1. World Health Organization. Global tuberculosis report 2023. Geneva. 2023.
2. Chandra P, Grigsby SJ, Philips JA. Immune evasion and provocation by
Mycobacterium tuberculosis.
Nat Rev Microbiol. 2022;20(12):750-766.DOI:
10.1038/s41579-022-00763-4.
3. Passos BBS, Araujo-Pereira M, Vinhaes CL, Amaral EP, Andrade BB. The role of ESAT-6 in tuberculosis immunopathology.
Front Immunol. 2024;15:1383098.DOI:
10.3389/fimmu.2024.1383098.
4. Ramon-Luing LA, Palacios Y, Ruiz A, Tellez-Navarrete NA, Chavez-Galan L. Virulence factors of
Mycobacterium tuberculosis as modulators of cell death mechanisms.
Pathogens. 2023;12(6):839.DOI:
10.3390/pathogens12060839.
5. Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M, et al. ESX-1 dependent impairment of autophagic flux by
Mycobacterium tuberculosis in human dendritic cells.
Autophagy. 2012;8(9):1357-1370.DOI:
10.4161/auto.20881.
6. Tiwari S, Casey R, Goulding CW, Hingley-Wilson S, Jacobs WR Jr. Infect and inject: How
Mycobacterium tuberculosis exploits its major virulence-associated Type VII secretion system, ESX-1.
Microbiol Spectr. 2019;7(3).DOI:
10.1128/microbiolspec.BAI-0024-2019.
7. Saini S, Gangwar A, Sharma R. Harnessing host-pathogen interactions for innovative drug discovery and host-directed therapeutics to tackle tuberculosis.
Microbiol Res. 2023;275:127466.DOI:
10.1016/j.micres.2023.127466.
8. Orgeur M, Sous C, Madacki J, Brosch R. Evolution and emergence of
Mycobacterium tuberculosis.
FEMS Microbiol Rev. 2024;48(2):fuae006.DOI:
10.1093/femsre/fuae006.
9. Franco-Paredes C, Marcos LA, Henao-Martinez AF, Rodriguez-Morales AJ, Villamil-Gomez WE, Gotuzzo E, et al. Cutaneous mycobacterial infections.
Clin Microbiol Rev. 2018;32(1):e00069-18.DOI:
10.1128/CMR.00069-18.
10. Moule MG, Cirillo JD.
Mycobacterium tuberculosis dissemination plays a critical role in pathogenesis.
Front Cell Infect Microbiol. 2020;10:65.DOI:
10.3389/fcimb.2020.00065.
11. Zumla A, Chakaya J, Centis R, D'Ambrosio L, Mwaba P, Bates M, et al. Tuberculosis treatment and management--an update on treatment regimens, trials, new drugs, and adjunct therapies.
Lancet Respir Med. 2015;3(3):220-234.DOI:
10.1016/S2213-2600(15)00063-6.
12. Dulberger CL, Rubin EJ, Boutte CC. The mycobacterial cell envelope - a moving target.
Nat Rev Microbiol. 2020;18(1):47-59.DOI:
10.1038/s41579-019-0273-7.
13. Singh R, Dwivedi SP, Gaharwar US, Meena R, Rajamani P, Prasad T. Recent updates on drug resistance in
Mycobacterium tuberculosis.
J Appl Microbiol. 2020;128:1547-1567.DOI:
10.1111/jam.14478.
14. Imaeda T, Kanetsuna F, Galindo B. Ultrastructure of cell walls of genus Mycobacterium.
J Ultrastruct Res. 1968;25(1):46-63.DOI:
10.1016/S0022-5320(68)80059-0.
15. Winder FG, Collins PB. Inhibition by isoniazid of synthesis of mycolic acids in
Mycobacterium tuberculosis.
J Gen Microbiol. 1970;63(1):41-48.DOI:
10.1099/00221287-63-1-41.
16. Abrahams KA, Besra GS. Synthesis and recycling of the mycobacterial cell envelope.
Curr Opin Microbiol. 2021;60:58-65.DOI:
10.1016/j.mib.2021.01.012.
17. Francis RJ, Robb G, McCann L, Khatri B, Keeble J, Dagg B, et al. Three-dimensional
in situ morphometrics of
Mycobacterium tuberculosis infection within lesions by optical mesoscopy and novel acid-fast staining.
Sci Rep. 2020;10(1):21774.DOI:
10.1038/s41598-020-78640-4.
18. Frando A, Boradia V, Grundner C. Regulatory intersection of two-component system and Ser/Thr protein kinase signaling in
Mycobacterium tuberculosis.
J Mol Biol. 2024;436(2):168379.DOI:
10.1016/j.jmb.2023.168379.
19. Oh Y, Lee HN, Ko EM, Jeong JA, Park SW, Oh JI. Mycobacterial regulatory systems involved in the regulation of gene expression under respiration-inhibitory conditions.
J Microbiol. 2023;61(3):297-315.DOI:
10.1007/s12275-023-00026-8.
20. Roy S, Ghatak D, Das P, BoseDasgupta S. ESX secretion system: The gatekeepers of mycobacterial survivability and pathogenesis.
Eur J Microbiol Immunol (Bp). 2020;10(4):202-209.DOI:
10.1556/1886.2020.00028.
21. Stupar M, Furness J, De Voss CJ, Tan L, West NP. Two-component sensor histidine kinases of
Mycobacterium tuberculosis: Beacons for niche navigation.
Mol Microbiol. 2022;117(5):973-985.DOI:
10.1111/mmi.14899.
22. Frando A, Grundner C. More than two components: complexities in bacterial phosphosignaling.
mSystems. 2024;9(5):e0028924.DOI:
10.1128/msystems.00289-24.
23. Luo G, Ming T, Yang L, He L, Tao T, Wang Y. Modulators targeting protein-protein interactions in
Mycobacterium tuberculosis.
Microbiol Res. 2024;284:127675.DOI:
10.1016/j.micres.2024.127675.
24. Verma A, Ghoshal A, Dwivedi VP, Bhaskar A. Tuberculosis: The success tale of less explored dormant
Mycobacterium tuberculosis.
Front Cell Infect Microbiol. 2022;12:1079569.DOI:
10.3389/fcimb.2022.1079569.
25. Kundu M, Basu J. Applications of transcriptomics and proteomics for understanding dormancy and resuscitation in
Mycobacterium tuberculosis.
Front Microbiol. 2021;12:642487.DOI:
10.3389/fmicb.2021.642487.
26. Kim MJ, Park KJ, Ko IJ, Kim YM, Oh JI. Different roles of DosS and DosT in the hypoxic adaptation of Mycobacteria.
J Bacteriol. 2010;192(19):4868-4875.DOI:
10.1128/JB.00550-10.
27. Yuan Y, Crane DD, Simpson RM, Zhu YQ, Hickey MJ, Sherman DR, et al. The 16-kDa alpha-crystallin (Acr) protein of
Mycobacterium tuberculosis is required for growth in macrophages.
Proc Natl Acad Sci U S A. 1998;95(16):9578-9583.DOI:
10.1073/pnas.95.16.9578.
28. Karbalaei M, Mosavat A, Soleimanpour S, Farsiani H, Ghazvini K, Amini AA, et al. Production and evaluation of Ag85B:HspX:hFcgamma1 immunogenicity as an Fc fusion recombinant multi-stage vaccine candidate against
Mycobacterium tuberculosis.
Curr Microbiol. 2024;81(5):127.DOI:
10.1007/s00284-024-03655-3.
29. Mansury D, Ghazvini K, Amel Jamehdar S, Badiee A, Tafaghodi M, Nikpoor AR, et al. Increasing cellular immune response in liposomal formulations of DOTAP encapsulated by fusion protein Hspx, PPE44, and EsxV, as a potential tuberculosis vaccine candidate. Rep Biochem Mol Biol. 2019;7(2):156-166.
30. Liu W, Li J, Niu H, Lin X, Li R, Wang Y, et al. Immunogenicity and protective efficacy of multistage vaccine candidates (Mtb8.4-HspX and HspX-Mtb8.4) against
Mycobacterium tuberculosis infection in mice.
Int Immunopharmacol. 2017;53:83-89.DOI:
10.1016/j.intimp.2017.10.015.
31. Dheda K, Mirzayev F, Cirillo DM, Udwadia Z, Dooley KE, Chang KC, et al. Multidrug-resistant tuberculosis.
Nat Rev Dis Primers. 2024;10(1):22.DOI:
10.1038/s41572-024-00504-2.
32. Grobbel HP, Merker M, Kohler N, Andres S, Hoffmann H, Heyckendorf J, et al. Design of multidrug-resistant tuberculosis treatment regimens based on DNA sequencing.
Clin Infect Dis. 2021;73(7):1194-1202.DOI:
10.1093/cid/ciab359.
34. Ahmed S, Raqib R, Guethmundsson GH, Bergman P, Agerberth B, Rekha RS. Host-directed therapy as a novel treatment strategy to overcome tuberculosis: targeting immune modulation.
Antibiotics (Basel). 2020;9(1):21.DOI:
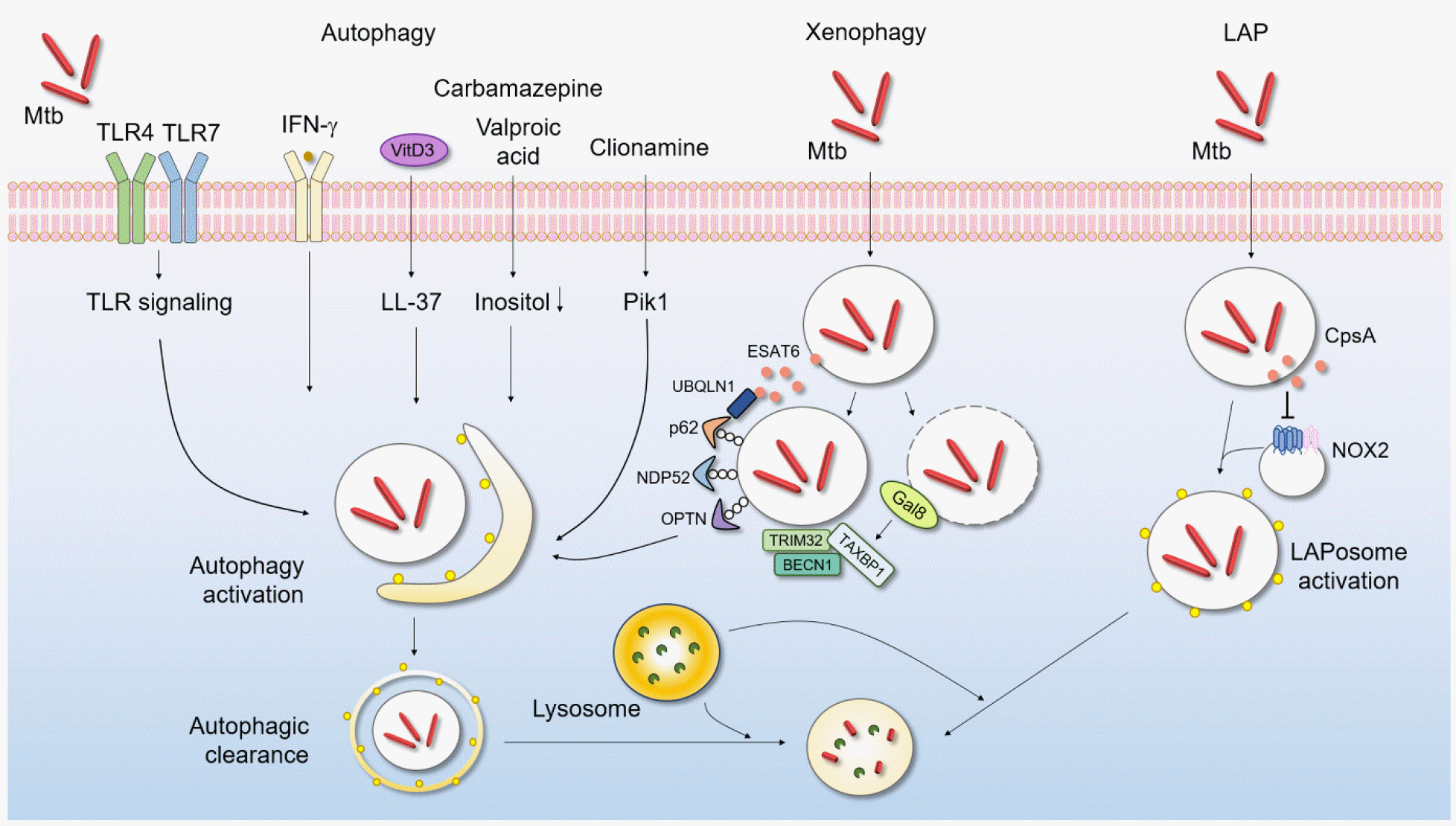
10.3390/antibiotics9010021.
35. Ayodele S, Kumar P, van Eyk A, Choonara YE. Advances in immunomodulatory strategies for host-directed therapies in combating tuberculosis.
Biomed Pharmacother. 2023;162:114588.DOI:
10.1016/j.biopha.2023.114588.
36. Kiran D, Podell BK, Chambers M, Basaraba RJ. Host-directed therapy targeting the
Mycobacterium tuberculosis granuloma: a review.
Semin Immunopathol. 2016;38(2):167-183.DOI:
10.1007/s00281-015-0537-x.
37. Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis.
Nat Rev Immunol. 2012;12(5):352-366.DOI:
10.1038/nri3211.
38. Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection.
Cell. 2009;136(1):37-49.DOI:
10.1016/j.cell.2008.11.014.
39. Weeratunga P, Moller DR, Ho LP. Immune mechanisms of granuloma formation in sarcoidosis and tuberculosis.
J Clin Invest. 2024;134(1):e175264.DOI:
10.1172/JCI175264.
40. Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos.
Immunity. 2002;17(6):693-702.DOI:
10.1016/S1074-7613(02)00475-2.
41. Belton M, Brilha S, Manavaki R, Mauri F, Nijran K, Hong YT, et al. Hypoxia and tissue destruction in pulmonary TB.
Thorax. 2016;71(12):1145-1153.DOI:
10.1136/thoraxjnl-2015-207402.
42. Rabinowitz JD, Enerback S. Lactate: the ugly duckling of energy metabolism.
Nat Metab. 2020;2(7):566-571.DOI:
10.1038/s42255-020-0243-4.
43. Emad A, Rezaian GR. Lactate dehydrogenase in bronchoalveolar lavage fluid of patients with active pulmonary tuberculosis.
Respiration. 1999;66(1):41-45.DOI:
10.1159/000029335.
44. Sharma PR, Jain S, Bamezai RN, Tiwari PK. Utility of serum LDH isoforms in the assessment of
Mycobacterium tuberculosis induced pathology in TB patients of Sahariya tribe.
Indian J Clin Biochem. 2010;25(1):57-63.DOI:
10.1007/s12291-010-0012-3.
45. Osada-Oka M, Goda N, Saiga H, Yamamoto M, Takeda K, Ozeki Y, et al. Metabolic adaptation to glycolysis is a basic defense mechanism of macrophages for
Mycobacterium tuberculosis infection.
Int Immunol. 2019;31(12):781-793.DOI:
10.1093/intimm/dxz048.
46. Elkington PT, D'Armiento JM, Friedland JS. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction.
Sci Transl Med. 2011;3(71):71ps6.DOI:
10.1126/scitranslmed.3001847.
47. Li Y, Wang Y, Liu X. The role of airway epithelial cells in response to mycobacteria infection.
Clin Dev Immunol. 2012;2012:791392.DOI:
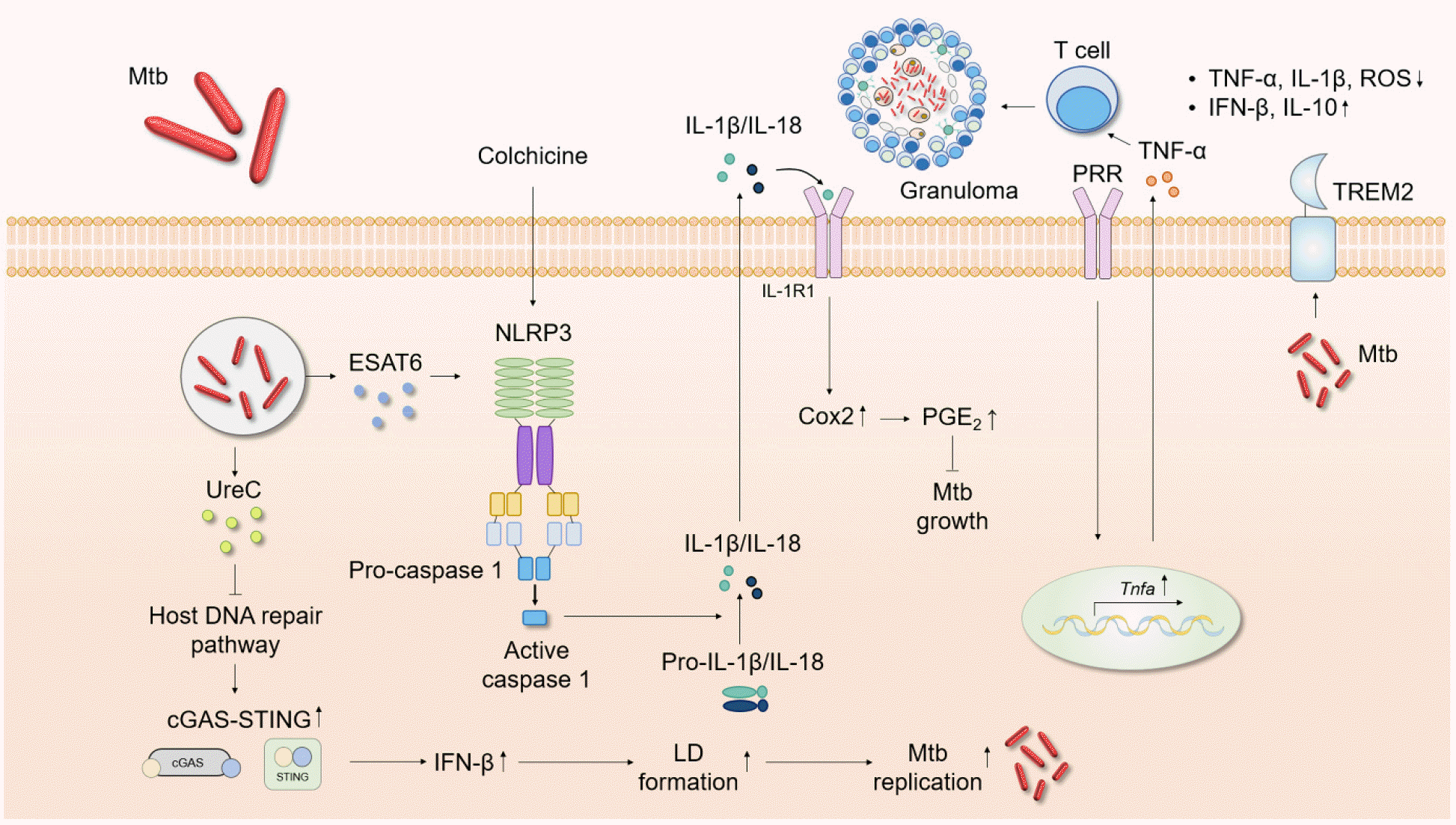
10.1155/2012/791392.
48. Ghio AJ, Carter JD, Dailey LA, Devlin RB, Samet JM. Respiratory epithelial cells demonstrate lactoferrin receptors that increase after metal exposure.
Am J Physiol. 1999;276(6):L933-40.DOI:
10.1152/ajplung.1999.276.6.L933.
49. Doss M, White MR, Tecle T, Hartshorn KL. Human defensins and LL-37 in mucosal immunity.
J Leukoc Biol. 2010;87(1):79-92.DOI:
10.1189/jlb.0609382.
50. Elkington PT, Emerson JE, Lopez-Pascua LD, O'Kane CM, Horncastle DE, Boyle JJ, et al.
Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch.
J Immunol. 2005;175(8):5333-5340.DOI:
10.4049/jimmunol.175.8.5333.
51. Hiemstra PS. Epithelial antimicrobial peptides and proteins: their role in host defence and inflammation.
Paediatr Respir Rev. 2001;2(4):306-310.DOI:
10.1053/prrv.2001.0165.
52. Lutay N, Hakansson G, Alaridah N, Hallgren O, Westergren-Thorsson G, Godaly G. Mycobacteria bypass mucosal NF-kB signalling to induce an epithelial anti-inflammatory IL-22 and IL-10 response.
PLoS One. 2014;9(1):e86466.DOI:
10.1371/journal.pone.0086466.
53. Kinhikar AG, Verma I, Chandra D, Singh KK, Weldingh K, Andersen P, et al. Potential role for ESAT6 in dissemination of
M. tuberculosis via human lung epithelial cells.
Mol Microbiol. 2010;75(1):92-106.DOI:
10.1111/j.1365-2958.2009.06959.x.
54. Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells--conditional innate immune cells.
J Hematol Oncol. 2013;6:61.DOI:
10.1186/1756-8722-6-61.
55. Baltierra-Uribe SL, Garcia-Vasquez Mde J, Castrejon-Jimenez NS, Estrella-Pinon MP, Luna-Herrera J, Garcia-Perez BE. Mycobacteria entry and trafficking into endothelial cells.
Can J Microbiol. 2014;60(9):569-577.DOI:
10.1139/cjm-2014-0087.
56. Garcia-Perez BE, Villagomez-Palatto DA, Castaneda-Sanchez JI, Coral-Vazquez RM, Ramirez-Sanchez I, Ordonez-Razo RM, et al. Innate response of human endothelial cells infected with mycobacteria.
Immunobiology. 2011;216(8):925-935.DOI:
10.1016/j.imbio.2011.01.004.
57. Lerner TR, Queval CJ, Fearns A, Repnik U, Griffiths G, Gutierrez MG. Phthiocerol dimycocerosates promote access to the cytosol and intracellular burden of
Mycobacterium tuberculosis in lymphatic endothelial cells.
BMC Biol. 2018;16(1):1.DOI:
10.1186/s12915-017-0471-6.
59. Polena H, Boudou F, Tilleul S, Dubois-Colas N, Lecointe C, Rakotosamimanana N, et al.
Mycobacterium tuberculosis exploits the formation of new blood vessels for its dissemination.
Sci Rep. 2016;6:33162.DOI:
10.1038/srep33162.
60. Saghazadeh A, Rezaei N. Vascular endothelial growth factor levels in tuberculosis: A systematic review and meta-analysis.
PLoS One. 2022;17(5):e0268543.DOI:
10.1371/journal.pone.0268543.
61. Matsuyama W, Hashiguchi T, Matsumuro K, Iwami F, Hirotsu Y, Kawabata M, et al. Increased serum level of vascular endothelial growth factor in pulmonary tuberculosis.
Am J Respir Crit Care Med. 2000;162(3 Pt 1):1120-1122.DOI:
10.1164/ajrccm.162.3.9911010.
62. Alatas F, Alatas O, Metintas M, Ozarslan A, Erginel S, Yildirim H. Vascular endothelial growth factor levels in active pulmonary tuberculosis.
Chest. 2004;125(6):2156-2159.DOI:
10.1378/chest.125.6.2156.
63. Datta M, Via LE, Kamoun WS, Liu C, Chen W, Seano G, et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery.
Proc Natl Acad Sci U S A. 2015;112(6):1827-1832.DOI:
10.1073/pnas.1424563112.
64. Harding J, Ritter A, Rayasam A, Fabry Z, Sandor M. Lymphangiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen.
Am J Pathol. 2015;185(2):432-445.DOI:
10.1016/j.ajpath.2014.09.020.
65. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors.
Nature. 2015;518(7540):547-551.DOI:
10.1038/nature13989.
66. Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages.
Immunity. 2015;42(4):665-678.DOI:
10.1016/j.immuni.2015.03.011.
67. Aegerter H, Lambrecht BN, Jakubzick CV. Biology of lung macrophages in health and disease.
Immunity. 2022;55(9):1564-1580.DOI:
10.1016/j.immuni.2022.08.010.
68. Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of
Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny.
J Exp Med. 2018;215(4):1135-1152.DOI:
10.1084/jem.20172020.
69. Gordon S. Alternative activation of macrophages.
Nat Rev Immunol. 2003;3(1):23-35.DOI:
10.1038/nri978.
70. Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, et al. Alveolar macrophages provide an early
Mycobacterium tuberculosis niche and initiate dissemination.
Cell Host Microbe. 2018;24(3):439-446. e4.DOI:
10.1016/j.chom.2018.08.001.
71. Redente EF, Higgins DM, Dwyer-Nield LD, Orme IM, Gonzalez-Juarrero M, Malkinson AM. Differential polarization of alveolar macrophages and bone marrow-derived monocytes following chemically and pathogen-induced chronic lung inflammation.
J Leukoc Biol. 2010;88(1):159-168.DOI:
10.1189/jlb.0609378.
72. Blumenthal A, Kobayashi T, Pierini LM, Banaei N, Ernst JD, Miyake K, et al. RP105 facilitates macrophage activation by
Mycobacterium tuberculosis lipoproteins.
Cell Host Microbe. 2009;5(1):35-46.DOI:
10.1016/j.chom.2008.12.002.
73. Sha S, Shi Y, Tang Y, Jia L, Han X, Liu Y, et al.
Mycobacterium tuberculosis Rv1987 protein induces M2 polarization of macrophages through activating the PI3K/Akt1/mTOR signaling pathway.
Immunol Cell Biol. 2021;99(6):570-585.DOI:
10.1111/imcb.12436.
74. Gong Z, Han S, Liang T, Zhang H, Sun Q, Pan H, et al.
Mycobacterium tuberculosis effector PPE36 attenuates host cytokine storm damage via inhibiting macrophage M1 polarization.
J Cell Physiol. 2021;236(11):7405-7420.DOI:
10.1002/jcp.30411.
75. Guo Q, Bi J, Li M, Ge W, Xu Y, Fan W, et al. ESX secretion-associated protein C from
Mycobacterium tuberculosis induces macrophage activation through the toll-like receptor-4/mitogen-activated protein kinase signaling pathway.
Front Cell Infect Microbiol. 2019;9:158.DOI:
10.3389/fcimb.2019.00158.
76. Ahmad F, Rani A, Alam A, Zarin S, Pandey S, Singh H, et al. Macrophage: a cell with many faces and functions in Tuberculosis.
Front Immunol. 2022;13:747799.DOI:
10.3389/fimmu.2022.747799.
77. Marino S, Cilfone NA, Mattila JT, Linderman JJ, Flynn JL, Kirschner DE. Macrophage polarization drives granuloma outcome during
Mycobacterium tuberculosis infection.
Infect Immun. 2015;83(1):324-338.DOI:
10.1128/IAI.02494-14.
78. Bo H, Moure UAE, Yang Y, Pan J, Li L, Wang M, et al.
Mycobacterium tuberculosis-macrophage interaction: molecular updates.
Front Cell Infect Microbiol. 2023;13:1062963.DOI:
10.3389/fcimb.2023.1062963.
79. Songane M, Kleinnijenhuis J, Netea MG, van Crevel R. The role of autophagy in host defence against
Mycobacterium tuberculosis infection.
Tuberculosis (Edinb). 2012;92(5):388-396.DOI:
10.1016/j.tube.2012.05.004.
80. Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis.
Yonsei Med J. 2009;50(1):1-11.DOI:
10.3349/ymj.2009.50.1.1.
81. Feng Y, Li M, Yangzhong X, Zhang X, Zu A, Hou Y, et al. Pyroptosis in inflammation-related respiratory disease.
J Physiol Biochem. 2022;78(4):721-737.DOI:
10.1007/s13105-022-00909-1.
82. Shin DM, Jeon BY, Lee HM, Jin HS, Yuk JM, Song CH, et al.
Mycobacterium tuberculosis Eis regulates autophagy, inflammation, and cell death through redox-dependent signaling.
PLoS Pathog. 2010;6(12):e1001230.DOI:
10.1371/journal.ppat.1001230.
83. Duan L, Yi M, Chen J, Li S, Chen W.
Mycobacterium tuberculosis eis gene inhibits macrophage autophagy through up-regulation of IL-10 by increasing the acetylation of histone H3.
Biochem Biophys Res Commun. 2016;473(4):1229-1234.DOI:
10.1016/j.bbrc.2016.04.045.
84. Singh A, Crossman DK, Mai D, Guidry L, Voskuil MI, Renfrow MB, et al.
Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response.
PLoS Pathog. 2009;5(8):e1000545.DOI:
10.1371/journal.ppat.1000545.
85. Moideen K, Kumar NP, Nair D, Banurekha VV, Bethunaickan R, Babu S. Heightened systemic levels of neutrophil and eosinophil granular proteins in pulmonary tuberculosis and reversal following treatment.
Infect Immun. 2018;86(6):e00008-18.DOI:
10.1128/IAI.00008-18.
86. Monteith AJ, Miller JM, Maxwell CN, Chazin WJ, Skaar EP. Neutrophil extracellular traps enhance macrophage killing of bacterial pathogens.
Sci Adv. 2021;7(37):eabj2101.DOI:
10.1126/sciadv.abj2101.
87. Blomgran R, Desvignes L, Briken V, Ernst JD.
Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells.
Cell Host Microbe. 2012;11(1):81-90.DOI:
10.1016/j.chom.2011.11.012.
88. Corleis B, Korbel D, Wilson R, Bylund J, Chee R, Schaible UE. Escape of
Mycobacterium tuberculosis from oxidative killing by neutrophils.
Cell Microbiol. 2012;14(7):1109-1121.DOI:
10.1111/j.1462-5822.2012.01783.x.
89. Repasy T, Martinez N, Lee J, West K, Li W, Kornfeld H. Bacillary replication and macrophage necrosis are determinants of neutrophil recruitment in tuberculosis.
Microbes Infect. 2015;17(8):564-574.DOI:
10.1016/j.micinf.2015.03.013.
90. Dallenga T, Repnik U, Corleis B, Eich J, Reimer R, Griffiths GW, et al.
M. tuberculosis-induced necrosis of infected neutrophils promotes bacterial growth following phagocytosis by macrophages.
Cell Host Microbe. 2017;22(4):519-530. e3.DOI:
10.1016/j.chom.2017.09.003.
91. Filio-Rodriguez G, Estrada-Garcia I, Arce-Paredes P, Moreno-Altamirano MM, Islas-Trujillo S, Ponce-Regalado MD, et al.
In vivo induction of neutrophil extracellular traps by
Mycobacterium tuberculosis in a guinea pig model.
Innate Immun. 2017;23(7):625-637.DOI:
10.1177/1753425917732406.
92. Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J 3rd, Fae KC, et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis.
J Clin Invest. 2014;124(3):1268-1282.DOI:
10.1172/JCI72030.
93. Kim TS, Jin YB, Kim YS, Kim S, Kim JK, Lee HM, et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions.
Autophagy. 2019;15(8):1356-1375.DOI:
10.1080/15548627.2019.1582743.
94. Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function.
J Exp Med. 2003;197(1):7-17.DOI:
10.1084/jem.20021229.
95. Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program.
J Immunol. 2003;171(9):4552-60.DOI:
10.4049/jimmunol.171.9.4552.
96. Magallanes-Puebla A, Espinosa-Cueto P, Lopez-Marin LM, Mancilla R. Mycobacterial glycolipid Di-O-acyl trehalose promotes a tolerogenic profile in dendritic cells.
PLoS One. 2018;13(12):e0207202.DOI:
10.1371/journal.pone.0207202.
97. Sondergaard JN, Laursen JM, Rosholm LB, Brix S.
Mycobacterium tuberculosis promotes Th17 expansion via regulation of human dendritic cells toward a high CD14 and low IL-12p70 phenotype that reprograms upon exogenous IFN-gamma.
Int Immunol. 2014;26(12):705-716.DOI:
10.1093/intimm/dxu085.
98. Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis.
Nat Rev Immunol. 2017;17(11):691-702.DOI:
10.1038/nri.2017.69.
99. Ulrichs T, Kosmiadi GA, Jorg S, Pradl L, Titukhina M, Mishenko V, et al. Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma.
J Infect Dis. 2005;192(1)):89-97.DOI:
10.1086/430621.
100. Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis.
J Immunol. 1999;162(9):5407-5416.DOI:
10.4049/jimmunol.162.9.5407.
101. Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against
Mycobacterium tuberculosis in mice.
Immunity. 1995;2(6):561-572.DOI:
10.1016/1074-7613(95)90001-2.
102. Ansari AW, Kamarulzaman A, Schmidt RE. Multifaceted impact of host C-C chemokine CCL2 in the immuno-pathogenesis of HIV-1/
M. tuberculosis co-infection. Front Immunol. 2013;4:312.DOI:
10.3389/fimmu.2013.00312.
103. Diedrich CR, O'Hern J, Wilkinson RJ. HIV-1 and the
Mycobacterium tuberculosis granuloma: A systematic review and meta-analysis.
Tuberculosis (Edinb). 2016;98:62-76.DOI:
10.1016/j.tube.2016.02.010.
104. Lu YJ, Barreira-Silva P, Boyce S, Powers J, Cavallo K, Behar SM. CD4 T cell help prevents CD8 T cell exhaustion and promotes control of
Mycobacterium tuberculosis infection.
Cell Rep. 2021;36(11):109696.DOI:
10.1016/j.celrep.2021.109696.
105. Serbina NV, Liu CC, Scanga CA, Flynn JL. CD8+ CTL from lungs of
Mycobacterium tuberculosis-infected mice express perforin
in vivo and lyse infected macrophages.
J Immunol. 2000;165(1):353-363.DOI:
10.4049/jimmunol.165.1.353.
106. Chavez-Galan L, Illescas-Eugenio J, Alvarez-Sekely M, Baez-Saldana R, Chavez R, Lascurain R. Tuberculosis patients display a high proportion of CD8(+) T cells with a high cytotoxic potential.
Microbiol Immunol. 2019;63(8):316-327.DOI:
10.1111/1348-0421.12724.
107. Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis.
J Exp Med. 2010;207(7):1409-1420.DOI:
10.1084/jem.20091885.
108. Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, et al. Cutting edge: regulatory T cells prevent efficient clearance of
Mycobacterium tuberculosis.
J Immunol. 2007;178(5):2661-2665.DOI:
10.4049/jimmunol.178.5.2661.
109. Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, et al. Expansion and function of
Foxp3-expressing T regulatory cells during tuberculosis.
J Exp Med. 2007;204(9):2159-2169.DOI:
10.1084/jem.20062105.
110. Irvine EB, O'Neil A, Darrah PA, Shin S, Choudhary A, Li W, et al. Robust IgM responses following intravenous vaccination with Bacille Calmette-Guerin associate with prevention of
Mycobacterium tuberculosis infection in macaques.
Nat Immunol. 2021;22(12):1515-1523.DOI:
10.1038/s41590-021-01066-1.
111. Watson A, Li H, Ma B, Weiss R, Bendayan D, Abramovitz L, et al. Human antibodies targeting a Mycobacterium transporter protein mediate protection against tuberculosis.
Nat Commun. 2021;12(1):602.DOI:
10.1038/s41467-021-20930-0.
112. Klionsky DJ. The molecular machinery of autophagy: unanswered questions.
J Cell Sci. 2005;118(Pt 1):7-18.DOI:
10.1242/jcs.01620.
113. Feng Y, Yao Z, Klionsky DJ. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy.
Trends Cell Biol. 2015;25(6):354-363.DOI:
10.1016/j.tcb.2015.02.002.
114. Yamamoto H, Zhang S, Mizushima N. Autophagy genes in biology and disease.
Nat Rev Genet. 2023;24(6):382-400.DOI:
10.1038/s41576-022-00562-w.
115. Kumar S, Jain A, Choi SW, Peixoto Duarte da Silva G, Allers L, Mudd MH, et al. Mammalian Atg8-family proteins are upstream regulators of the lysosomalsystem by controlling MTOR and TFEB.
Autophagy. 2020;16(12):2305-2306.DOI:
10.1080/15548627.2020.1837423.
116. Kim YS, Silwal P, Kim SY, Yoshimori T, Jo EK. Autophagy-activating strategies to promote innate defense against mycobacteria.
Exp Mol Med. 2019;51(12):1-10.DOI:
10.1038/s12276-019-0290-7.
117. Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and
Mycobacterium tuberculosis survival in infected macrophages.
Cell. 2004;119(6):753-766.DOI:
10.1016/j.cell.2004.11.038.
118. Delgado M, Singh S, De Haro S, Master S, Ponpuak M, Dinkins C, et al. Autophagy and pattern recognition receptors in innate immunity.
Immunol Rev. 2009;227(1):189-202.DOI:
10.1111/j.1600-065X.2008.00725.x.
119. Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity.
Immunity. 2007;27(1):135-144.DOI:
10.1016/j.immuni.2007.05.022.
120. Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells.
Science. 2007;315(5817):1398-1401.DOI:
10.1126/science.1136880.
121. Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy.
EMBO J. 2008;27(7):1110-1121.DOI:
10.1038/emboj.2008.31.
122. Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin.
Cell Host Microbe. 2009;6(3):231-243.DOI:
10.1016/j.chom.2009.08.004.
123. Schiebler M, Brown K, Hegyi K, Newton SM, Renna M, Hepburn L, et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of
Mycobacterium tuberculosis through inositol depletion.
EMBO Mol Med. 2015;7(2):127-139.DOI:
10.15252/emmm.201404137.
124. Persaud R, Li SC, Chao JD, Forestieri R, Donohue E, Balgi AD, et al. Clionamines stimulate autophagy, inhibit
Mycobacterium tuberculosis survival in macrophages, and target Pik1.
Cell Chem Biol. 2022;29(5):870-882. e11.DOI:
10.1016/j.chembiol.2021.07.017.
125. Khaminets A, Behl C, Dikic I. Ubiquitin-dependent and independent signals in selective autophagy.
Trends Cell Biol. 2016;26(1):6-16.DOI:
10.1016/j.tcb.2015.08.010.
126. McEwan DG. Host-pathogen interactions and subversion of autophagy.
Essays Biochem. 2017;61(6):687-697.DOI:
10.1042/EBC20170058.
127. Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, et al. Phosphorylation of the autophagy receptor optineurin restricts
Salmonella growth.
Science. 2011;333(6039):228-233.DOI:
10.1126/science.1205405.
128. Jo EK, Yuk JM, Shin DM, Sasakawa C. Roles of autophagy in elimination of intracellular bacterial pathogens.
Front Immunol. 2013;4:97.DOI:
10.3389/fimmu.2013.00097.
129. Castrejon-Jimenez NS, Leyva-Paredes K, Hernandez-Gonzalez JC, Luna-Herrera J, Garcia-Perez BE. The role of autophagy in bacterial infections.
Biosci Trends. 2015;9(3):149-159.DOI:
10.5582/bst.2015.01035.
130. Bell SL, Lopez KL, Cox JS, Patrick KL, Watson RO. Galectin-8 senses phagosomal damage and recruits selective autophagy adapter TAX1BP1 to control
Mycobacterium tuberculosis infection in macrophages.
mBio. 2021;12(4):e0187120.DOI:
10.1128/mBio.01871-20.
131. Sakowski ET, Koster S, Portal Celhay C, Park HS, Shrestha E, Hetzenecker SE, et al. Ubiquilin 1 promotes IFN-gamma-induced xenophagy of
Mycobacterium tuberculosis.
PLoS Pathog. 2015;11(7):e1005076.DOI:
10.1371/journal.ppat.1005076.
132. Romagnoli A, Di Rienzo M, Petruccioli E, Fusco C, Palucci I, Micale L, et al. The ubiquitin ligase TRIM32 promotes the autophagic response to
Mycobacterium tuberculosis infection in macrophages.
Cell Death Dis. 2023;14(8):505.DOI:
10.1038/s41419-023-06026-1.
133. Lee YJ, Kim JK, Jung CH, Kim YJ, Jung EJ, Lee SH, et al. Chemical modulation of SQSTM1/p62-mediated xenophagy that targets a broad range of pathogenic bacteria.
Autophagy. 2022;18(12):2926-2945.DOI:
10.1080/15548627.2022.2054240.
134. Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide.
Cell Death Differ. 2005;12 Suppl 2:1535-1541.DOI:
10.1038/sj.cdd.4401728.
135. Pena-Martinez C, Rickman AD, Heckmann BL. Beyond autophagy: LC3-associated phagocytosis and endocytosis.
Sci Adv. 2022;8(43):eabn1702.DOI:
10.1126/sciadv.abn1702.
136. Durgan J, Lystad AH, Sloan K, Carlsson SR, Wilson MI, Marcassa E, et al. Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine.
Mol Cell. 2021;81(9):2031-2040. e8.DOI:
10.1016/j.molcel.2021.03.020.
137. Heckmann BL, Green DR. LC3-associated phagocytosis at a glance.
J Cell Sci. 2019;132(5):jcs222984.DOI:
10.1242/jcs.222984.
138. Koster S, Upadhyay S, Chandra P, Papavinasasundaram K, Yang G, Hassan A, et al.
Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA.
Proc Natl Acad Sci U S A. 2017;114(41):E8711-E8720.DOI:
10.1073/pnas.1707792114.
139. Ge P, Lei Z, Yu Y, Lu Z, Qiang L, Chai Q, et al.
M. tuberculosis PknG manipulates host autophagy flux to promote pathogen intracellular survival.
Autophagy. 2022;18(3):576-594.DOI:
10.1080/15548627.2021.1938912.
140. Bedard M, van der Niet S, Bernard EM, Babunovic G, Cheng TY, Aylan B, et al. A terpene nucleoside from
M. tuberculosis induces lysosomal lipid storage in foamy macrophages.
J Clin Invest. 2023;133(6):e161944.DOI:
10.1172/JCI161944.
141. Paik S, Kim KT, Kim IS, Kim YJ, Kim HJ, Choi S, et al. Mycobacterial acyl carrier protein suppresses TFEB activation and upregulates miR-155 to inhibit host defense.
Front Immunol. 2022;13:946929.DOI:
10.3389/fimmu.2022.946929.
142. Strong EJ, Wang J, Ng TW, Porcelli SA, Lee S.
Mycobacterium tuberculosis PPE51 inhibits autophagy by suppressing Toll-like receptor 2-dependent signaling.
mBio. 2022;13(3):e0297421.DOI:
10.1128/mbio.02974-21.
143. Strong EJ, Ng TW, Porcelli SA, Lee S.
Mycobacterium tuberculosis PE_PGRS20 and PE_PGRS47 proteins inhibit autophagy by interaction with Rab1A.
mSphere. 2021;6(4):e0054921.DOI:
10.1128/mSphere.00549-21.
144. Garcia-Bengoa M, Meurer M, Goethe R, Singh M, Reljic R, von Kockritz-Blickwede M. Role of phagocyte extracellular traps during
Mycobacterium tuberculosis infections and tuberculosis disease processes.
Front Microbiol. 2023;14:983299.DOI:
10.3389/fmicb.2023.983299.
145. Diatlova A, Linkova N, Lavrova A, Zinchenko Y, Medvedev D, Krasichkov A, et al. Molecular markers of rarly immune response in tuberculosis: prospects of application in predictive medicine.
Int J Mol Sci. 2023;24(17):13261.DOI:
10.3390/ijms241713261.
146. Ashenafi S, Loreti MG, Bekele A, Aseffa G, Amogne W, Kassa E, et al. Inflammatory immune profiles associated with disease severity in pulmonary tuberculosis patients with moderate to severe clinical TB or anemia.
Front Immunol. 2023;14:1296501.DOI:
10.3389/fimmu.2023.1296501.
147. Sudbury EL, Clifford V, Messina NL, Song R, Curtis N.
Mycobacterium tuberculosis-specific cytokine biomarkers to differentiate active TB and LTBI: A systematic review.
J Infect. 2020;81(6):873-881.DOI:
10.1016/j.jinf.2020.09.032.
148. Dorhoi A, Kaufmann SH. Tumor necrosis factor alpha in mycobacterial infection.
Semin Immunol. 2014;26(3):203-209.DOI:
10.1016/j.smim.2014.04.003.
149. Ray JC, Flynn JL, Kirschner DE. Synergy between individual TNF-dependent functions determines granuloma performance for controlling
Mycobacterium tuberculosis infection.
J Immunol. 2009;182(6):3706-3717.DOI:
10.4049/jimmunol.0802297.
150. Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection.
J Immunol. 2002;168(9):4620-4627.DOI:
10.4049/jimmunol.168.9.4620.
151. Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD, Group B. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report.
Arthritis Rheum. 2003;48(8):2122-2127.DOI:
10.1002/art.11137.
152. Francisco NM, Hsu NJ, Keeton R, Randall P, Sebesho B, Allie N, et al. TNF-dependent regulation and activation of innate immune cells are essential for host protection against cerebral tuberculosis.
J Neuroinflammation. 2015;12:125.DOI:
10.1186/s12974-015-0345-1.
153. Vanden Driessche K, Persson A, Marais BJ, Fink PJ, Urdahl KB. Immune vulnerability of infants to tuberculosis.
Clin Dev Immunol. 2013;2013:781320.DOI:
10.1155/2013/781320.
154. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics.
Nat Rev Immunol. 2019;19(8):477-489.DOI:
10.1038/s41577-019-0165-0.
155. Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, et al.
Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome.
Cell Microbiol. 2010;12(8):1046-1063.DOI:
10.1111/j.1462-5822.2010.01450.x.
156. Amaral EP, Riteau N, Moayeri M, Maier N, Mayer-Barber KD, Pereira RM, et al. Lysosomal cathepsin release is required for NLRP3-inflammasome activation by
Mycobacterium tuberculosis in infected macrophages.
Front Immunol. 2018;9:1427.DOI:
10.3389/fimmu.2018.01427.
157. Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection.
Cell Microbiol. 2008;10(9):1866-1878.DOI:
10.1111/j.1462-5822.2008.01177.x.
158. Silverio D, Goncalves R, Appelberg R, Saraiva M. Advances on the role and applications of interleukin-1 in tuberculosis.
mBio. 2021;12(6):e0313421.DOI:
10.1128/mBio.03134-21.
159. Winchell CG, Mishra BB, Phuah JY, Saqib M, Nelson SJ, Maiello P, et al. Evaluation of IL-1 blockade as an adjunct to linezolid therapy for tuberculosis in mice and macaques.
Front Immunol. 2020;11:891.DOI:
10.3389/fimmu.2020.00891.
160. Shah S, Bohsali A, Ahlbrand SE, Srinivasan L, Rathinam VA, Vogel SN, et al. Cutting edge:
Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-beta and AIM2 inflammasome-dependent IL-1beta production via its ESX-1 secretion system.
J Immunol. 2013;191(7):3514-3518.DOI:
10.4049/jimmunol.1301331.
161. Wong KW, Jacobs WR Jr. Critical role for NLRP3 in necrotic death triggered by
Mycobacterium tuberculosis.
Cell Microbiol. 2011;13(9):1371-1384.DOI:
10.1111/j.1462-5822.2011.01625.x.
162. Eklund D, Welin A, Andersson H, Verma D, Soderkvist P, Stendahl O, et al. Human gene variants linked to enhanced NLRP3 activity limit intramacrophage growth of
Mycobacterium tuberculosis.
J Infect Dis. 2014;209(5):749-753.DOI:
10.1093/infdis/jit572.
163. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases.
Nat Rev Drug Discov. 2012;11(8):633-652.DOI:
10.1038/nrd3800.
164. Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans.
Semin Immunol. 2013;25(6):469-484.DOI:
10.1016/j.smim.2013.10.008.
165. Panda V, Ashar H, Srinath S. Antioxidant and hepatoprotective effect of Garcinia indica fruit rind in ethanol-induced hepatic damage in rodents.
Interdiscip Toxicol. 2012;5(4):207-213.DOI:
10.2478/v10102-012-0034-1.
166. Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis.
Clin Infect Dis. 2006;43(6):717-722.DOI:
10.1086/506935.
167. Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, et al. Caspase-1 independent IL-1beta production is critical for host resistance to
mycobacterium tuberculosis and does not require TLR signaling
in vivo.
J Immunol. 2010;184(7):3326-3330.DOI:
10.4049/jimmunol.0904189.
168. Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice.
Lab Invest. 2000;80(5):759-767.DOI:
10.1038/labinvest.3780079.
169. Sugawara I, Yamada H, Hua S, Mizuno S. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection.
Microbiol Immunol. 2001;45(11):743-750.DOI:
10.1111/j.1348-0421.2001.tb01310.x.
170. Bohrer AC, Tocheny C, Assmann M, Ganusov VV, Mayer-Barber KD. Cutting Edge: IL-1R1 mediates host resistance to
Mycobacterium tuberculosis by trans-protection of infected cells.
J Immunol. 2018;201(6):1645-1650.DOI:
10.4049/jimmunol.1800438.
171. Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to
Mycobacterium tuberculosis infection.
J Immunol. 2007;179(2):1178-1189.DOI:
10.4049/jimmunol.179.2.1178.
172. Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk.
Nature. 2014;511(7507):99-103.DOI:
10.1038/nature13489.
173. Kwon KW, Kim LH, Kang SM, Lee JM, Choi E, Park J, et al. Host-directed anti-mycobacterial activity of colchicine, an anti-gout drug, via strengthened host innate resistance reinforced by the IL-1beta/PGE
2 axis.
Br J Pharmacol. 2022;179(15):3951-3969.DOI:
10.1111/bph.15838.
174. Dorhoi A, Nouailles G, Jorg S, Hagens K, Heinemann E, Pradl L, et al. Activation of the NLRP3 inflammasome by
Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis.
Eur J Immunol. 2012;42(2):374-384.DOI:
10.1002/eji.201141548.
175. Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system.
Cytokine. 2014;70(1):11-20.DOI:
10.1016/j.cyto.2014.05.024.
176. Gabay C. Interleukin-6 and chronic inflammation.
Arthritis Res Ther. 2006;8 Suppl 2 (Suppl 2):S3.DOI:
10.1186/ar1917.
177. Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications.
Nat Rev Rheumatol. 2017;13(7):399-409.DOI:
10.1038/nrrheum.2017.83.
178. Gupte AN, Kumar P, Araujo-Pereira M, Kulkarni V, Paradkar M, Pradhan N, et al. Baseline IL-6 is a biomarker for unfavourable tuberculosis treatment outcomes: a multisite discovery and validation study.
Eur Respir J. 2022;59(4):2100905.DOI:
10.1183/13993003.00905-2021.
179. Chowdhury IH, Choudhuri S, Sen A, Bhattacharya B, Ahmed AM, Hazra A, et al. Serum interleukin 6 (IL-6) as a potential biomarker of disease progression in active pulmonary tuberculosis following anti-tuberculosis drug therapy.
Mol Immunol. 2015;63(2):601-602.DOI:
10.1016/j.molimm.2014.09.006.
180. Maseko TG, Ngubane S, Letsoalo M, Rambaran S, Archary D, Samsunder N, et al. Higher plasma interleukin - 6 levels are associated with lung cavitation in drug-resistant tuberculosis.
BMC Immunol. 2023;24(1):26.DOI:
10.1186/s12865-023-00563-2.
181. Liu Y, Li X, Liu W, Liu Y, Zhong Z, Wang L, et al. IL-6 release of Rv0183 antigen-stimulated whole blood is a potential biomarker for active tuberculosis patients.
J Infect. 2018;76(4):376-382.DOI:
10.1016/j.jinf.2017.11.004.
182. Antas P, Borchert J, Ponte C, Lima J, Georg I, Bastos M, et al. Interleukin-6 and -27 as potential novel biomarkers for human pleural tuberculosis regardless of the immunological status.
Microbes Infect. 2024;26(1-2):105238.DOI:
10.1016/j.micinf.2023.105238.
183. Kruthika P. Role of IL 6 as a biomarker in the diagnosis of tuberculous meningitis - A systematic review.
Int J Mycobacteriol. 2022;11(3):229-235.DOI:
10.4103/ijmy.ijmy_101_22.
184. Boni FG, Hamdi I, Koundi LM, Shrestha K, Xie J. Cytokine storm in tuberculosis and IL-6 involvement.
Infect Genet Evol. 2022;97:105166.DOI:
10.1016/j.meegid.2021.105166.
185. Dutta RK, Kathania M, Raje M, Majumdar S. IL-6 inhibits IFN-gamma induced autophagy in
Mycobacterium tuberculosis H37Rv infected macrophages.
Int J Biochem Cell Biol. 2012;44(6):942-954.DOI:
10.1016/j.biocel.2012.02.021.
186. Jung BG, Wang X, Yi N, Ma J, Turner J, Samten B. Early secreted antigenic target of 6-kDa of
Mycobacterium tuberculosis stimulates IL-6 production by macrophages through activation of STAT3.
Sci Rep. 2017;7:40984.DOI:
10.1038/srep40984.
187. Rottenberg ME, Carow B. SOCS3 and STAT3, major controllers of the outcome of infection with
Mycobacterium tuberculosis.
Semin Immunol. 2014;26(6):518-532.DOI:
10.1016/j.smim.2014.10.004.
188. Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SH. Lethal tuberculosis in interleukin-6-deficient mutant mice.
Infect Immun. 1997;65(11):4843-4849.DOI:
10.1128/iai.65.11.4843-4849.1997.
189. Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to
Mycobacterium tuberculosis infection.
Infect Immun. 2000;68(6):3322-3326.DOI:
10.1128/IAI.68.6.3322-3326.2000.
190. Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses.
Clin Immunol. 2009;130(1):27-33.DOI:
10.1016/j.clim.2008.08.018.
191. Linge I, Tsareva A, Kondratieva E, Dyatlov A, Hidalgo J, Zvartsev R, et al. Pleiotropic effect of IL-6 produced by B-Lymphocytes during early phases of adaptive immune responses against TB infection.
Front Immunol. 2022;13:750068.DOI:
10.3389/fimmu.2022.750068.
192. Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines.
Infect Immun. 1997;65(2):623-629.DOI:
10.1128/iai.65.2.623-629.1997.
193. Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, et al. CXCR5
+ T helper cells mediate protective immunity against tuberculosis.
J Clin Invest. 2013;123(2):712-726.DOI:
10.1172/JCI65728.
194. Liu S, Guan L, Peng C, Cheng Y, Cheng H, Wang F, et al.
Mycobacterium tuberculosis suppresses host DNA repair to boost its intracellular survival.
Cell Host Microbe. 2023;31(11):1820-1836. e10.DOI:
10.1016/j.chom.2023.09.010.
195. Ding Y, Bei C, Xue Q, Niu L, Tong J, Chen Y, et al. Transcriptomic analysis of mycobacterial infected macrophages reveals a high MOI specific type I IFN signaling.
Infect Immun. 2023;91(7):e0015523.DOI:
10.1128/iai.00155-23.
196. Bobba S, Howard NC, Das S, Ahmed M, Tang L, Thirunavukkarasu S, et al.
Mycobacterium tuberculosis carrying the rifampicin drug-resistance-conferring
rpoB mutation H445Y is associated with suppressed immunity through type I interferons.
mBio. 2023;14(5):e0094623.DOI:
10.1128/mbio.00946-23.
197. Dabla A, Liang YC, Rajabalee N, Irwin C, Moonen CGJ, Willis JV, et al. TREM2 promotes immune rvasion by Mycobacterium tuberculosis in human macrophages.
mBio. 2022;13(4):e0145622.DOI:
10.1128/mbio.01456-22.
198. Naik SK, McNehlan ME, Mreyoud Y, Kinsella RL, Smirnov A, Chowdhury CS, et al. Type I IFN signaling in the absence of IRGM1 promotes
M. tuberculosis replication in immune cells by suppressing T cell responses.
bioRxiv. 2023;2023.DOI:
10.1101/2023.10.03.560720.
199. Kotov DI, Lee OV, Fattinger SA, Langner CA, Guillen JV, Peters JM, et al. Early cellular mechanisms of type I interferon-driven susceptibility to tuberculosis.
Cell. 2023;186(25):5536-5553. e22.DOI:
10.1016/j.cell.2023.11.002.
200. Moreira-Teixeira L, Mayer-Barber K, Sher A, O'Garra A. Type I interferons in tuberculosis: Foe and occasionally friend.
J Exp Med. 2018;215(5):1273-1285.DOI:
10.1084/jem.20180325.
201. Zarogoulidis P, Kioumis I, Papanas N, Manika K, Kontakiotis T, Papagianis A, et al. The effect of combination IFN-alpha-2a with usual antituberculosis chemotherapy in non-responding tuberculosis and diabetes mellitus: a case report and review of the literature.
J Chemother. 2012;24(3):173-177.DOI:
10.1179/1973947812Y.0000000005.
202. Bax HI, Freeman AF, Ding L, Hsu AP, Marciano B, Kristosturyan E, et al. Interferon alpha treatment of patients with impaired interferon gamma signaling.
J Clin Immunol. 2013;33(5):991-1001.DOI:
10.1007/s10875-013-9882-5.
203. Rivas-Santiago CE, Guerrero GG. IFN-alpha boosting of
Mycobacterium bovis Bacillus Calmette Guerin-vaccine promoted Th1 type cellular response and protection against
M. tuberculosis infection.
Biomed Res Int. 2017;2017:8796760.DOI:
10.1155/2017/8796760.
204. Groschel MI, Sayes F, Shin SJ, Frigui W, Pawlik A, Orgeur M, et al. Recombinant BCG expressing ESX-1 of
Mycobacterium marinum combines low virulence with cytosolic immune signaling and improved TB protection.
Cell Rep. 2017;18(11):2752-2765.DOI:
10.1016/j.celrep.2017.02.057.
205. McNab FW, Ewbank J, Howes A, Moreira-Teixeira L, Martirosyan A, Ghilardi N, et al. Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-gamma for production of IL-12 and bacterial killing in
Mycobacterium tuberculosis-infected macrophages.
J Immunol. 2014;193(7):3600-3612.DOI:
10.4049/jimmunol.1401088.
206. Howes A, Taubert C, Blankley S, Spink N, Wu X, Graham CM, et al. Differential production of type I IFN determines the reciprocal levels of IL-10 and proinflammatory cytokines produced by C57BL/6 and BALB/c macrophages.
J Immunol. 2016;197(7):2838-2853.DOI:
10.4049/jimmunol.1501923.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download