clump flagging by the BC-6800Plus hematology analyzer using integrated digital image analysis.
Methods
We prospectively collected 132 whole blood samples suspected of platelet clumping (102 in EDTA and 30 in sodium citrate) from 88 individuals. We compared platelet counts determined using the platelet count by impedance (PLT-I) function of the DxH 900 hematology analyzer and the PLT-I or optical platelet count (PLT-O) function of the BC-6800Plus. Platelet clumping was verified through manual inspection and the MC-80 digital image analyzer.
Results
Among the 132 whole blood samples, 43 EDTA samples showed platelet clumping. The DxH 900 PLT-I and BC-6800Plus PLT-I results demonstrated a strong correlation (r=0.711) for the EDTA samples but only a moderate correlation with the BC-6800Plus PLT-O results (r=0.506 and 0.545, respectively). The BC-6800Plus PLT-O results were consistent with the sodium citrate platelet counts, with a median dissociation rate of 102.5% (range, 74.9%–123.1%). The DxH 900 and BC-6800Plus analyzers had sensitivity values of 0.79 and 0.72, respectively, for platelet-clump flagging. When integrating the MC-80 digital image analysis results, the sensitivity of BC-6800Plus improved to 0.89 (standard mode) or 1.0 (PLT-Pro mode).
Conclusions
BC-6800Plus PLT-O measurement results are close to the actual values obtained by platelet deaggregation with PTCP samples. Integrating the BC-6800Plus with a digital imaging analyzer effectively improved the diagnosis of PTCP and reduced the requirement for additional laboratory procedures.
EDTA is the most suitable anticoagulant for whole blood samples used for automated complete blood count (CBC) tests. EDTA inhibits blood clotting by chelating calcium in the blood, thereby preserving the integrity of blood cell morphology and ensuring optimal staining for peripheral blood smear analysis [1]. EDTA is highly compatible with most automated hematology analyzers and consistently delivers reliable and uniform results. However, pseudothrombocytopenia (PTCP) caused by EDTA-induced platelet clumping creates problems in CBC testing.
PTCP was first described in 1969 [2], and its prevalence was well documented in the 1990s. The incidences of PTCP in the general outpatient and hospitalized populations have been estimated to be 0.1% [3] and 2.0% [4], respectively. However, within the subset of outpatients presenting with isolated thrombocytopenia, the incidence of PTCP is significantly higher, at 15.3% [5]. Consequently, clinical laboratories in tertiary care hospitals dedicated to treating critically ill patients encounter PTCP relatively frequently.
In cases where PTCP is suspected in blood samples, manual confirmation in a peripheral blood smear is imperative. Once EDTA-induced PTCP is confirmed, supplementary procedures or additional blood collection are required to ensure accurate platelet counts, prolonging the laboratory turnaround time and delaying the reporting of results. The BC-6800Plus hematology analyzer (Mindray Bio-Medical Electronics, Shenzhen, China) has the unique feature of dispersing preformed platelet aggregates. The CAL 8000 automated hematology workstation (Mindray) is a cellular analysis line composed of a BC-6800Plus hematology analyzer, an SC-120 slide maker, and an MC-80 digital image analyzer. This integrated system helps visually identify PTCP and ensures accurate platelet counts.
We assessed the platelet-counting performance of the BC-6800Plus hematology analyzer using samples affected by EDTA-induced PTCP. We primarily examined the platelet-counting performance of the optical platelet count (PLT-O) channel of the BC-6800Plus in PTCP samples. Next, we evaluated the usefulness of the “PLT clumps” flag generated by the BC-6800P system to gauge its effectiveness in identifying platelet clumps. Finally, we explored the potential of integrating BC-6800Plus results with those of the MC-80 digital image analyzer to enhance the detection of platelet clumps.
We prospectively collected 132 clinical whole blood samples from 88 patients between July and December of 2022. The inclusion criteria were as follows: 1) samples showing true-positive PTCP or the presence of fibrin or interfering substances on a cellular scattergram among those flagged for platelet clumps by the DxH 900 analyzer, 2) samples from patients with a history of PTCP, and 3) samples from newly admitted patients with thrombocytopenia (platelet count <150×109/L) confirmed to have platelet clumps based on manual microscopic inspection.
All 132 samples were collected in K2 EDTA (Beckton Dickinson, Plymouth, UK; N=102) or sodium citrate (Beckton Dickson; N=30) tubes. Among the 102 EDTA samples analyzed, 43 EDTA samples from 29 patients were confirmed to exhibit true PTCP, whereas the remaining 59 EDTA samples were negative for PTCP. To match the EDTA samples, we collected 30 samples in sodium citrate tubes from 24 patients.
As a negative-control group, we included 100 EDTA samples with low platelet counts (<150×109/L) and no platelet clumps to assess the diagnostic performance of the platelet clump-flagging feature.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Ewha Womans University Medical Center (approval number SEUMC 2022-05-031-003), with an exemption from obtaining informed consent.
Three automated platelet-counting methods were compared: DxH 900 (Beckman Coulter, Miami, FL, USA) platelet count by impedance (PLT-I), BC-6800Plus PLT-I, and BC-6800Plus PLT-O. Samples that met the inclusion criteria were selected using the DxH 900 automated blood cell analyzer. Platelet-count slides were prepared using the DxH 900 hematology analyzer and DxH 900 SMS II 1.0 (Beckman Coulter).
We tested the selected samples using the BC-6800Plus hematology analyzer within 4 hrs after establishing their eligibility. We identified platelet clumps containing over five aggregated platelets manually using microscopy, adhering to the definition provided by the Groupe Francophone d’Hématologie Cellulaire [6].
Using the same set of samples, we obtained platelet counts, generated slides, and performed automated image analysis using the CAL 8000 workstation. BC-6800Plus performs PLT-O measurements using a specific fluorescent dye in CBC, differential (DIFF), and reticulocyte (RET) (CDR) mode through a comprehensive three-dimensional analysis involving forward scatter, side scatter, and fluorescence (SF Cube cellular analysis) [7, 8]. Using this technology in CDR mode enabled accurate measurement of platelet counts by effectively resolving platelet aggregation. Biochemically, a reactive solution containing amikacin is introduced to obstruct platelet regulatory pathways and directly facilitate platelet deaggregation. Mechanically, the system maintains a constant temperature in a strongly alkaline environment with a pH of 9–10 and performs vortexing at 1,400 rpm to promote the dissociation of platelet aggregates [7].
The DxH 900 system can identify platelet clumps in the nucleated red blood cell (NRBC) channel, which uses low-angle light scatter and axial light loss to count NRBCs. Based on the NRBC scattergrams, the system flags the presence of giant platelets or platelet clumping.
The BC-6800Plus system can identify platelet clumps based on the platelet-distribution histogram with a low platelet count and irregularities in the PLT-I channel. When triggered, the platelet-clumping flag indicates a discrepancy between the PLT-O and PLT-I results. In addition, a ghost image characterized by a 45° angle rise appears at the lower end of the DIFF channel. A similar phenomenon is evident in the white blood cell–NRBC, NRBC, and basophils (WNB) channel. Analysts can exclude interference from RBC fragments or giant platelets in the RET channel.
Platelet clumping was confirmed by manual microscopic examination of the prepared slides. Digital image analysis to detect platelet clumps was performed for 88 EDTA samples from 78 patients using the MC-80 image analyzer in standard mode. Additional digital image assessments were performed to capture images in the ideal, tail, and edge zones in PLT-Pro mode.
We comparatively analyzed platelet-count results obtained using the BC-6800Plus PLT-O, DxH 900 PLT-I, and BC-6800Plus PLT-I methods. We determined Pearson correlation coefficients (r) to assess the degree of correlation, with the following interpretations: ≤0.30, negligible correlation; 0.30–0.50, low positive correlation; 0.50–0.70, moderate positive correlation; 0.70–0.90, high positive correlation; and ≥0.90, very high positive correlation [9]. The actual platelet counts were derived from the samples tested using sodium citrate tubes. When multiple samples were collected from the same patient, only the first sample was used to evaluate the platelet-clump flagging performance. Agreement was assessed using weighted kappa values, according to the criteria established by Landis and Koch: ≤0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and ≥0.81, almost perfect agreement [10]. We analyzed the sensitivity, specificity, likelihood ratio (LR), and odds ratio of the platelet-clump flagging feature of both devices and of the integrated MC-80 and BC-6800Plus results. As a practical guideline for interpretation, positive likelihood ratios (+LRs) >10 were considered as strong evidence supporting a diagnosis, ratios of 5–10 as moderate evidence, and those of 2–5 as weak evidence [11]. To diagnose platelet clumps using the MC-80 analyzer, we used cutoffs of ≥5 platelet clumps in standard mode and ≥15 in PLT-Pro mode, using Analyse-it version 5.90 (Analyse-it Software, Leeds, UK).
Manual microscopic examination confirmed the presence of true platelet clumping in 43 EDTA samples. With the DxH 900 system, platelet clumps manifested as a distinct light-green mass located in the upper-left portion of the NRBC channel (Fig. 1A). In contrast, with the BC-6800Plus, platelet clumps exhibited a distinctive sawtooth appearance and appeared larger than typical platelets in the platelet-distribution curve (Fig. 1B). The platelet clumps were verified from a three-dimensional perspective across the WNB, DIFF, and RET channels (Fig. 1C–1E).
MC-80 digital image analysis in standard mode helped capture both the shape and quantity of platelet clumps. Using the MC-80 PLT-Pro function, additional confirmation of platelet clumps located in the edge and tail zones was obtained.
For the 43 EDTA samples with true platelet clumping, the BC-6800Plus PLT-O results exhibited higher values than the DxH 900 PLT-I and BC-6800Plus PLT-I results (Fig. 2). The DxH 900 PLT-I and BC-6800Plus PLT-I results exhibited a strong correlation (r=0.711). In contrast, the DxH 900 PLT-I and BC-6800Plus PLT-I results demonstrated moderate positive correlations with the BC-6800Plus PLT-O results (r=0.506 and 0.545, respectively).
Of the 14 samples showing platelet counts of <50×109/L during DxH 900 PLT-I or BC-6800Plus PLT-I analysis, 11 exhibited platelet counts of >50×109/L during BC-6800Plus PLT-O analysis (Supplemental Data Table S1).
Of the 30 samples collected concurrently in EDTA and sodium citrate tubes, the first 22 samples shown in Fig. 3 (going from left to right) exhibited comparable results between the BC-6800Plus PLT-O and DxH 900 PLT-I methods (Fig. 3). The dissociation rate, calculated as BC-6800Plus PLT-O (EDTA) count/DxH 900 PLT-I (sodium citrate) count ×100, was calculated for these samples [12]. The median dissociation rate of these 22 samples with platelet clumping was 102.5% (range, 74.9%–123.1%). For the remaining eight samples represented in Fig. 3 (samples 93, 87, 71, 97, 90, 80, 31, and 40), where platelet aggregation was not adequately resolved using the BC-6800Plus PLT-O mode, the median dissociation rate was 52.2% (range, 19.8%–70.4%).
Notably, with samples 61 and 87 (Supplemental Data Table S1), platelet clumping was observed in the sodium citrate tubes; thus, the BC-6800Plus PLT-O (sodium citrate) results were 23% and 87% higher than the DxH 900 PLT-I (citrate) results, respectively, causing an increase in the platelet counts (from 310×109/L to 390×109/L for sample 61 and from 151×109/L to 283×109/L for sample 87), as confirmed by microscopic examination.
We comparatively analyzed the accuracy of both instruments in platelet-clump flagging (Table 1). We analyzed 188 samples, including 100 negative controls, among which 29 were identified as true positives. DxH 900 and BC-6800Plus demonstrated similar sensitivity in detecting platelet clumps, with values of 0.79 and 0.72, respectively. However, their specificity values were comparatively low, at 0.69 and 0.77, respectively. Platelet clump flagging by DxH 900 and BC-6800Plus showed substantial agreement, with a kappa value of 0.73 (95% confidence interval, 0.63–0.83).
Next, we analyzed the platelet-clumping results of 178 samples (including 100 negative controls) obtained using the BC-6800Plus instrument with the MC-80 analyzer, revealing 26 true positives (Table 1). The sensitivity (0.89 and 1.00, respectively) and specificity (0.99 and 0.99, respectively) significantly improved when we used thresholds of ≥5 and ≥15 platelet clumps in the standard and PLT-Pro modes, respectively.
Evaluation of the LR revealed that all four methods had odds ratio values >1.0 (Table 1), providing initial positive evidence of platelet clumping. When the PLT-clumps flag was triggered, both DxH 900 and BC-6800Plus generated relatively weak indications (+LRs of 2.57 and 3.20, respectively), whereas the threshold-based MC-80 digital image results offered strong evidence of platelet clumping (+LRs of 67.23 and 76.00, respectively). Consequently, by integrating digital image analysis, the diagnostic odds ratio significantly increased from 8.61–8.97 to 575.00–∞ (Table 1).
We evaluated the digital image analysis results of 44 samples that were flagged erroneously by the DxH 900 or BC-6800Plus system. The distinct causative factors in these cases were as follows: fibrin interference was identified in eight samples, large or giant platelets were detected in 24 samples, RBC microcytosis was observed in five samples, and the precise etiology remained indeterminate in seven samples.
MC-80 digital analysis confirmed the presence of large and giant platelets (Fig. 4). The characteristics of platelet clumps could be confirmed more accurately in PLT-pro mode than in standard mode. Pronounced platelet clumps were observed in a few samples for which the BC-6800Plus PLT-O method did not achieve adequate platelet clump deaggregation and were classified as artifacts upon digital image analysis (Fig. 5). A false-positive result was obtained in standard mode with the MC-80 analyzer but tested negative in PLT-Pro mode. This discrepancy was attributed to platelet satellitism.
This was the first study to identify and resolve PTCP samples by integrating an automated hematology analyzer with a digital image analyzer. The results confirmed that the BC-6800Plus PLT-O system could yield accurate platelet-clumping results in EDTA-induced PTCP without additional sodium citrate-anticoagulated CBC testing, although inaccurate results were obtained for some samples. While the sensitivity and specificity of platelet-clump flagging by the CBC analyzers were only 70%–80% because of false-negative and false-positive findings, they significantly increased to 100% and 99%, respectively, by incorporating imaging information obtained using the MC-80 image analyzer. Notably, the BC-6800Plus PLT-O system, in conjunction with the MC-80 analyzer, effectively detected actual platelet clumps even in CBC tests with blood anticoagulated with sodium citrate.
Lardinois et al. [13] reported that the incidence of EDTA-PTCP was increased by hospitalization, infection, male sex with an age above 50 yrs, pregnancy, medications, and underlying diseases. However, the association between EDTA-PTCP and particular diseases remains unclear. In addition to EDTA, common causes of PTCP include drug therapies, EDTA-independent cold agglutinins, platelet activation during blood collection, and undercounting of megathrombocytes [14, 15]. In a laboratory setting, identifying the cause of PTCP in samples can be challenging. The primary objective of a diagnostic hematology laboratory is to provide accurate platelet results, leveraging established knowledge to identify and validate PTCP samples while suggesting preventive measures [6, 13].
Given that PTCP is inherently associated with the use of EDTA as an anticoagulant in CBC testing, accurately detecting PTCP is imperative. Detection partially relies on flags generated by the CBC instrument, particularly those based on platelet-distribution curves and cellular scattergrams [3, 6, 13, 15, 16]. Deviations in platelet-count results compared with previous data or between EDTA and citrate tubes can indicate PTCP. The Mindray BC-6800Plus features two platelet-measurement methods: PLT-I and PLT-O. To increase the possibility of identifying PTCP, results obtained in both modes have to be compared, particularly for patients with low platelets.
Various studies have been conducted to explore PTCP detection, with varying sensitivity and specificity found with different devices. The sensitivity and specificity of the Sysmex instrument (Sysmex, Kobe, Japan) were 0.626 and 0.947, respectively, when platelet aggregation was induced using adenosine diphosphate in citrate-anticoagulated whole blood samples [17]. In a comparative analysis of the usefulness of platelet-clump flagging among the Sysmex, Beckman Coulter, and ADVIA (Siemens Healthcare Diagnostics, Eschborn, Germany) CBC analyzers, the Sysmex analyzer demonstrated the highest sensitivity of 67% [18]. BC-6800, the predecessor of the BC-6800Plus, exhibited a sensitivity of 94% and specificity of 87% for platelet-clump flagging [19]. Platelet clumping occurs very rarely, and its incidence rate varies with population characteristics, resulting in diverse results on flagging efficiency. In this study, the DxH 900 and BC-6800Plus demonstrated platelet-clump flagging sensitivity and specificity values of approximately 70%, likely because of variations in the analyzed population.
We observed increased false-positive and false-negative rates in samples suspected of platelet clumping. In clinical-laboratory settings, a suspicion of platelet clumping can be the sole means of their detection. A substantial portion of false positives (32/44) resulted from fibrin formation or the presence of large or giant platelets. These phenomena are primarily attributed to issues during sample collection or inherent sample characteristics rather than EDTA-induced PTCP. Notably, visual identification is challenging when microagglutination occurs, requiring microscopic observations for accurate assessment. In our laboratory, when dealing with low platelet counts, we evaluated platelet-distribution curves and peripheral blood smears. Moreover, we document patients with previously confirmed PTCP in the laboratory information system for communication among technicians. This proactive approach enabled us to identify more PTCP cases based on our laboratory criteria, complementing our use of CBC equipment flags.
Numerous approaches, including aminoglycoside supplementation, have been proposed for accurate reporting of results from PTCP samples [20]. Although the optimal approach is immediate measurement upon collection, practical constraints often limit the implementation of this ideal method. Alternatively, a proposed method involves testing samples while maintaining them at 37°C [21, 22]. For precise platelet measurements, optical measurements using a fluorescent dye are recommended over impedance measurements [23, 24]. To improve accuracy, the use of anticoagulants other than EDTA is advised [3, 25]. However, platelet clumping is reported in 15%–20% of PTCP cases, even in citrate-treated whole blood samples. Employing alternative anticoagulants may pose challenges in outpatient settings owing to the requirement for additional blood collection. The BC-6800Plus instrument demonstrated efficacy and usefulness in reducing the need for blood re-collection by dissociating pre-existing platelet clumps through combined chemical and physical interventions.
The prominent study findings are owing to the improved accuracy of PTCP diagnosis in patients under suspicion facilitated by the CAL 8000 hematology workstation. Implementing a systematic workflow tailored to samples with potential PTCP, as used in our laboratory, minimizes the likelihood of missing cases. Our approach integrates PLT-O into PLT-I with reflexive testing based on predefined criteria, eliminating the need for numerous stages as depicted in flowcharts from other research groups [6, 13]. Our workflow involves a slide maker and the MC-80 PLT-Pro function. Automated slide readings and comprehensive data analyses expedite image analysis, thus eliminating the need for examiner intervention during testing. Consequently, this approach reduces the need for blood re-collection and significantly diminishes both time and manual labor, enabling prompt and precise result reporting.
The main limitation of our study is the temporal discrepancy between the DxH 900 and BC-6800Plus tests. Platelet clumping can manifest after 2 hrs in whole blood samples collected in EDTA [26, 27]. However, in our study, samples tested with the BC-6800Plus analyzer had a wait time of up to 4 hrs until processing after the DxH 900 analyzer, resulting in a tendency for lower platelet counts in the BC-6800Plus PLT-I mode than in the DxH 900 PLT-I mode (Fig. 2). Furthermore, the bonds in the platelet clumps may have strengthened over the wait time and therefore may have been inadequately disrupted in the BC-6800Plus PLT-O mode.
In conclusion, the Mindray BC-6800Plus in PLT-O mode can report results close to the actual values obtained by platelet deaggregation, even in cases of pre-existing EDTA-induced platelet aggregation, avoiding the need for repeated blood sampling. As CBC analyzers can yield false-positive and false-negative results, the diagnosis of PTCP can be effectively improved by combining the results with image-analysis results. The findings of this study provide valuable insights into the performance and capabilities of the BC-6800Plus analyzer integrated with the MC-80 digital image analyzer in handling PTCP samples, improving the accuracy and reliability of CBC testing.
Notes
AUTHOR CONTRIBUTIONS
So MK contributed to study conceptualization, investigation, formal analysis, and writing – original draft. Huh J contributed to study supervision and writing – review & editing. Kim S contributed to study supervision. Park S contributed to project administration, supervision, funding acquisition, conceptualization, methodology, and writing – review & editing.
Appendix
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.3343/alm.2023.0460
References
1. Banfi G, Salvagno GL, Lippi G. 2007; The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med. 45:565–76. DOI: 10.1515/CCLM.2007.110. PMID: 17484616.
2. Gowland E, Kay HE, Spillman JC, Williamson JR. 1969; Agglutination of platelets by a serum factor in the presence of EDTA. J Clin Pathol. 22:460–4. DOI: 10.1136/jcp.22.4.460. PMID: 4978997. PMCID: PMC474212.
3. Bartels PC, Schoorl M, Lombarts AJ. 1997; Screening for EDTA-dependent deviations in platelet counts and abnormalities in platelet distribution histograms in pseudothrombocytopenia. Scand J Clin Lab Invest. 57:629–36. DOI: 10.3109/00365519709055287. PMID: 9397495.
4. Vicari A, Banfi G, Bonini PA. 1988; EDTA-dependent pseudothrombocytopaenia: a 12-month epidemiological study. Scand J Clin Lab Invest. 48:537–42. DOI: 10.3109/00365518809085770. PMID: 3146133.
5. Silvestri F, Virgolini L, Savignano C, Zaja F, Velisig M, Baccarani M. 1995; Incidence and diagnosis of EDTA-dependent pseudothrombocytopenia in a consecutive outpatient population referred for isolated thrombocytopenia. Vox Sang. 68:35–9. DOI: 10.1159/000462884. PMID: 7725669.
6. Baccini V, Geneviève F, Jacqmin H, Chatelain B, Girard S, Wuilleme S, et al. 2020; Platelet counting: ugly traps and good advice. Proposals from the French-speaking Cellular Hematology Group (GFHC). J Clin Med. 9:808. DOI: 10.3390/jcm9030808. PMID: 32188124. PMCID: PMC7141345. PMID: 704f5f47e8944dcf89f9bf3558e2f964.
7. Deng J, Chen Y, Zhang S, Li L, Shi Q, Liu M, et al. 2020; Mindray SF-Cube technology: an effective way for correcting platelet count in individuals with EDTA dependent pseudo thrombocytopenia. Clin Chim Acta. 502:99–101. DOI: 10.1016/j.cca.2019.12.012. PMID: 31863740.
8. Guo P, Cai Q, Mao M, Lin H, Chen L, Wu F, et al. 2022; Performance evaluation of the new platelet measurement channel on the BC-6800 Plus automated hematology analyzer. Int J Lab Hematol. 44:281–7. DOI: 10.1111/ijlh.13753. PMID: 34873856.
9. Mukaka MM. 2012; Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 24:69–71.
10. Landis JR, Koch GG. 1977; The measurement of observer agreement for categorical data. Biometrics. 33:159–74. DOI: 10.2307/2529310. PMID: 843571.
11. Jaeschke R, Guyatt GH, Sackett DL. 1994; Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 271:703–7. DOI: 10.1001/jama.1994.03510330081039. PMID: 8309035.
12. Bao Y, Wang J, Wang A, Bian J, Jin Y. 2020; Correction of spurious low platelet counts by optical fluorescence platelet counting of BC-6800 hematology analyzer in EDTA-dependent pseudo thrombocytopenia patients. Transl Cancer Res. 9:166–72. DOI: 10.21037/tcr.2019.12.58. PMID: 35117170. PMCID: PMC8798320.
13. Lardinois B, Favresse J, Chatelain B, Lippi G, Mullier F. 2021; Pseudothrombocytopenia-a review on causes, occurrence and clinical implications. J Clin Med. 10:594. DOI: 10.3390/jcm10040594. PMID: 33557431. PMCID: PMC7915523. PMID: d53aef395a6d4be6951f486001d28eb1.
14. Wahed A, Quesada A, Dasgupta A. 2020. Sources of errors in hematology and coagulation. Hematology and Coagulation. 2nd ed. Academic Press;Cambridge: p. 277–91. DOI: 10.1016/B978-0-12-814964-5.00018-8.
15. Zandecki M, Genevieve F, Gerard J, Godon A. 2007; Spurious counts and spurious results on haematology analysers: a review. Part I: platelets. Int J Lab Hematol. 29:4–20. DOI: 10.1111/j.1365-2257.2006.00870.x. PMID: 17224004.
16. Gulati G, Uppal G, Gong J. 2022; Unreliable automated complete blood count results: causes, recognition, and resolution. Ann Lab Med. 42:515–30. DOI: 10.3343/alm.2022.42.5.515. PMID: 35470271. PMCID: PMC9057813.
17. Xu P, Fang K, Chen X, Liu Y, Dong Z, Zhu J, et al. 2022; The flagging features of the Sysmex XN-10 analyser for detecting platelet clumps and the impacts of platelet clumps on complete blood count parameters. Clin Chem Lab Med. 60:748–55. DOI: 10.1515/cclm-2021-1226. PMID: 35212492.
18. Sandhaus LM, Osei ES, Agrawal NN, Dillman CA, Meyerson HJ. 2002; Platelet counting by the Coulter LH 750, Sysmex XE 2100, and Advia 120: a comparative analysis using the RBC/platelet ratio reference method. Am J Clin Pathol. 118:235–41. DOI: 10.1309/MK3G-MC3V-P06R-PNV2. PMID: 12162684.
19. Fuster Ó, Andino B, Laiz B. 2016; Performance evaluation of low platelet count and platelet clumps detection on Mindray BC-6800 hematology analyzer. Clin Chem Lab Med. 54:e49–51. DOI: 10.1515/cclm-2015-0409. PMID: 26154195.
20. Sakurai S, Shiojima I, Tanigawa T, Nakahara K. 1997; Aminoglycosides prevent and dissociate the aggregation of platelets in patients with EDTA-dependent pseudothrombocytopenia. Br J Haematol. 99:817–23. DOI: 10.1046/j.1365-2141.1997.4773280.x. PMID: 9432027.
21. Bizzaro N. 1995; EDTA-dependent pseudothrombocytopenia: a clinical and epidemiological study of 112 cases, with 10-year follow-up. Am J Hematol. 50:103–9. DOI: 10.1002/ajh.2830500206. PMID: 7572988.
22. Casonato A, Bertomoro A, Pontara E, Dannhauser D, Lazzaro AR, Girolami A. 1994; EDTA dependent pseudothrombocytopenia caused by antibodies against the cytoadhesive receptor of platelet gpIIB-IIIA. J Clin Pathol. 47:625–30. DOI: 10.1136/jcp.47.7.625. PMID: 8089218. PMCID: PMC502090.
23. Pan LL, Chen CM, Huang WT, Sun CK. 2014; Enhanced accuracy of optical platelet counts in microcytic anemia. Lab Med. 45:32–6. DOI: 10.1309/LM7QPULDM5IHBO3L. PMID: 24719982.
24. Schoorl M, Schoorl M, Oomes J, van Pelt J. 2013; New fluorescent method (PLT-F) on Sysmex XN2000 hematology analyzer achieved higher accuracy in low platelet counting. Am J Clin Pathol. 140:495–9. DOI: 10.1309/AJCPUAGGB4URL5XO. PMID: 24045545.
25. Schuff-Werner P, Steiner M, Fenger S, Gross HJ, Bierlich A, Dreissiger K, et al. 2013; Effective estimation of correct platelet counts in pseudothrombocytopenia using an alternative anticoagulant based on magnesium salt. Br J Haematol. 162:684–92. DOI: 10.1111/bjh.12443. PMID: 23808903. PMCID: PMC3796857.
26. Schuff-Werner P, Mansour J, Gropp A. 2020; Pseudo-thrombocytopenia (PTCP). A challenge in the daily laboratory routine? J Lab Med. 44:295–304. DOI: 10.1515/labmed-2020-0099. PMID: a1c836d5515d4a1fbca5625079d29397.
27. Shreiner DP, Bell WR. 1973; Pseudothrombocytopenia: manifestation of a new type of platelet agglutinin. Blood. 42:541–9. DOI: 10.1182/blood.V42.4.541.541. PMID: 4205032.
Fig. 1
True platelet clumps detected using different automated hematology analyzers. (A) DxH 900 NRBC channel. (B–E) BC-6800Plus. (B) Platelet-distribution histogram. (C) WNB channel. (D) DIFF channel. (E) RET channel. Panel (E) confirms the lack of RBC interference. Arrows indicate platelet clumps.
Abbreviations: NRBC, nucleated red blood cell; DIFF, differential; RET, reticulocyte.
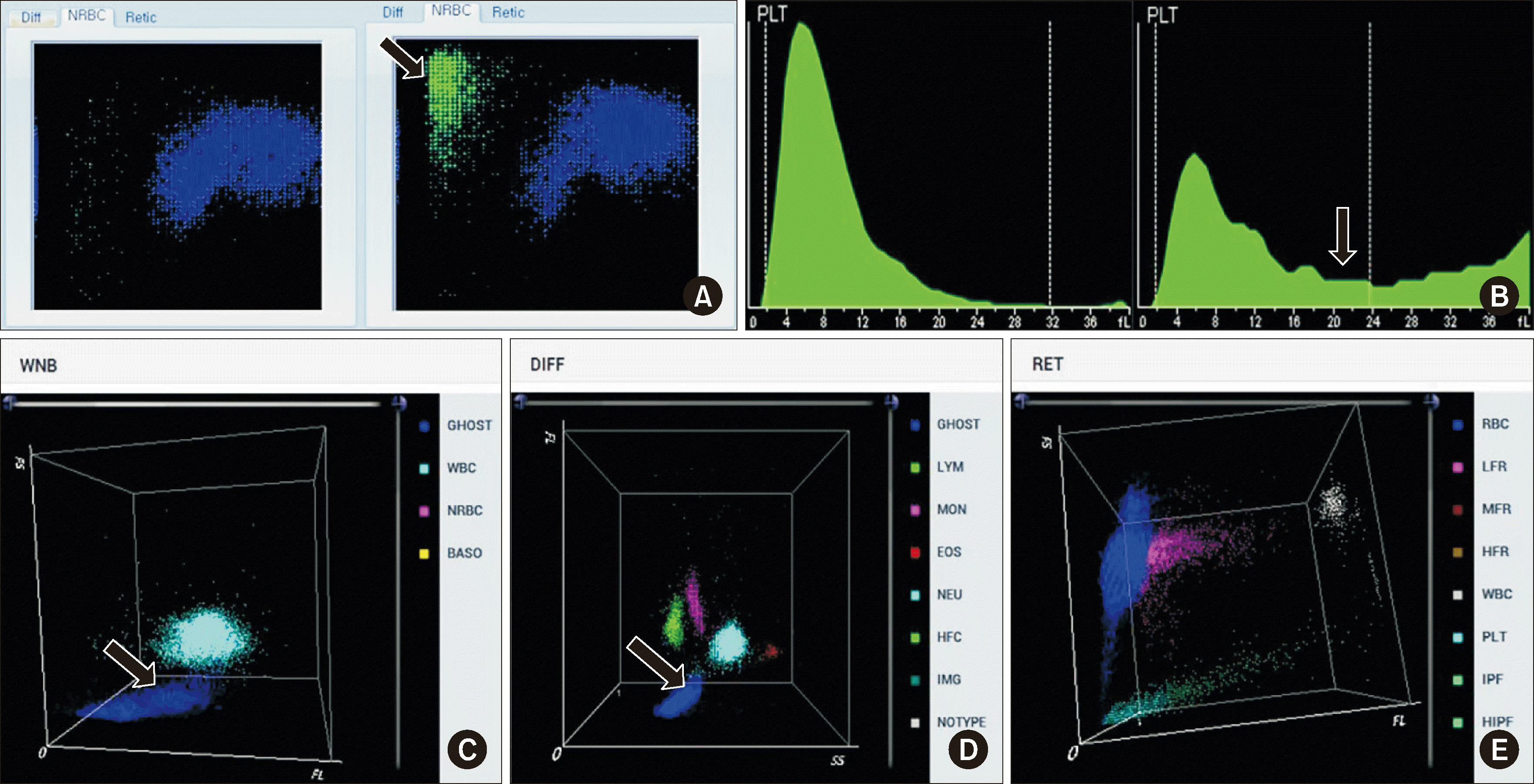
Fig. 2
Comparison of platelet counts measured using the DxH 900 PLT-I, BC-6800Plus PLT-I, and BC-6800Plus PLT-O method with EDTA tubes from patients with pseudothrombocytopenia (N=43).
Abbreviations: PLT-I, platelet count by impedance; PLT-O, optical platelet count.
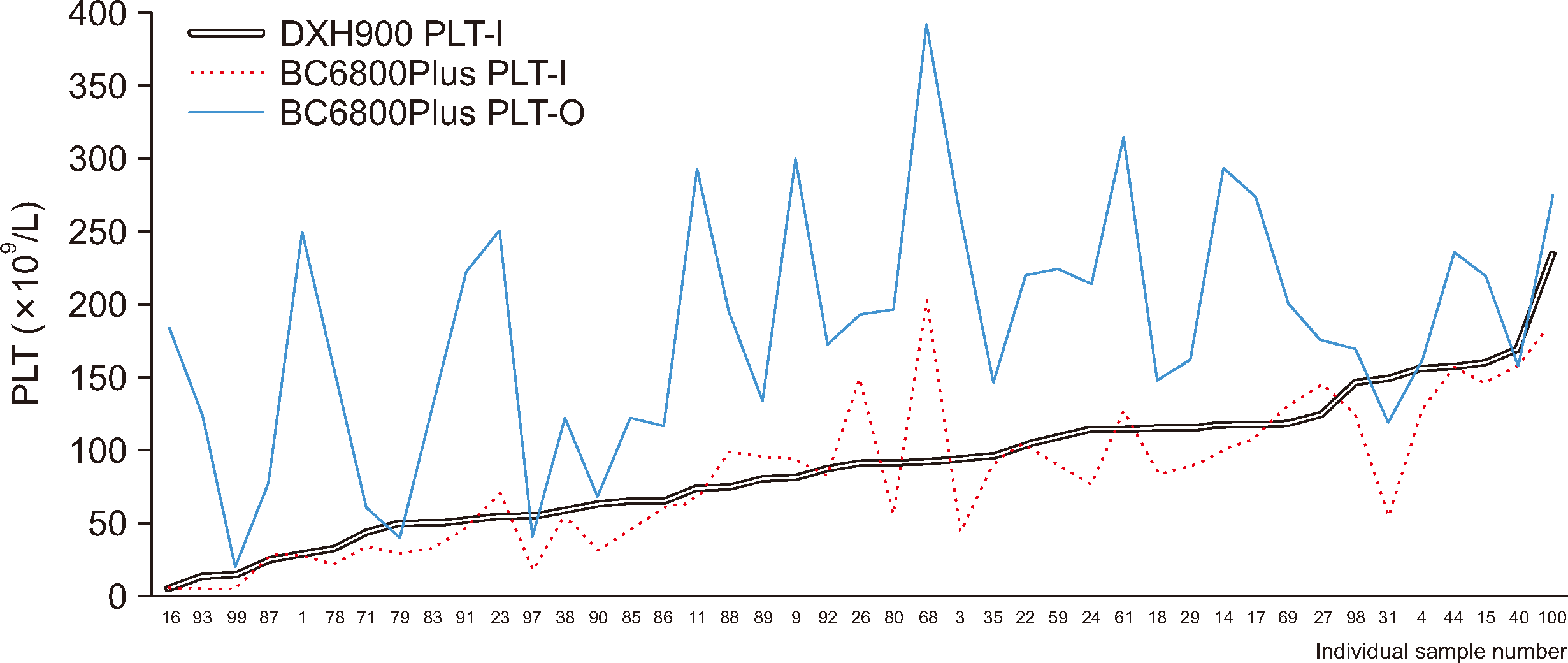
Fig. 3
Comparison of platelet counts measured using the BC-6800Plus PLT-O method with EDTA tubes and the DxH 900 PLT-I method with sodium citrate tubes from patients with pseudothrombocytopenia (N=30).
Abbreviations: PLT-O, optical platelet count; PLT-I, platelet count by impedance.
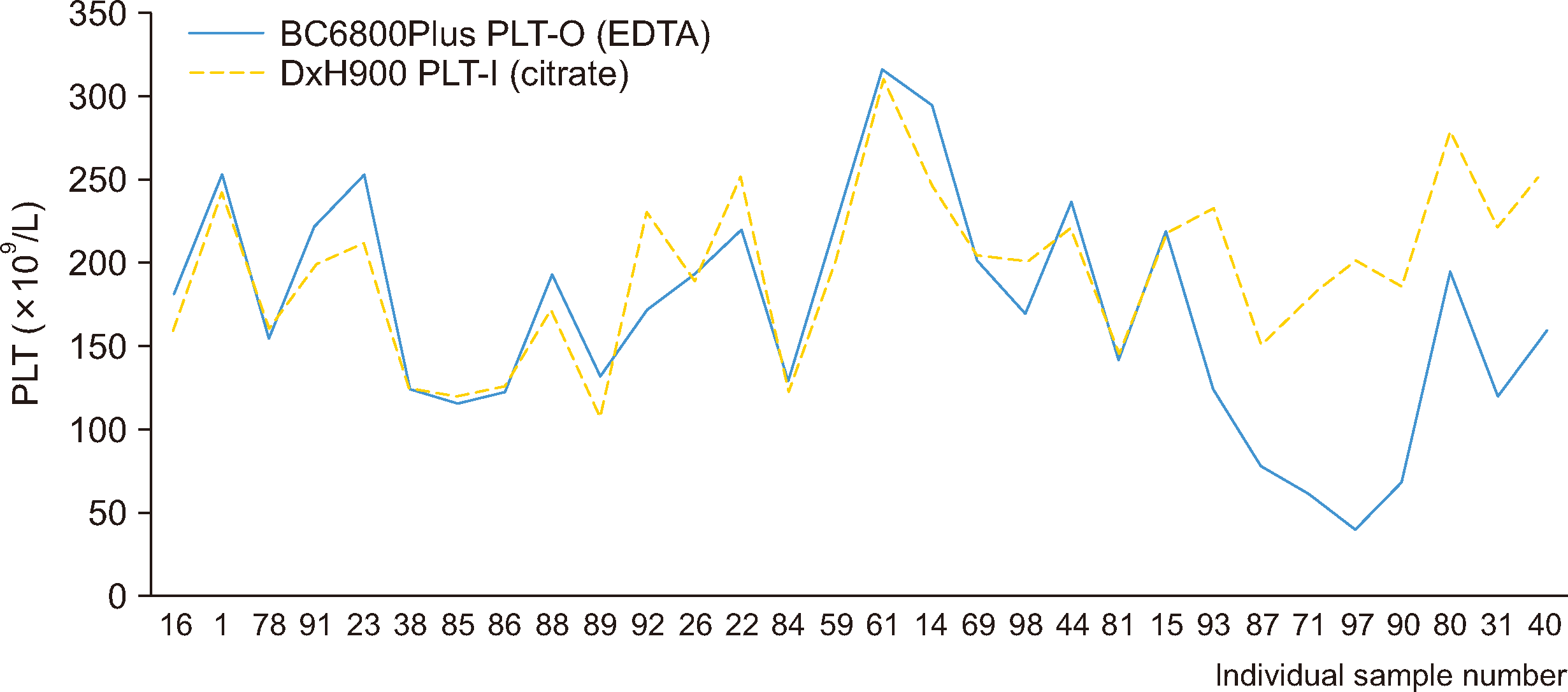
Fig. 4
Presence of giant and large platelets confirmed by MC-80 image analysis of PLT clumps flagged by the DxH 900 analyzer. (A) BC-6800Plus platelet-distribution histogram. (B and C) MC-80 standard mode. (D) MC-80 PLT-Pro mode.
Abbreviation: PLT, platelet.
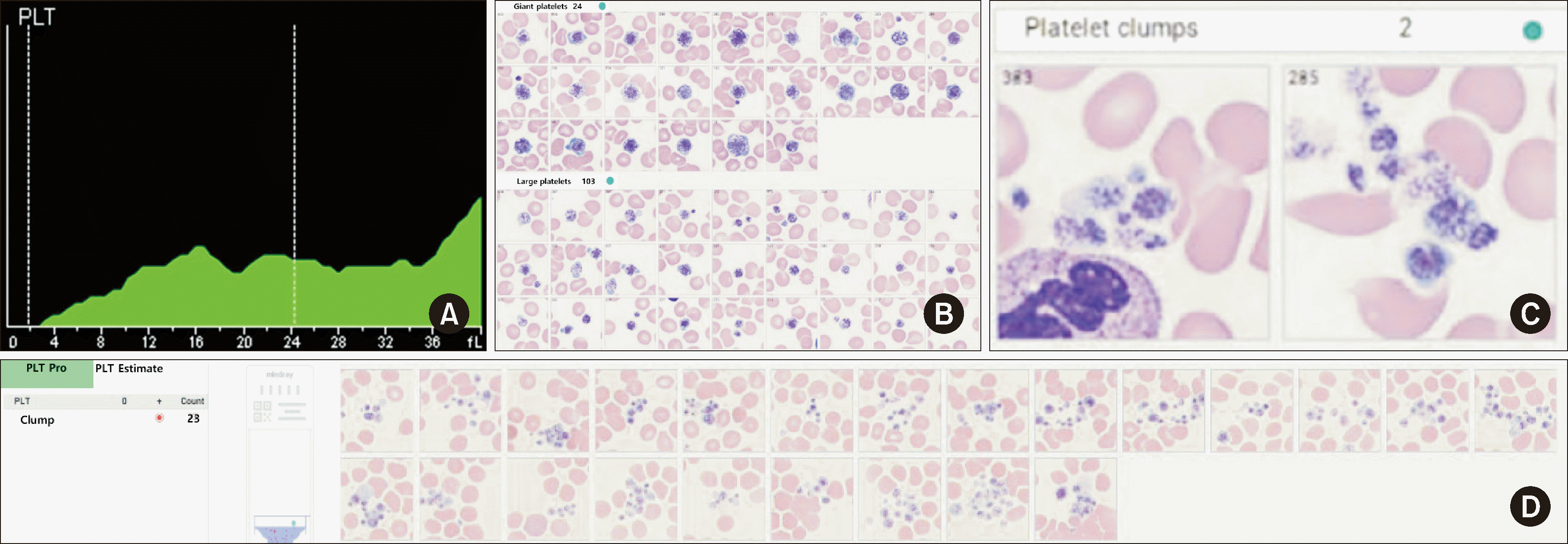
Fig. 5
Presence of heavy platelets confirmed by MC-80 image analysis of PLT clumps flagged by the D×H 900 analyzer. (A) BC-6800Plus platelet-distribution histogram. (B and C) MC-80 standard mode. (D) MC-80 PLT-Pro mode. In panel (B), the platelet clumps are classified as artifacts.
Abbreviation: PLT, platelet.
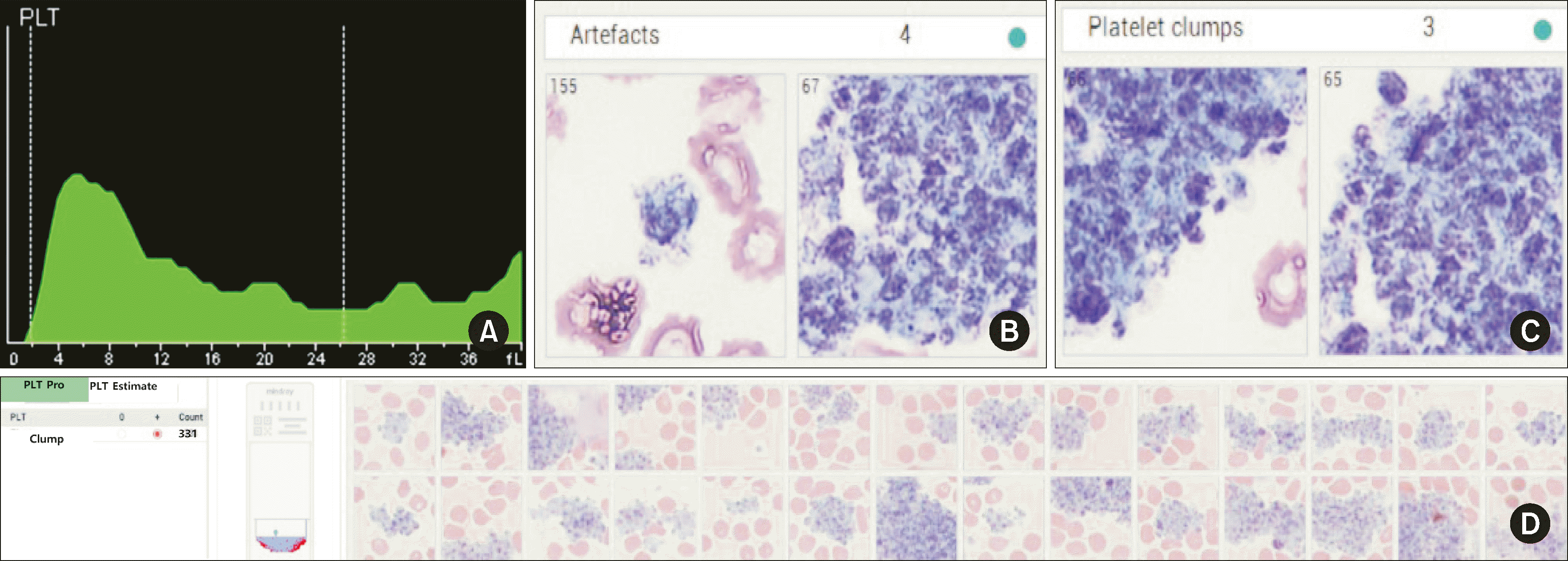
Table 1
Diagnostic performance of platelet clump flagging by DxH 900, BC-6800Plus, and MC-80 integrated with BC-6800Plus
| Diagnostic performance | DxH 900 (N=188) | BC-6800Plus (N=188) | BC-6800Plus + MC-80 in standard mode* (N=178) | BC-6800Plus + MC-80 in PLT-Pro mode† (N=178) |
|---|---|---|---|---|
| True positive | 23 | 21 | 23 | 26 |
| True negative | 110 | 123 | 150 | 150 |
| False positive | 49 | 36 | 2 | 2 |
| False negative | 6 | 8 | 3 | 0 |
| Sensitivity (95% CI) | 0.79 (0.62–0.90) | 0.72 (0.54–0.85) | 0.89 (0.71–0.96) | 1.00 (0.87–1.00) |
| Specificity (95% CI) | 0.69 (0.62–0.76) | 0.77 (0.70–0.83) | 0.99 (0.95–1.00) | 0.99 (0.95–1.00) |
| Positive likelihood ratio (95% CI) | 2.57 (1.86–3.43) | 3.20 (2.17–4.55) | 67.23 (18.79–245.98) | 76.00 (21.41–276.56) |
| Negative likelihood ratio (95% CI) | 0.30 (0.14–0.56) | 0.36 (0.19–0.60) | 0.12 (0.04–0.29) | 0.00 (0.00–0.13) |
| Diagnostic odds ratio (95% CI) | 8.61 (3.37–21.90) | 8.97 (3.71–21.60) | 575.00 (96.83–3,347.09) | + ∞ (344.56 to −/+ ∞) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download