INTRODUCTION
Artificial intelligence (AI) has become invaluable in healthcare for disease diagnosis, treatment planning, and clinical decision-making. AI uses algorithms that emulate the human brain to learn, synthesize, analyze, generalize, and solve problems using natural language processing (NLP), machine learning, deep learning, and large language models [
1]. The accuracy and performance of AI algorithms depend on the quality and quantity of data they process. Maximizing the potential of AI algorithms requires feeding them substantial data from various healthcare systems. Unfortunately, most healthcare systems operate with different data formats and document architectures, compounding issues with unstructured, non-standardized data, which require increased storage space and processing time. Moreover, processing such data can introduce errors that may skew analysis results, highlighting the need for data interoperability in AI algorithms.
Herein, we examined the importance of interoperability, particularly semantic interoperability based on terminology standards, the global adoption of terminology standards, collaborative activities in terminology standard development, and proposed strategies to improve their usability in laboratory medicine.
INTEROPERABILITY AND STANDARDS
Interoperability refers to the ability of two or more systems to exchange and utilize information, including foundational, structural, semantic, and organizational levels [
2]. Foundational interoperability involves the most basic level of data receipt without requiring interpretation by the receiving system. Structural interoperability specifies the format and structure of the data. Fast Healthcare Interoperability Resources (FHIR) is an emerging standard for structural interoperability. Semantic interoperability ensures unambiguous data exchange, with key standards including the International Statistical Classification of Diseases (ICD), Systematized Nomenclature of Medicine - Clinical Terms (SNOMED CT), and Logical Observation Identifiers Names and Codes (LOINC). Organizational interoperability secures data exchange among organizations beyond the technical context. Standards include governance, policy, social, and legal rules and regulations to facilitate the secure exchange of data within and among organizations.
TERMINOLOGY STANDARDS
Terminology standards are crucial for ensuring structured and meaningful data exchange. ICD, SNOMED CT, and LOINC are the most widely used terminology standards.
The ICD is a global statistical classification for coding the causes and consequences of diseases and deaths. WHO designed the ICD to enhance the comparability of morbidity and mortality data internationally [
3]. ICD-10 codes vary from three to seven characters. The first three characters designate the disease category. The next three characters indicate the related etiology, anatomic site, severity, or other clinical details. The seventh character is used to document care episodes for injuries and other conditions with external causes. ICD-10 has a mono-hierarchical structure with residual categories for diseases not classified elsewhere.
SNOMED CT is the most comprehensive multilingual clinical terminology globally, including over 366,000 concepts with multiple descriptions and logical definitions organized hierarchically [
4]. The core components of SNOMED CT are concepts, descriptions, and relationships. Each concept represents a unique clinical meaning and is organized hierarchically to reflect different levels of granularity. Descriptions, which are human-readable terms associated with concepts, are categorized as fully specified names (FSNs) that fully capture the meaning of a concept and synonyms that offer alternative expressions of the concept. A relationship represents an association between concepts and is classified as either a subtype (IS-A) or an attribute. Relationships facilitate the logical definition of concepts for computational processing. SNOMED CT encompasses a broad spectrum of clinical concepts such as diseases, procedures, medications, and outcomes for documentation in electronic health records (EHRs).
LOINC is a standardized terminology for describing laboratory and clinical observations [
5]. Laboratory LOINC includes tests, measurements, or observations about a specimen, covering domains such as chemistry, hematology, cell counts, and antibiotic susceptibilities. Clinical LOINC includes tests, measurements, and observations about patients, including vital signs, hemodynamics, and radiological findings. Currently, there are over 100,000 LOINC codes, each comprising six parts that represent the component, property measured, timing, type of sample, type of scale, and method used. LOINC assigns three names to each concept: the six-part fully specified name, the clinician-friendly long common name, and a short name suitable for column headers in reports.
ADOPTION OF TERMINOLOGY STANDARDS
According to a survey of 27 member countries by the Organisation for Economic Co-operation and Development (OECD) in 2021, the adoption of EHRs has increased since 2016 [
6]. In EHRs, patient data are coded in a structured manner using terminology standards. The survey revealed that multiple terminology standards are used for the same data category in many countries. For example, ICD is used for capturing diagnoses in 21 countries. Conversely, Australia and Switzerland use SNOMED CT and Greece, Hungary, and the United States (US) use both terminologies to record diagnoses. For medication, the Anatomical Therapeutic Chemical (ATC) classification is used in 16 of the 27 countries. Australia and Switzerland use SNOMED CT, and other countries use locally developed terminology standards. For surgical procedures, eight of the 27 countries use ICD codes, such as ICD-9-CM and ICD-10-PCS; five use SNOMED CT; and the remainder use national codes. Laboratory tests are captured using LOINC in 16 countries, whereas SNOMED CT is used in Iceland, Slovenia, and Sweden.
Table 1 presents the adoption of health terminology by domain in six OECD member countries.
COLLABORATIVE ACTIVITIES IN TERMINOLOGY STANDARD DEVELOPMENT
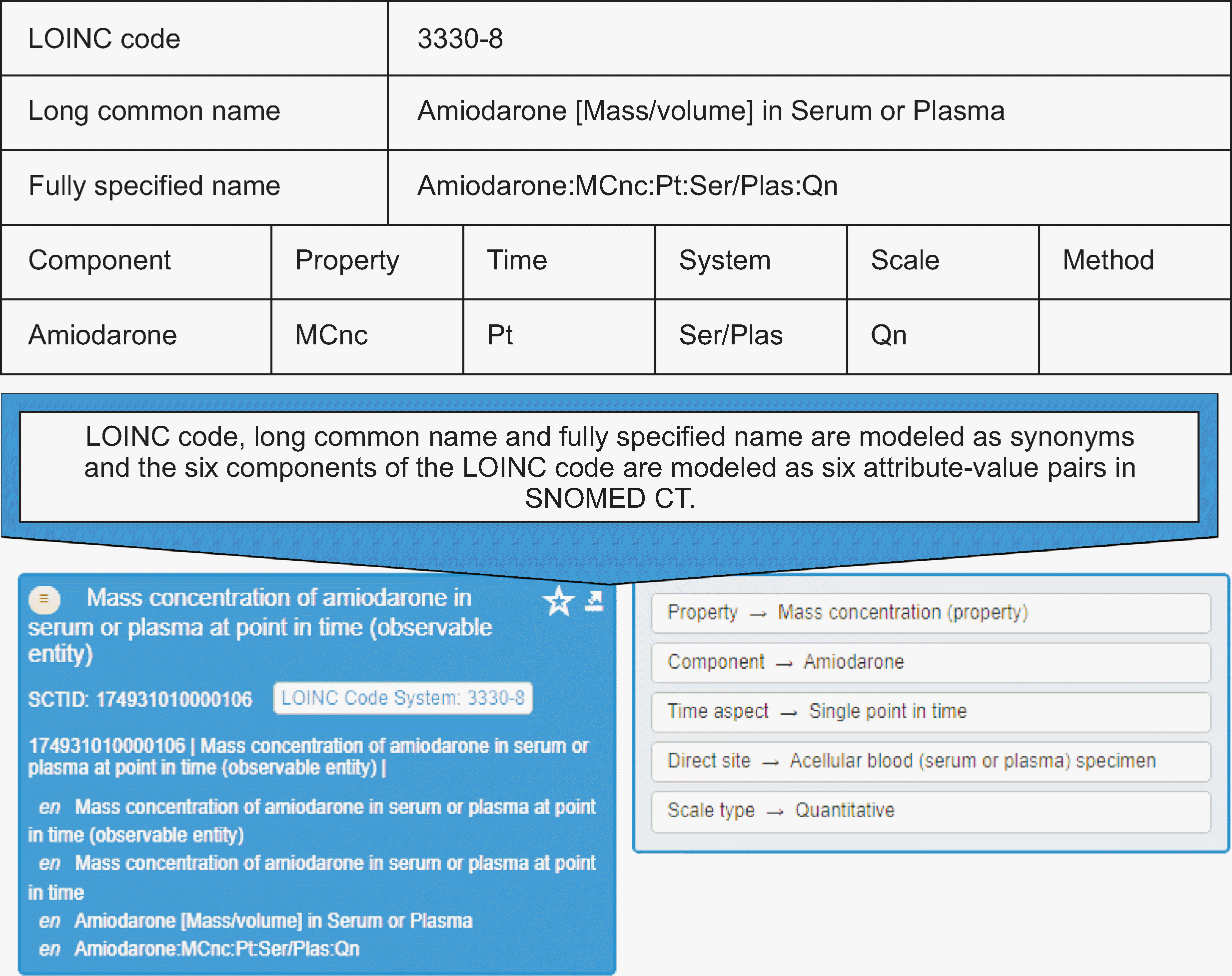
Collaboration among developers of terminology standards has increased over the past few years. In 2022, the Regenstrief Institute and SNOMED International (SI) agreed to develop a LOINC extension in which each LOINC concept has an equivalent SNOMED CT concept [
7]. The SNOMED CT concept equivalent of a LOINC is fully defined, implying that each LOINC part has an equivalent SNOMED CT concept (
Fig. 1). LOINC extensions in the SNOMED CT format are available at
https://loincsnomed.org/browser. A LOINC concept can be searched using a text string, LOINC name, or SNOMED CT concept ID. The LOINC extension links LOINC, which provides laboratory content in an understandable format, and SNOMED CT, which provides the computable framework together in a complementary way. The LOINC extension aims to reduce duplication of efforts by providers who use different combinations of LOINC and SNOMED CT and leverages the rich semantic/ontological representation of SNOMED CT for the analysis of laboratory reports, irrespective of whether they use LOINC or SNOMED CT. Another collaborative effort is to map SNOMED CT to classifications such as ICD [
8]. The WHO and SI mapped SNOMED CT concepts to ICD-10 and published a reference set via the SNOMED CT browser. Other efforts include mapping SNOMED CT to Current Procedural Terminology (CPT) in the US and mapping SNOMED CT to Office of Population Censuses and Surveys Classification of Interventions and Procedures-4 (OPCS-4) and ICD-10 in the United Kingdom (UK).
BiNDing TerMINOLOGY STANDARDS TO STRUCTURAL STANDARDS
A global initiative was undetaken to combine structural and semantic standards, thereby enhancing the interoperability of data from diverse sources; for instanceHL7 FHIR was integrated with SNOMED CT and LOINC. For example, terminology binding of US Core Data for Interoperability (USCDI) using US Core Profiles in the US Core Implementation Guide [
9,
10]. The USCDI represents the basic building blocks of healthcare interoperability, including a standardized set of data classes, data elements, and associated terminology standards such as SNOMED CT and LOINC. The US Core Implementation Guide provides detailed technical specifications and implementation guidance to support USCDI adoption. Australia, Canada, the UK, and Korea are adopting similar approaches.
STRATEGIES FOR ADOPTING TERMINOLOGY STANDARDS IN LABORATORY MEDICINE
While terminology standards are essential for ensuring the semantic interoperability of healthcare data, their adoption presents challenges [
11]. The availability of multiple terminology standards for representing laboratory medicine complicates achieving a consensus on a singular standard. This dilemma may be addressed by allowing a range of complementary terminologies and accepting the inherent differences among them. LOINC codes are suitable for coding laboratory tests, whereas SNOMED CT is appropriate for coding non-numerical results. Data interoperability may be facilitated by cross-mapping LOINC and SNOMED CT or by adopting SNOMED CT as a reference terminology to act as an interlingua among various standards.
Terminology standards inevitably change in content and structure owing to the evolution of medical knowledge, the need for concept refinement or disambiguation, content redundancy, and changes in code or meaning. As most terminology standards are designed to accommodate these evolutions, clinicians may propose the addition of new concepts. Often, the descriptions used in terminology standards are not commonly used by clinicians (e.g., “Urea nitrogen:MCnc:Pt:Ser/Plas:Qn”), rendering their use cumbersome. Clinicians may also suggest incorporating custom-made terms (e.g., “BUN measurement”) as synonyms to enhance the practicality of the standards.
The application of terminology standards at the point of care may burden healthcare providers. This burden can be alleviated by introducing a better EHR interface with personalized templates for commonly used terms and a terminology search engine that retrieves appropriate terms and recognizes common synonyms. Additionally, developing NLP algorithms to match local terms with standardized terminology and mapping SNOMED CT to other terminologies or classifications in healthcare information systems, such as ICD-10 and LOINC, can streamline processes by automatically correlating the clinical data recorded by clinicians in SNOMED CT with other classifications or terminologies.
In summary, the quantity and quality of data are crucial for data-driven AI in healthcare. Ensuring data quality with semantic interoperability requires the unambiguous collection, sharing, and use of data facilitated by adherence to terminology standards. The usability of terminology standards in laboratory medicine can be improved by accepting a range of complementary terminology standards, active participation in the evolution of the content and structure of these standards, and alleviating the burden on healthcare providers by developing better interfaces or incorporating natural language processing.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download