Abstract
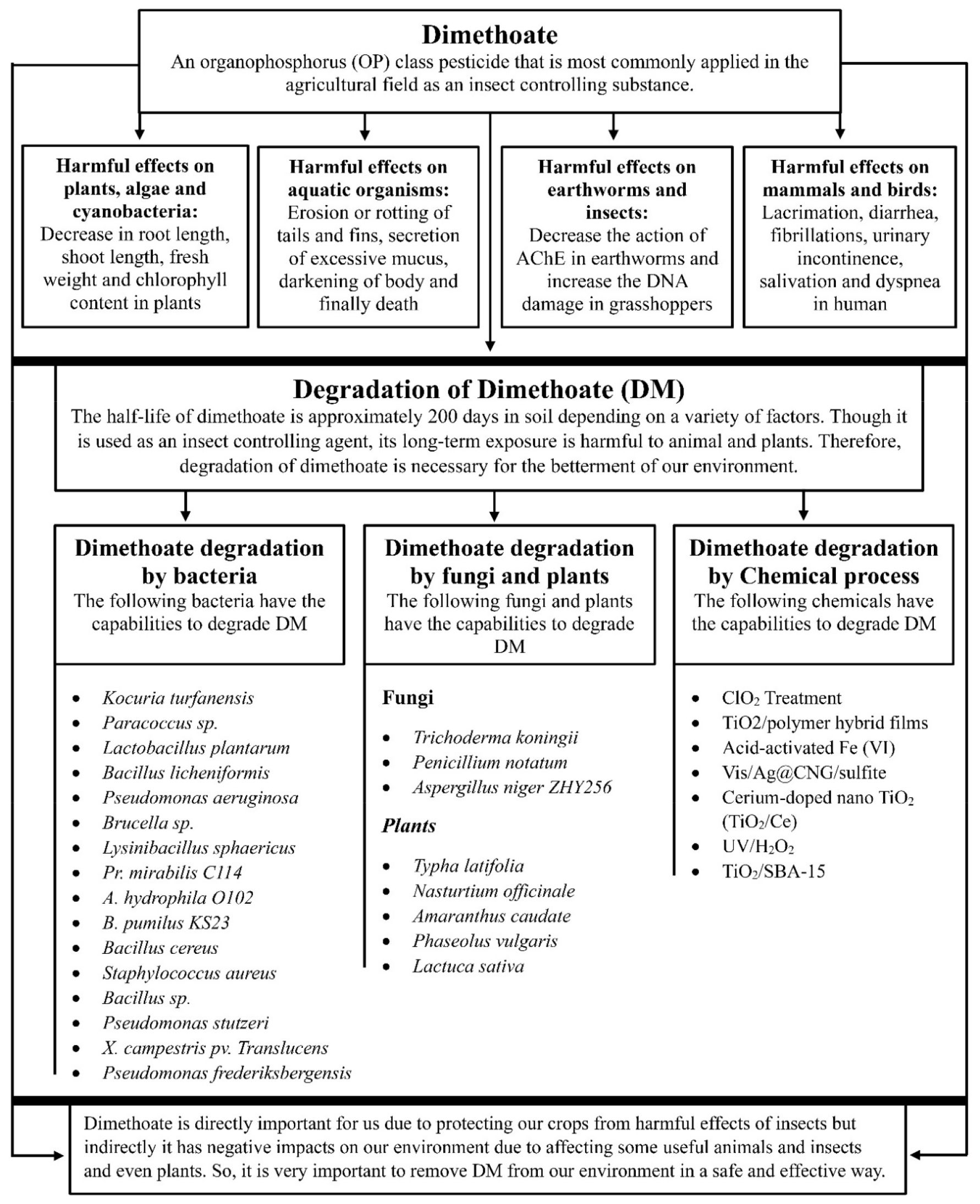
Dimethoate, an organophosphorus pesticide, is widely used in our crops to protect from different kinds of insects. This pesticide causes severe pollution of soil and groundwater and affects aquatic organisms, bees, plants, birds, earthworms, humans, and other animals by inhibiting largely acetylcholinesterase (AChE) enzymes. Its half-life is around 200 days in soil depending on temperature, pH, microorganisms, and organic contents, indicating its accumulation in soil after every use in agricultural fields. The primary metabolite of this pesticide, omethoate, is highly toxic than dimethoate. Thus, the pesticide needs to be degraded to protect our environment from its harmful effects. Biodegradation has received increased attention as a safe, eco-friendly, and cost-effective technique to decontaminate soil and water. Bacteria such as Kocuria turfanensis, Lactobacillus plantarum, Pseudomonas aeruginosa, Lysinibacillus sphaericus, Brucella sp., and Bacillus licheniformis, and fungi such as Aspergillus niger, Trichoderma koningii, and Penicillium notatum, and plants such as Typha latifolia, Nasturtium officinale, Amaranthus caudate, Phaseolus vulgari, and Lactuca sativa, are used to degrade dimethoate. In addition to biodegradation, a chemical process exists where different chemicals, including TiO2, ClO2, and acid-activated Fe (VI), are used. The combination of a chemical process with radiation displays increased efficiency of dimethoate degradation. Although several methods of degrading dimethoate pesticides are available, all processes are not convenient and eco-friendly. In this review, we have addressed the safe, eco-friendly, and convenient method of degrading dimethoate to protect our environment from its harmful effects.
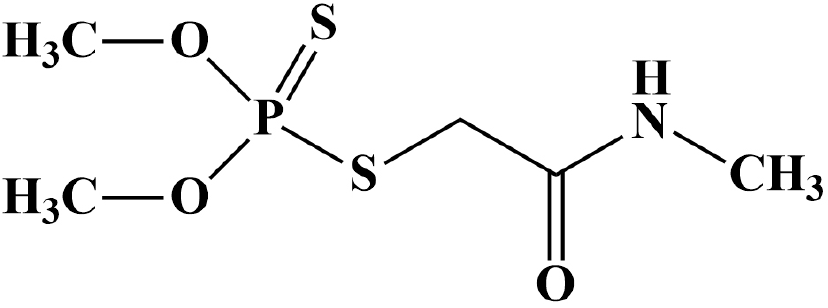
Organophosphorus (OP) class pesticides are most commonly applied to the agricultural field as an insect repellent (1). The group of OP class pesticides contains approximately 150 different types of chemicals (2). OP pesticides cause severe pollution in the environment because these are sometimes transported from their site of targets to the aquatic environments by leaching of soil, spray, air drift, accidental spills, or surface runoff (3, 4). OP pesticides exhibit high toxicity to aquatic animals such as fishes and other invertebrates (5). The dairy cattle exposed to pesticide-contaminated water, feed, or air might contain pesticides, especially OPs, in their milk because of their solubility in fat (6). Dimethoate (O,O-dimethyl S-methylcarbamoylmethyl phosphorodithioate, C5H12NO3PS2), a systemic OP class pesticide (Fig. 1) was introduced in 1956 and used on different crops, such as fruits, citrus, potatoes, rice, cotton, olives, vegetables, cabbage, tea, and tobacco to control several insects such as whiteflies, pea midges, aphids, sawy, thrips, suckers, mites, and wheat bulby (1, 7, 8). The United States Environmental Protection Agency (USEPA) has marked dimethoate (DM) in a third contaminant candidate list due to its harmful effects on human health (9). DM is absorbed readily in plant tissues and is recommended at concentration ranges from 300 to 600 ppm on several crops such as fruits, cereals, and vegetables (10). The solubility of DM in water is 39,800 mg/L and adsorption coefficient (Koc) in the soil is 20 (11). It exerts poisoning effects by absorbing through the stomach and skin, following which it interferes with the action of acetylcholinesterase (AChE), an enzyme necessary for appropriate functioning of both human and insect nervous systems (12). The residues of DM and its metabolites have been found in soil, vegetables, and fruits and also in cow’s milk (7). The maximum permissible amount of DM in drinking water is 0.006 mg/L (13). The half-life (T1/2) of DM is approximately 200 days in soil depending on different factors such as organic matter, contents of microorganisms, pH, and temperature (6). The primary oxidized metabolite of DM is omethoate (O,O-dimethyl-S-methylcarbamoylmethylthiophosphate), which exerts higher neurotoxicity compared to DM (6). The reduction of the double bond between phosphorous and sulfur (P = S) in the DM molecule is responsible for its metabolism (8). Numerous degradation pathways exist for OPs such as microbial treatment, photolytic oxidation, microwave irradiation, nano-filtration, hydrolysis, and other biological techniques (5, 9). DM degradation by microbial treatment is one of the safest, cheapest, eco-friendly, and efficient methods to clean up contaminated environments (6, 7, 14). The purpose of the review is to identify safe and convenient methods of degrading DM pesticides to protect our environment from their harmful effects (Fig. 2).
Effects on aquatic organisms: The adverse effects of DM are attributed to its high solubility in water and low adsorption coefficient in soil (11). Aquatic organisms, such as guppy, Daphnia magna, and zebrafish displayed more susceptibility to the emulsifiable concentrate of DM (10%) with 48 h LC50 values of 16, 0.83 and 7.5 mg/L, respectively (11). Dogan and Can exposed adult rainbow trout (O. mykiss) to DM concentrations of 0.3675 and 0.7350 mg/L after 10 days of the experiment. They found that behavioral changes such as erratic and hysteric swimming, circling movement, loss of appetite and equilibrium, convulsion, and remaining motionless were more severe at a concentration of 0.7350 mg/L (15). The clinically observed toxic signs were erosion or rotting of tails and fins, secretion of excessive mucus, and darkening of body surface color (15). The freshwater species, Gammarus pulex when exposed to DM for 96 h, demonstrated an average LC50 value of 167 μg/L (16). A study reported that 24 h exposure to DM at a concentration of 10 and 20 μg/L significantly reduced glutathione (GSH) levels and superoxide dismutase (SOD) enzyme activity but significantly increased malondialdehyde (MDA) levels in Gammarus sp. (16). The catfish, Heteropneustes fossilis, when exposed to DM, exerted altered behavioral responses including abrupt jerky and erratic swimming, frequent gulping and surfacing, restlessness, drooping of fins, and finally death (17). DM remarkably changes the levels of nucleic acid, total protein, free amino acid, and protease activity in the ovotestis and hepatopancreas tissues of the adult freshwater snails, Lymnaea acuminata (18).
Effects on plants, algae, and cyanobacteria: The treatment of pigeon pea plants with DM concentrations of 10, 20, 40, and 80 ppm showed a significant decrease in the root length, dry weight, shoot length, fresh weight, chlorophyll a content, and chlorophyll b content (19). Treatment of algae (Ge-Xian-Mi) with 3000 μM DM significantly declined the content of chlorophyll a, allophycocyanin (APC), and phycocyanin (CPC) (4). The presence of 2000 μM DM for 96 h remarkably decreased the gross photosynthetic rate of Ge-Xian-Mi colonies (4). Moreover, extreme concentrations of DM inhibit the growth and are speculated to cause the ultimate death of cyanobacterium Synechocystis sp. (20). A particular concentration (≥ 100 µM) of this insecticide strongly influences the efficiency of photosynthesis in Chlorella vulgaris cells (21). Treatment of seedlings of bitter gourd (Momordica charantia L.) with 100 and 200 ppm DM showed a concentration-dependent decline in growth, whereas low concentration of DM (50 ppm) showed an increase in seedling growth (22). DM has been known to enhance the consumption of respiratory O2 in cyanobacteria and affect the integrity and fluidity of the lipid membrane (19). It can significantly reduce transpiration, stomatal conductance, and photosynthesis (19). Treatment of seedlings of cowpea (V. unguiculata L.) with 100 and 200 ppm DM more adversely affected the roots than the shoots (10). In addition, it remarkably declined the root length, the dry and fresh mass of the shoot, leaf area, content of chlorophyll a, and content of chlorophyll b. (10). DM has been implicated in hormonal imbalance, leaf function deterioration, root osmotic imbalance, and causing problems in plant respiration (23).
Effects on earthworms and insects: The alimentary surfaces of earthworms are continuously exposed to different types of contaminants as they ingest a huge amount of soil or particular fractions of soil (24). The LC50 of Octolasion lacteum and Eisenia andrei earthworms were found to be 1.98±0.25 μg/cm2 and 10.36±1.39 μg/cm2, respectively after 24 h exposure to DM (24). Higher concentrations of DM significantly inhibited the activity of AChE and catalase in both earthworm species (24). The exposure of the bow-winged grasshoppers to DM enhanced DNA damage in their hemocytes (2). Ten days of treatment of E. andrei earthworms with a 3 mg/kg dose of DM greatly inhibited carboxylesterase (CES) activity but increased the levels of glutathione (GSH) (25). The fourth instar larvae of Kiefferulus calligaster and Chironomus riparius showed remarkable inhibition of the cholinesterase enzyme following the treatment with DM doses of 7.1 mg/L and 4.5 mg/L, respectively (11). Exposure of honey bees to 0.2 ppm DM for 3 weeks caused spastic movements and reduced larvae development and egg production (26). Furthermore, DM caused 80% inhibition of the brain acetylcholine-esterase activity when exposed for 48 h at an amount of 20 ng per bee (26). Atkins and Kellum reported that the LD50 of larvae (aged 5-6 days) was 0.66 µg per larva (27). In contrast, Gough et al. reported that the LD50 of adult honey bees was 0.18 µg per bee, indicating higher acute toxicity of adult bees compared to larvae (27).
Effects on mammals and birds: DM at a dose of 16 mg/kg exerted no significant decrease in body weight (BW); however, it showed a dose-dependent decrease in spleen weight of female Swiss albino mice (28). In addition, DM remarkably decreased lymphocyte proliferation responses of mice to B-cell mitogen (LPS) and T-cell mitogen (PHA) (28). The exposure of male Wistar albino rats to higher doses of DM may cause infertility because of severe atrophy in the seminiferous tubules (29). DM at a dose of 20 mg/kg remarkably decreased testis weight of test rats (29). The exposure of rats to DM resulted in hemorrhage and edema in the testes interstitial tissue (29). The DM (0.2 g/L)-treated adult Wistar rats showed a decrease in the intake of food by 25%, consumption of water by 23%, levels of vitamin E by 75%, and a remarkable inhibition of erythrocyte AChE activity by 59% (30). A single dose of DM (10 or 30 mg/kg) reduced the activity of serum and brain AChE in mice (31). The exposure of male broiler chicks to daily 4 and 8 mg/kg doses of DM for 4 weeks exhibited a remarkable decrease in serum triiodothyronine (T3) and thyroxine (T4) levels (32). The Japanese quail (Coturnix japonica) when treated with 75 mg/kg dose of DM showed an 85% decrease in brain AChE activity (11). The birds Adult Robin, Song Sparrow, and Chipping Sparrow showed a reduction in the mean blood serum cholinesterase activity when exposed to DM (33). The exposure of red-winged blackbirds to different doses of DM showed varied symptoms such as ataraxia at a dose range 1.75 to 2.00 mg/kg BW, neuromuscular dysfunctions (including tremors, hyperexcitability, muscular fatigue, etc.) at a dose 2.5 mg/kg BW or more, vomiting and dyspnea at a dose range 3.00 to 4.00 mg/kg BW, and death or muscle paralysis at a dose 4.90 mg/kg BW or more (34). The poisoning signs of DM include lacrimation, diarrhea, fibrillations, urinary incontinence, salivation, and dyspnea (35). DM shows different LD50 values in different animals such as 80 mg/kg in sheep, more than 100 mg/kg in dogs, and more than 50 mg/kg in horse (35). Daily 40 mg/kg dose of DM for 3 weeks in guineapigs exhibited a reduction in erythrocyte cholinesterase by 19 to 23% than normal (35).
Kocuria turfanensis: Kocuria turfanensis is gram-positive coccus and is deposited under accession no. KU521338.1 in GenBank (1). This strain degraded 78.22% of DM after 21 days of treatment in MSM (mineral salts medium) medium (containing 1% DM in 100 mL of MSM media, incubated in a shaker at 37°C and 120 rpm) (1). This strain utilizes DM as its sole source of carbon and energy (1).
Paracoccus sp.: This strain (designated as Paracoccus sp. Lgjj-3) was isolated from a wastewater treatment plant and exhibited high effectiveness in degrading DM (7). This bacterial strain in the MSM media (containing 100 mg/L DM in 100 mL of MSM, incubated in a rotary shaker at 30°C and 150 rpm) effectively degraded DM in 6 h (7).
Lactobacillus plantarum: Degradation studies of DM were performed in bovine milk (containing 50 mg/kg DM, incubation temperature 37°C) in the presence of this isolate, and it demonstrated 81.28% degradation of DM within 24 h (6). This strain efficiently degrades DM and its primary metabolite omethoate, making this strain useful for application in fermented food to remove DM (6).
Xanthomonas campestris pv. translucens: The biomass production of this strain increased in the MSM medium containing DM (12). This strain degraded approximately 97.8% of DM in the MSM media (containing 5 ppm DM in 100 mL MSM, incubation temperature 30°C, and shaker rotation 150 rpm) within 32 days (12). DM in the presence of this strain demonstrated a half-life (T1/2) of 15.86 days in water (12).
Bacillus licheniformis: This bacterial strain was isolated from the intestine of freshwater Labeo rohita fish and studied in MS (mineral salt) solution containing DM as the sole source of carbon and energy (36). Incubation of this isolate at 28°C for 24 h in MS solution showed that it exhibited utilizing capacity of DM up to 2 mg/mL, with a maximum rate of growth at 0.45 mg/mL (36). The strain B. licheniformis F102 utilized 100 mg/L DM in MS solution (incubation temperature 28°C) on day 4 (37).
Pseudomonas aeruginosa: The strain P. aeruginosa MCMB-427 was isolated from the sugarcane rhizosphere and studied in Davis Mingiolis medium containing 0.5 g/L DM as the sole carbon and energy source (38). This strain exhibited more than 95% degradation efficacy for DM after 8 days of incubation (38). The researcher demonstrated that the DM degradation capacity of this strain is present in the 6.6 kbp plasmid pDMD427 (38). The strain P. aeruginosa W171 showed 96% DM degradation in MS medium (incubation temperature 28°C) on day 7 (37).
Brucella sp.: The strain Brucella sp. PS4 was isolated from the rhizospheric soil samples of cotton and was deposited under accession no. MZ322506 in GenBank (23). DM at 50 ppm in the MSM medium showed 88% degradation of DM on day 3 and DM at 100 ppm in the MSM medium showed 83% degradation of DM on day 7 in the presence of this strain (23). The researcher reported that the optimum values of temperature and pH for the growth of this strain were 35°C and 6, respectively (23).
Lysinibacillus sphaericus: This strain was deposited under accession no. MF967404 in GenBank (39). This strain can tolerate up to 500 mg/L concentration of DM in MS broth and demonstrated degrading capability within a pH and temperature range of 4-12 and 15-37°C, respectively (39).
Other reported bacteria: Bacterial strains Proteus mirabilis C114, Aeromonas hydrophila O102, and Bacillus pumilus KS23 were isolated from the intestine of Labeo rohita and were reported to degrade DM in the MS medium (incubation temperature 28°C and time 7 days) at a rate of 72%, 83%, and 71%, respectively (37). Pseudomonas frederiksbergensis has also been reported to degrade DM, as evident from its high efficacy and rapid degradation rate (40). Bacillus cereus (GenBank accession no. MF967405) can tolerate 500 mg/L concentration of DM in MS broth (incubation temperature 28°C) (39). The bacteria Staphylococcus aureus and Bacillus sp. have been reported to efficiently degrade DM at 40°C (41). The bacterial strain Pseudomonas stutzeri exhibited a good degradation rate of DM at 35°C (42).
Fungi: The strain Aspergillus niger ZHY256 was isolated from sewage and cotton field soil and cultured in a basal salt medium for enzyme production (43). This strain produced an enzyme that (estimated molecular mass 66 kDa) successfully degraded the P-S linkage of DM (43). Copper (Cu) ion can effectively activate the activity of the enzyme and barium (Ba) ion can effectively stimulate the production of the enzyme (43). In addition, fungal strains Trichoderma koningii and Penicillium notatum have been reported to degrade DM because they utilize the pesticide as a source of carbon (44).
Plants: Phytoremediation is an aesthetically pleasing process that uses plants to remove contaminants from water and soil (45). In addition, DM was degraded in soil by certain plants such as Typha latifolia, Nasturtium officinale, Amaranthus caudate, Phaseolus vulgaris, and Lactuca sativa (14). Typha latifolia is a perennial and wetland plant that is found in different places such as brackish water, freshwater, or deep marshes (46). Nasturtium officinale is perennial and grows in both aquatic and semi-aquatic areas such as pond margins, brooks, boggy, ditches, or marshy ground (47). The bean Phaseolus vulgaris is cultivated mainly in Brazil, China, India, Mexico, and the USA and grows symbiotically with Rhizobium (a bacterium that fixes N2) (48). Lactuca sativa is a group of leafy vegetables that are cultivated mainly in China, USA, India, Spain, Italy, and Japan (49).
ClO2 Treatment: DM (10 mg/L) treatment with 5 mg/L and 10 mg/L concentration of ClO2 exhibited DM degradation of 87-93% and 88-98%, respectively, in the presence of light (50). In contrast, treatment with 5 mg/L and 10 mg/L concentrations of ClO2 showed DM degradation of 85-92% and 86-93%, respectively, in the absence of light (50). A 24 h treatment of DM with a 10 mg/L dose of ClO2 exhibited a high degradation efficiency of 89-97% at pH 7.00 (50).
TiO2/polymer hybrid films: The immobilized catalysts (PAH/PSS/TiO2) are used where the photocatalyst TiO2 (Degussa Aeroxide P25 TiO2) is the final layer (13). When the immobilized catalysts containing 1 g/L and 2 g/L of TiO2 were irradiated for 300 min with a 400-W UV lamp, the amount of DM remaining was 31.7 and 5.1%, respectively (13). The increase in the catalyst activity (TiO2 at 4 g/L) exhibited complete degradation of DM when irradiated with a 400-W UV lamp for 300 min (13).
Acid-activated Fe (VI): The DM (initial concentration 43.65 µM) removal rate was 26% when Fe (VI) was used alone for 20 min, 85% when acid-activated Fe (VI) was used for 1 min, and 90% when acid-activated Fe (VI) under simulated sunlight was used for 1 min (51). DM was completely removed with 20 min treatment of acid-activated Fe (VI) (51).
Vis/Ag@CNG/sulfite: The Ag nanoparticles decorated graphene oxide-supported graphite nitrogen carbide with sulfite activation under visible light (Vis/Ag@CNG/sulfite) demonstrated an efficiency of DM removal 98 and 93% for initial DM concentration of 5 mg/L and 10 mg/L, respectively (52). The initial sulfite concentration of 1 mM exhibited 83% degradation efficiency, and the 2 mM sulfite concentration was selected as the best concentration due to economic circumstances (52). The Ag@CNG at a concentration of 0.1 g/L showed a DT degradation efficiency of 79% in the Vis/Ag@CNG/sulfite system and the optimal concentration for the catalyst was selected as 0.2 g/L (52). The presence of sulfite in the Vis/Ag@CNG system showed 15.8 times higher DM degradation efficacy than the Vis/Ag@CNG system alone (52).
Cerium-doped nano TiO2 (TiO2/Ce): The TiO2/Ce exhibited a rapid DM degradation rate in Bok choy plants with a 1.6 times shorter half-life (53). The TiO2/Ce hydrosol exerted a remarkable effect on the increase in reactive oxygen species (ROS), which consequently enhanced the degradation of DM in Bok choy plants (53).
UV/H2O2: When 1 mg/L concentration of DM was exposed to UV radiation for 60 min, it exhibited 95% degradation of DM (54). The combined action of UV and H2O2 (at an optimum concentration of 100 mg/L) displayed higher efficacy than UV treatment alone and degraded almost 100% DM (1 mg/L) in 240 min (54).
TiO2/SBA-15: The 26% TiO2/SBA-15 photocatalysts (surface area 386 m2/g) completely degraded DM (30 mg/mL) within 7 h and exhibited 62% more catalytic activity than the pure form of TiO2 (55). This enhanced catalytic activity is attributed to the synergism of TiO2 and the mesoporous material SBA-15 (55). The electron delocalization and adsorption properties of the mesoporous material (SBA-15) are largely responsible for the enhanced degradation rate of DM (55).
DM protects the crops from the harmful effects of insects but negatively impacts the environment, including animals, insects, and plants. Therefore, it is crucial to remove DM from our environment. Several organisms (including bacteria, fungi, and plants), chemicals (including TiO2, Acid-activated Fe (VI), ClO2, etc.), and radiation exhibit the degradation capability of DM; however, all processes are not safe and convenient. Biodegradation is safe and more convenient than all other degradation processes because the pesticide is degraded by bacteria, fungi, and plants. Because it is necessary to remove the pesticides from our environment, we should focus on improving the biodegradation process to keep our environment safe from the harmful effects of DM.
References
1. Barot KJ, Chaudhari K. Analysis of dimethoate degradation by Kocuria turfanensis using GC-MS. Asian J Microbiol Biotechnol Environ Sci. 2020;22:107-110.
2. Karpeta-Kaczmarek J, Kubok M, Dziewiȩcka M, Sawczyn T, Augustyniak M. The level of DNA damage in adult grasshoppers Chorthippus biguttulus (Orthoptera, Acrididae) following dimethoate exposure is dependent on the insects' habitat. Environ Pollut. 2016;215:266-272.DOI: 10.1016/j.envpol.2016.05.032.
3. Salem AB, Azzouz S, Mougou A, Salghi R, Chaabane H, Fattouch S. Biochemical characterisation and bioremediation study of dimethoate and chlorpyrifos tolerant bacterial strains isolated from an agricultural soil. J New Sci. 2016;33(3):1901-1909.
4. Chen Z, Juneau P, Qiu B. Effects of three pesticides on the growth, photosynthesis and photoinhibition of the edible cyanobacterium Ge-Xian-Mi (Nostoc). Aquat Toxicol. 2007;81(3):256-265.DOI: 10.1016/j.aquatox.2006.12.008.
5. Abdel-megeed A, El-nakieb FA. Bioremediation of Dimethoate by Effective Micro-Organisms in Egyptian Contaminated Water. Terrestrial Aquatic Environ Toxicol. 2006;2:490-8.
6. Yuan S, Yang F, Yu H, Xie Y, Guo Y, Yao W. Biodegradation of the organophosphate dimethoate by Lactobacillus plantarum during milk fermentation. Food Chem. 2021;360:130042.DOI: 10.1016/j.foodchem.2021.130042.
7. Li R, Zheng J, Wang R, Song Y, Chen Q, Yang X, et al. Biochemical degradation pathway of dimethoate by Paracoccus sp . Lgjj-3 isolated from treatment wastewater. Int Biodeterior Biodegradation. 2010;64(1):51-57.DOI: 10.1016/j.ibiod.2009.10.007.
8. Liang Y, Zeng F, Qiu G, Lu X, Liu X, Gao H. Co-metabolic degradation of dimethoate by Raoultella sp. X1. Biodegradation. 2009;20(3):363-373.DOI: 10.1007/s10532-008-9227-x.
9. Tian F, Liu W, Guo G, Qiang Z, Zhang C. Kinetics and mechanism of dimethoate chlorination during drinking water treatment. Chemosphere. 2014;103:181-187.DOI: 10.1016/j.chemosphere.2013.11.061.
10. Mishra V, Srivastava G, Prasad SM, Abraham G. Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate. Pestic Biochem Physiol. 2008;92(1):30-37.DOI: 10.1016/j.pestbp.2008.05.003.
11. Van Scoy A, Pennell A, Zhang X. Environmental fate and toxicology of dimethoate. Rev Environ Contam Toxicol. 2016;237:53-70.DOI: 10.1007/978-3-319-23573-8_3.
12. Derbalah A, Massoud A, El-mehasseb I, Allah MS, Ahmed MS, Al-brakati A, et al. Microbial detoxification of dimethoate and methomyl residues in aqueous media. Water. 2021;13(8):1117.DOI: 10.3390/w13081117.
13. Priya DN, Modak JM, Trebse P, Zabar R, Raichur AM. Photocatalytic degradation of dimethoate using LbL fabricated TiO2/polymer hybrid films. J Hazard Mater. 2011;195:214-222.DOI: 10.1016/j.jhazmat.2011.08.030.
14. Nawaz K, Hussain K, Choudary N, Majeed A, Ilyas U, Ghani A, et al. Eco-friendly role of biodegradation against agricultural pesticides hazards. African J Microbiol Res. 2011;5(3):177-183.
15. Dogan D, Can C. Hematological, biochemical, and behavioral responses of Oncorhynchus mykiss to dimethoate. Fish Physiol Biochem. 2011;37(4):951-958.DOI: 10.1007/s10695-011-9492-1.
16. Serdar O. The effect of dimethoate pesticide on some biochemical biomarkers in Gammarus pulex. Environ Sci Pollut Res Int. 2019;26(21):21905-21914.DOI: 10.1007/s11356-019-04629-w.
17. Pandey RK, Singh RN, Singh S, Singh NN, Das VK. Acute toxicity bioassay of dimethoate on freshwater airbreathing catfish, Heteropneustes fossilis (Bloch). J Environ Biol. 2009;30:437-440.
18. Tripathi PK, Singh A. Toxic effects of dimethoate and carbaryl pesticides on protein metabolism of the freshwater snail Lymnaea acuminata. Bull Environ Contam Toxicol. 2003;70(1):146-152.DOI: 10.1007/s00128-002-0168-5.
19. Pandey JK, Dubey G, Gopal R. Study the effect of insecticide dimethoate on photosynthetic pigments and photosynthetic activity of pigeon pea: Laser-induced chlorophyll fluorescence spectroscopy. J Photochem Photobiol B. 2015;151:297-305.DOI: 10.1016/j.jphotobiol.2014.08.014.
20. Mohapatra PK, Schiewer U. Effect of dimethoate and chlorfenvinphos on plasma membrane integrity of Synechocystis sp. PCC 6803. Ecotoxicol Environ Saf. 1998;41(3):269-274.DOI: 10.1006/eesa.1998.1708.
21. Jena S, Acharya S, Mohapatra PK. Variation in effects of four OP insecticides on photosynthetic pigment fluorescence of Chlorella vulgaris Beij. Ecotoxicol Environ Saf. 2012;80:111-117.DOI: 10.1016/j.ecoenv.2012.02.016.
22. Mishra V, Srivastava G, Prasad SM. Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci Hortic. 2009;120(3):373-378.DOI: 10.1016/j.scienta.2008.11.024.
23. Ahmad S, Chaudhary HJ, Damalas CA. Microbial detoxification of dimethoate through mediated hydrolysis by Brucella sp. PS4: molecular profiling and plant growth-promoting traits. Environ Sci Pollut Res int. 2022;29(2):2420-2431.DOI: 10.1007/s11356-021-15806-1.
24. Velki M, Hackenberger BK. Species-specific differences in biomarker responses in two ecologically different earthworms exposed to the insecticide dimethoate. Comp Biochem Physiol Part C Toxicol Pharmacol. 2012;156(2):104-112.DOI: 10.1016/j.cbpc.2012.05.001.
25. Velki M, Hackenberger BK. Inhibition and recovery of molecular biomarkers of earthworm Eisenia andrei after exposure to organophosphate dimethoate. Soil Biol Biochem. 2013;57:100-108.DOI: 10.1016/j.soilbio.2012.09.018.
26. Christen V, Joho Y, Vogel M, Fent K. Transcriptional and physiological effects of the pyrethroid deltamethrin and the organophosphate dimethoate in the brain of honey bees (Apis mellifera). Environ Pollut. 2019;244:247-256.DOI: 10.1016/j.envpol.2018.10.030.
27. Aupinel P, Fortini D, Michaud B, Marolleau F, Tasei JN, Odoux JF. Toxicity of dimethoate and fenoxycarb to honey bee brood (Apis mellifera), using a newin vitro standardized feeding method. Pest Manag Sci. 2007;63(11):1090-1094.DOI: 10.1002/ps.1446.
28. Aly NM, El-Gendy KS. Effect of dimethoate on the immune system of female mice. J Environ Sci Health B. 2000;35(1):77-86.DOI: 10.1080/03601230009373255.
29. Sayim F. Histopathological effects of dimethoate on testes of rats. Bull Environ Contam Toxicol. 2007;78(6):479-484.DOI: 10.1007/s00128-007-9196-5.
30. Ben Amara I, Soudani N, Hakim A, Bouaziz H, Troudi A, Zeghal KM, et al. Dimethoate-induced oxidative damage in erythrocytes of female adult rats: Possible protective effect of vitamin e and selenium supplemented to diet. Toxicol Ind Health. 2012;28(8):222-237.DOI: 10.1177/0748233711410909.
31. Long SM, Dawson A, Shore RF. A comparison of the effects of single and repeated exposure to an organophosphate insecticide on acetylcholinesterase activity in mammals. Environ Toxicol Chem. 2006;25(7):1857-1863.DOI: 10.1897/05-533R.1.
32. Maiti PK, Gupta P, Chaurasia SS, Kar A. Dimethoate induced lipid peroxidation and inhibition of type- I iodothyronine 5'-monodeiodinase activity in young cockerel. Bull Environ Contam Toxicol. 1996;57(2):335-340.DOI: 10.1007/s001289900195.
33. Rondeau G, Desgranges JL. Effects of insecticide use on breeding birds in Christmas tree plantations in Quebec. Ecotoxicology. 1995;4(5):281-298.DOI: 10.1007/BF00118595.
34. Brunet R, Girard C, Cyr A. Comparative study of the signs of intoxication and changes in activity level of red-winged blackbirds (Agelaius phoeniceus) exposed to dimethoate. Agric Ecosyst Environ. 1997;64(3):201-209.DOI: 10.1016/S0167-8809(97)00038-8.
35. Sanderson DM, Edson EF. Toxicologic properties of the organophosphorus insecticide dimethoate. Br J Ind Med. 1964;21(1):52-64.DOI: 10.1136/oem.21.1.52.
36. Mandal MD, Mandal S, Pal NK. Plasmid-mediated dimethoate degradation by Bacillus licheniformis isolated from a fresh water fish Lobeo rohita. J Biomed Biotechnol. 2005;2005(3):280-286.DOI: 10.1155/JBB.2005.280.
37. DebMandal M, Mandal S, Pal NK. Kinetics of dimethoate biodegradation in bacterial system. Microbiol Res. 2011;2(2):e20.DOI: 10.4081/mr.2011.e20.
38. Deshpande NM, Dhakephalkar PK, Kanekar PP. Plasmid-mediated dimethoate degradation in pseudomonas aeruginosa MCMB-427. Lett Appl Microbiol. 2001;33(4):275-279.DOI: 10.1046/j.1472-765X.2001.00995.x.
39. Saleh M, Youssef AF, Muhammed Y. The potentiality of Lysinibacillus sphaericus DM-3 and Bacillus cereus DM-5 in degrading dimethoate. Egypt J Bot. 2018;58(2):217-232.DOI: 10.21608/ejbo.2018.2446.1151.
40. Al-Qurainy F, Abdel-Megeed A. Phytoremediation and detoxification of two organophosphorous pesticides residues in Riyadh area. World Appl Sci. J. 2009;6(7):987-998.
41. Singh S, Iyer PR. Isolation and characterization of microorganisms that degrade dimethoate. J Bioremediat Biodegrad. 2017;08:410.DOI: 10.4172/2155-6199.1000410.
42. Shalaby EA. Prospects of effective microorganisms technology in wastes treatment in Egypt. Asian Pac J Trop Biomed. 2011;1(3):243-248.DOI: 10.1016/S2221-1691(11)60035-X.
43. Liu YH, Chung YC, Xiong Y. Purification and characterization of a dimethoate-degrading enzyme of Aspergillus niger ZHY256, isolated from sewage. Appl Environ Microbiol. 2001;67(8):3746-3749.DOI: 10.1128/AEM.67.8.3746-3749.2001.
44. Lone MA, Wani MR. Degradation of dimethoate and pyrethroid by using fungal strains isolated from the rhizosphere of Juglans regia L. in the northern region of Jammu and Kashmir, India. Int J Pharma Bio Sci. 2012;3(4):716-723.
45. Parte SG, Mohekar AD, Kharat AS. Microbial degradation of pesticide: A review. African J Microbiol Res. 2017;11(24):992-1012.DOI: 10.5897/AJMR2016.8402.
46. Tsyusko OV, Smith MH, Sharitz RR, Glenn TC. Genetic and clonal diversity of two cattail species, Typha latifolia and T. angustifolia (Typhaceae), from Ukraine. Am J Bot. 2005;92(7):1161-1169.DOI: 10.3732/ajb.92.7.1161.
47. Klimek-Szczykutowicz M, Szopa A, Ekiert H. Chemical composition, traditional and professional use in medicine, application in environmental protection, position in food and cosmetics industries, and biotechnological studies of Nasturtium officinale (watercress) - a review. Fitoterapia. 2018;129:283-292.DOI: 10.1016/j.fitote.2018.05.031.
48. Fernández-Luqueño F, Reyes-Varela V, Martínez-Suárez C, Salomón-Hernández G, Yáñez-Meneses J, Ceballos-Ramírez JM, et al. Effect of different nitrogen sources on plant characteristics and yield of common bean (Phaseolus vulgaris L.). Bioresour Technol. 2010;101(1):396-403.DOI: 10.1016/j.biortech.2009.07.058.
49. Křístková E, Doležalová I, Lebeda A, Vinter V, Novotná A. Description of morphological characters of lettuce (Lactuca sativa L.) genetic resources. Hortic Sci. 2008;35(3):113-129.DOI: 10.17221/4/2008-HORTSCI.
50. Pergal MV, Kodranov ID, Dojčinović B, Avdin VV, Stanković DM, Petković BB, et al. Evaluation of azamethiphos and dimethoate degradation using chlorine dioxide during water treatment. Environ Sci Pollut Res. 2020;27(21):27147-27160.DOI: 10.1007/s11356-020-09069-5.
51. Lu J, Cui Z, Deng X, Liang Z, Chai S, Fan J, et al. Rapid degradation of dimethoate and simultaneous removal of total phosphorus by acid-activated Fe(VI) under simulated sunlight. Chemosphere. 2020;258:127265.DOI: 10.1016/j.chemosphere.2020.127265.
52. Deng X, Chen R, Zhao Z, Cui F, Xu X. Graphene oxide-supported graphitic carbon nitride microflowers decorated by sliver nanoparticles for enhanced photocatalytic degradation of dimethoate via addition of sulfite: Mechanism and toxicity evolution. Chem Eng J. 2021;425:131683.DOI: 10.1016/j.cej.2021.131683.
53. Liu X, Li Y, Zhou X, Luo K, Hu L, Liu K, et al. Photocatalytic degradation of dimethoate in Bok choy using cerium-doped nano titanium dioxide. PLoS One. 2018;13(5):e0197560.DOI: 10.1371/journal.pone.0197560.
54. Gandhi K, Lari S, Tripathi D, Kanade G. Advanced oxidation processes for the treatment of chlorpyrifos, dimethoate and phorate in aqueous solution. J Water Reuse Desalin. 2016;6(1):195-203.DOI: 10.2166/wrd.2015.062.
55. Li G, Wang B, Xu WQ, Han Y, Sun Q. Rapid TiO2/SBA-15 synthesis from ilmenite and use in photocatalytic degradation of dimethoate under simulated solar light. Dye Pigment. 2018;155:265-275.DOI: 10.1016/j.dyepig.2018.03.058.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download