I. Introduction
Tissue regeneration in wound healing encompasses a coordinated and interdependent set of events involving various cells, a proper blood supply, an apt scaffold, and signaling biomolecules
1. Among them, fibroblasts and platelets play an active role in orchestrating these crucial processes
1. Fibroblasts contribute to wound contraction and remodeling via the production of extracellular matrix (ECM), and platelets play a vital role in tissue repair by releasing abundant cytokines and growth factors, which are also involved in the regulation of fibroblastic activity
2.
Platelet concentrates have been used extensively in dentistry for many years, proving valuable in diverse regenerative applications
3. Platelet-rich fibrin (PRF), an autologous platelet concentrate, aids in tissue regeneration by serving as a three-dimensional scaffold of fibrin, constituting leukocytes, macrophages, neutrophils, and platelets. In addition, PRF is a natural reservoir of factors responsible for adhesion, coagulation, and angiogenesis
4,5. PRF has been employed as a therapeutic agent in oral and maxillofacial surgeries such as exodontia, implantology, management of oro-antral communications, and cleft reconstructions and in surgical periodontics such as guided tissue/bone regeneration (GTR/GBR) and recession coverage to promote bone and soft tissue regeneration during wound healing
6-9.
Over the years, several protocols of PRF were advocated which have shown distinction in the biological properties of PRF such as stability, cellularity, cell distribution and release of growth factors. A recently introduced protocol of PRF, albumin PRF (Alb-PRF)
3, or extended PRF
10 has gained popularity due to its prolonged degradation time. In this protocol, after centrifugation of blood, the plasma devoid of platelets is collected and heated to produce denatured albumin, which is mixed with liquid PRF to form Alb-PRF. The resultant PRF membrane has a denser protein structure organization with prolonged resorption properties (4-6 months) due to the denatured albumin, while re-addition of the platelet-rich layer offers cellular and growth factor contents
3,11. To date, Alb-PRF has been employed as a barrier membrane in management of extraction sites, explanted sites, and zygomatic implant surgeries and also as a bio filler for lateral sinus augmentation, gingival recession coverage, and facial scar reduction
10.
Several studies have evaluated the effects of various biomolecules in combination with PRF to enhance its efficacy either by improving fibrin cross-linking or utilizing it as a local delivery system for biomolecules
12. Ascorbic acid (AA), i.e., vitamin C, is a powerful antioxidant that plays a role in many important processes in the body including collagen synthesis, ECM formation, immune function, cell differentiation, promotion of the growth and regeneration of stem cells, and inhibition of cellular senescence
2,13. AA is particularly important for healing of both soft and hard tissue wounds as it promotes the growth of fibroblasts, which are crucial for tissue repair. Research has demonstrated that oral vitamin C accelerates the healing process after surgery or tooth extraction
2. Combination of AA and PRF in the management of intra-bony defects has also shown significant improvement in periodontal parameters
1.
With this background, we hypothesized that the combination of Alb-PRF and AA would prove beneficial in promoting regeneration and would healing. Therefore, the aim of this in vitro trial was to evaluate the effect of AA augmented Alb-PRF (AA Alb-PRF) on wound healing activity of human gingival fibroblasts (HGFs) and to determine if it can be utilized as a biomaterial for oral and maxillofacial and periodontal surgeries.
IV. Discussion
The present in vitro trial investigated the effects of AA Alb-PRF on wound healing activity of HGFs by assessing cell viability through MTT assay and cell migration through scratch assay and transwell migration assay.
Wound healing encompasses a complex and coordinated set of cellular and biochemical events. First is activation of platelets and fibrin clot formation, resulting in hemostasis. This is followed by infiltration of neutrophils and macrophages, leading to an inflammatory phase
14. Fibroblasts then migrate and proliferate to establish a new ECM that subsequently matures
14. These processes are regulated by various chemokines and cytokines, such as platelet-derived growth factor, transforming growth factor-beta 1, vascular endothelial growth factor, interleukin-6, interleukin-1β, and tumor necrosis factor-alpha, as well as the necessary nutrients for uneventful healing
14,15.
PRF as an autologous surgical adjuvant is known to promote and accelerate soft and hard tissue wound healing and regeneration due to its potential to facilitate optimal concentrations of platelets, fibrin, growth factors, leukocytes, and macrophages
7,16. The degranulation of platelets from PRF releases supraphysiological levels of the aforementioned growth factors into the wound site to promote healing
7. The renewal of periodontal cells is also promoted by these growth factors, leading to tissue regeneration
5.
Several techniques have been noted to enhance the durability and efficacy of PRF either by additional physical (ultraviolet light, heat) or chemical methods (glucose, glutaraldehyde, etc.)
17. Association of biomaterials with albumin has demonstrated favorable modulation in the fibrin network ultrastructure and permeability, resulting in formation of fibers with increased thickness and a coarse nodular appearance
18. Earlier studies have established that heating and denaturing albumin modifies its three-dimensional structure through the creation of new hydrogen and disulfide bonds. These modifications lead to drastic changes in resorption properties and improved stability over time
11. The combination of denatured albumin and liquid PRF produces Alb-PRF, reportedly having a denser and more stable organization of the protein structure, extended resorption properties, enhanced volume stability (21 days), and an increased duration of release of cells and growth factors (up to 10 days)
3,10,11,19. The combination has demonstrated improved cellular viability and proliferation of gingival fibroblasts
11 and can be considered as an alternative for autologous PRF membranes, with longer stability and resorption time.
During wound healing, especially in the inflammatory phase, the higher level of catabolism leads to increased uptake of various micronutrients, including AA (vitamin C)
14. AA is an essential micronutrient for healing but is not synthesized naturally in the human body. Studies have shown that AA levels decrease significantly (up to 70%) at the site of injury and do not fully recover even after two weeks
20. The micronutrient helps eliminate free radicals, aids in collagen production, modulates immune cell functions, and is necessary for fibroblast proliferation and angiogenesis. Through these processes, AA is rapidly depleted and therefore needs to be supplemented
14,21.
In a clinical trial, Elbehwashy et al.
1 incorporated AA in the blood to prepare PRF for treatment of intra-osseous periodontal defects and reported a substantial improvement in periodontal parameters. The improvement was attributed to the sustained release of AA during surgical wound healing and its subsequent regenerative effects on the resident periodontal cells, in addition to PRF growth factors
1. AA at a concentration of 250 µM was employed for the preparation of AA Alb-PRF in the present study based on an
in vitro trial that reported maximum proliferation of the gingival stem cells at that concentration
13.
We hypothesized that AA Alb-PRF would increase the wound healing activity of HGFs and can be employed as a potential regenerative biomaterial in maxillofacial and periodontal surgeries. To the best of our knowledge, this is the first in vitro study examining the wound healing activity of HGFs under the influence of AA Alb-PRF.
In the present
in vitro trial, cell viability and proliferation were assessed with MTT assay. That assay spectrophotometrically measures the reduction of yellow MTT to purple-blue formazan by mitochondrial succinate dehydrogenase produced by metabolically active cells. The greater is the activity of the cells, the greater is the intensity of purple color, and the higher is the absorbance, indicating higher cell viability
22. The scratch assay inspects the ability of a particular cell line to migrate to and close a wound in a confluent monolayer of cells, simulating
in vivo cell migration, while the transwell migration assay offers the distinct advantage to analyze migration in response to a chemotactic gradient across a filter membrane
23,24.
Results of previous research have shown that the size of the PRF membranes obtained from male and female patients varies significantly (17%) due to differences in hematocrit values
25. To reduce this variability and maintain consistency in the average size of the PRF obtained, only male participants were included in the present trial.
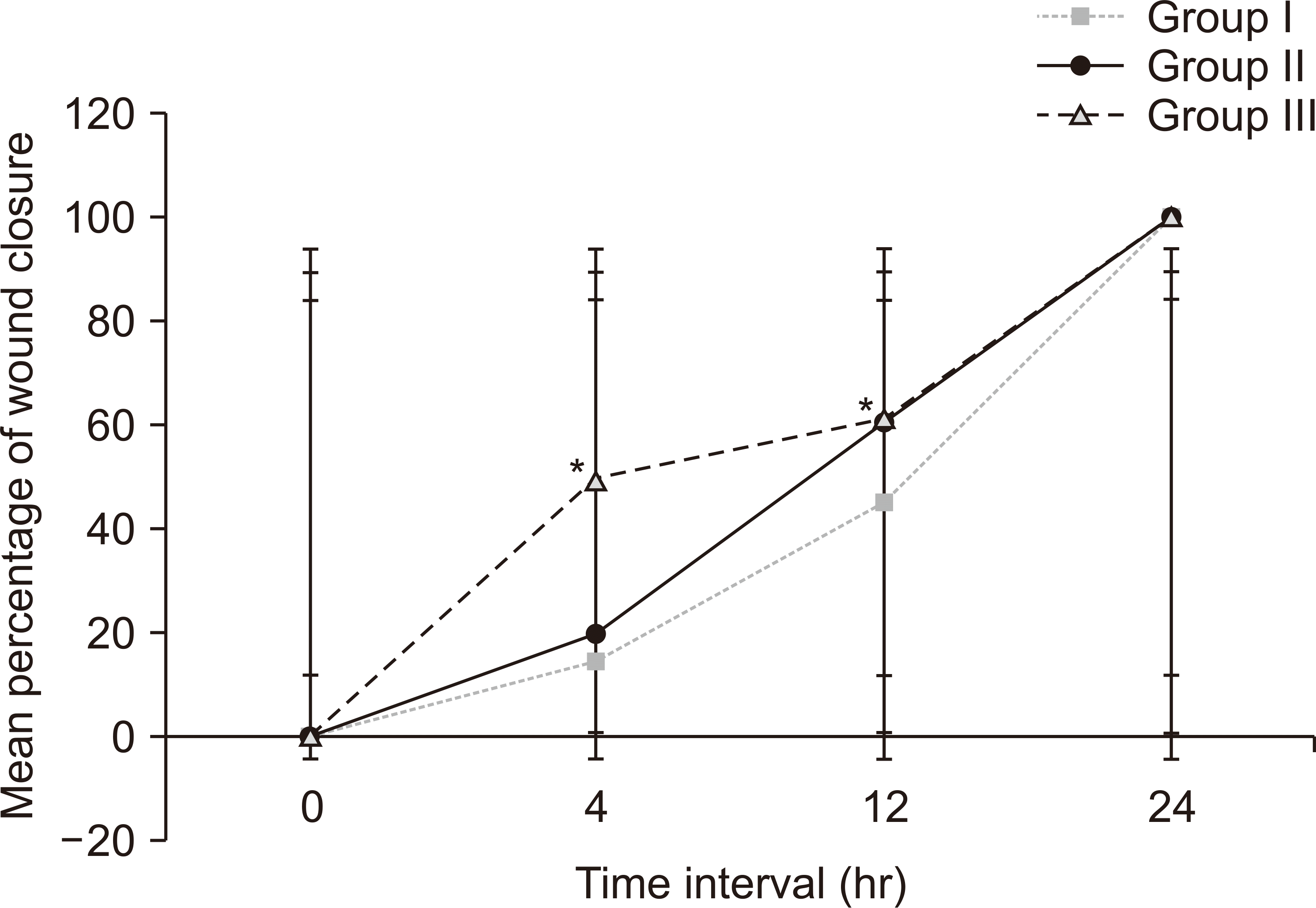
Maximum viability (128%, OD: 0.684±0.003) and fastest wound closure demonstrated by HGFs in group III could be substantiated by confluence of the stimulatory effects of AA along with growth factors released from Alb-PRF. Studies have shown that AA boosts the proliferative and regenerative potentials of gingival fibroblasts along with their ability to produce ECM and remodeling via increased expression of urokinase-type plasminogen activator, hyaluronan-mediated motility receptor, and IL-6
13. AA also redirects quiescent fibroblasts into the cell cycle and promotes migration
13. At 4 hours, 49.92% closure is suggestive of the aforementioned stimulatory effects of AA that could have resulted in accelerated initial wound closure. Our results are comparable with those of an
in vitro study by Chaitrakoonthong et al.
2, where local rinsing with 20 µg/mL AA stimulated gingival fibroblastic viability and migration. The growth factors released at the wound site can act as mitogens and chemoattractants for dermal fibroblasts and gingival and periodontal cells, increasing their proliferation and migration
26. Group II also showed increased fibroblastic viability (114%), migration, and wound closure, accredited to the mitogenic activity of growth factors released by Alb-PRF. This is comparable to an
in vitro trial by Fujioka-Kobayashi et al.
3.
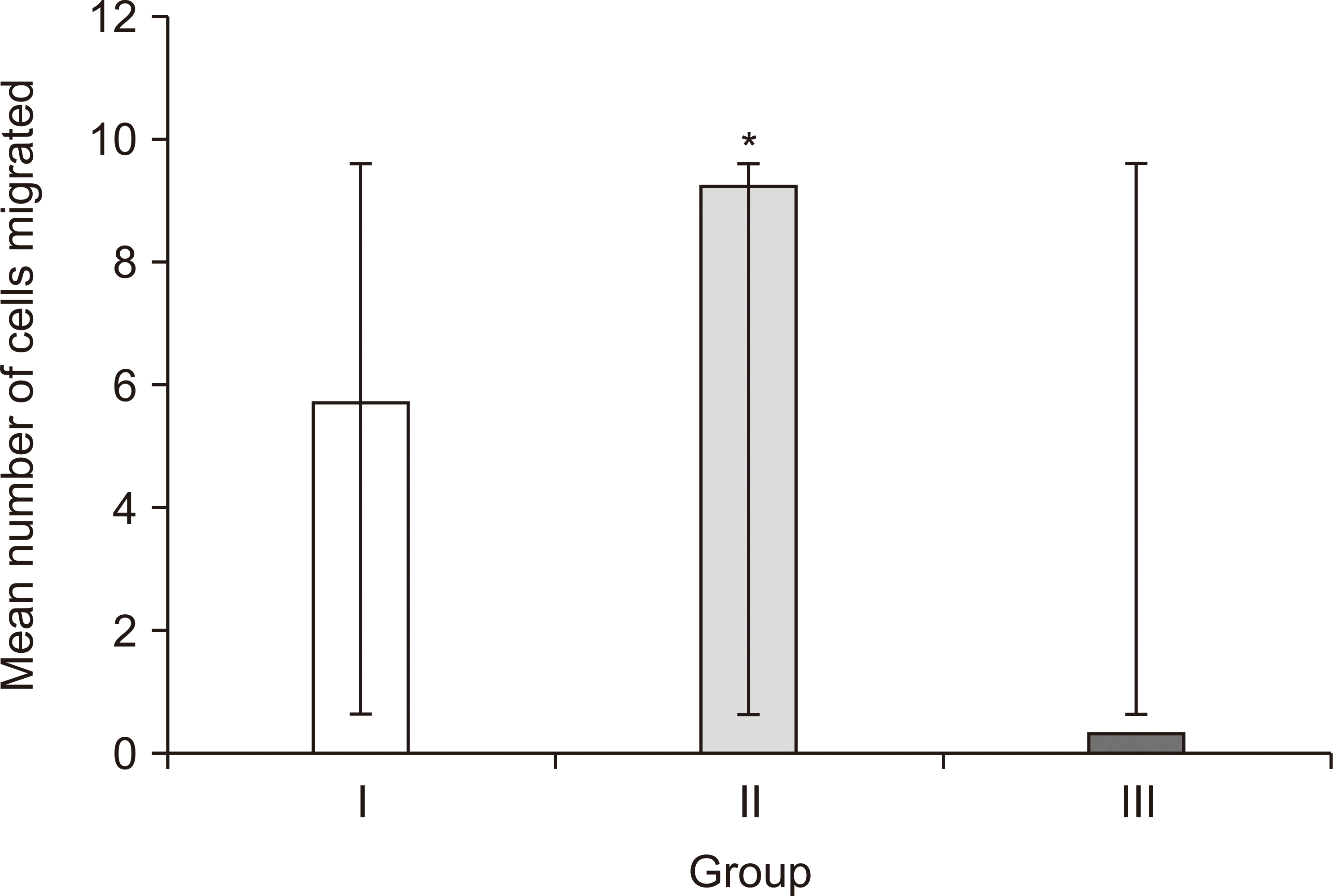
Migration of HGFs across the transwell membrane was the lowest in group III compared to the other two groups, and the difference was statistically significant. Chaitrakoonthong et al.
2 reported that while rinsing the fibroblasts with higher concentration of AA (50 μg/mL) reduced their viability and wound closure ability, it promoted their ECM protein expression, thus depicting a dose dependent switch in the fibroblastic activity. Another
in vitro study, by Baranyi et al.
27, reported that supplementation of AA (100 µM) to the culture medium was found to reduce the migration capability of fibroblasts and therefore to reduce wound closure. From the findings of these various studies it can be postulated that the fibroblastic activity varies depending on the concentration and mode of administration of AA. It is noteworthy to consider this factor while interpreting the outcome of our study. As mentioned previously, the addition of AA in group III improved viability of HGFs, and they demonstrated rapid initial wound closure (49.92% at 4 hours). However, it resulted in decreased transwell migration, which was possibly attributable to enhanced collagen and ECM production at the employed concentration of 250 µM.
Apart from the chemotactic and mitogenic actions of TGF-β1, it is a vital factor involved in the transformation of fibroblasts to myofibroblasts, particularly during remodeling of the granulation tissue
28,29. Myofibroblasts are pivotal in orchestrating the production of granulation tissue, and they exhibit an amplified capacity for granulation tissue contraction and remodeling, eventually resulting in wound closure and/or scar formation
30. A scientific investigation documented that co-culture of human dermal fibroblasts with TGF-β1 coupled with AA initiated a switch to a myofibroblast phenotype
27,31. Also, assessment of growth factor release from Alb-PRF by Fujioka-Kobayashi et al.
3 revealed a significant upsurge in the concentration of TGF-β1 released for up to 10 days. In line with previously listed research studies, it can be hypothesized that AA along with TGF-β1 released from AA Alb-PRF could have accelerated the myofibroblastic differentiation, which probably increased the ECM production and ultimately reduced migration of HGFs across the transwell membrane in group III. However, it is important to note that their viability was not negatively impacted.
Thus, our
in vitro trial strongly suggests that AA Alb-PRF is a potential biocompatible material for gingival fibroblast-mediated healing and regeneration in the wound microenvironment. As an autologous and stable local delivery agent for AA, enriched with cells and growth factors, Alb-PRF may facilitate wound regeneration
1,3. Augmentation with AA would synergistically promote the wound healing activity of gingival fibroblasts due the antioxidant and mitogenic properties, while aiding in collagen synthesis. Although the utilization of AA Alb-PRF would add to the cost compared to classic periodontal surgery methods, its benefits outweigh and justify the added expense.
When interpreting the results and deriving conclusions in the current
in vitro trial, it is crucial to consider a few perspectives. The harvested HGFs and blood samples used for cell culture and in the Alb-PRF preparations were sourced from non-autologous donors. Earlier investigations have highlighted that blood with physiological differences in the number and distribution of platelets, leukocytes, and erythrocytes among blood samples and associated variables like stress, nutrition, sex hormones, and compromised immunity might influence the quality and quantity of PRF and have an impact on study outcomes
32. Similarly,
in vitro cell culture conditions, particularly the media supplements, also can phenotypically alter the primary cells, although it remains uncertain whether a comparable alteration occurs in an
in vivo environment
27.
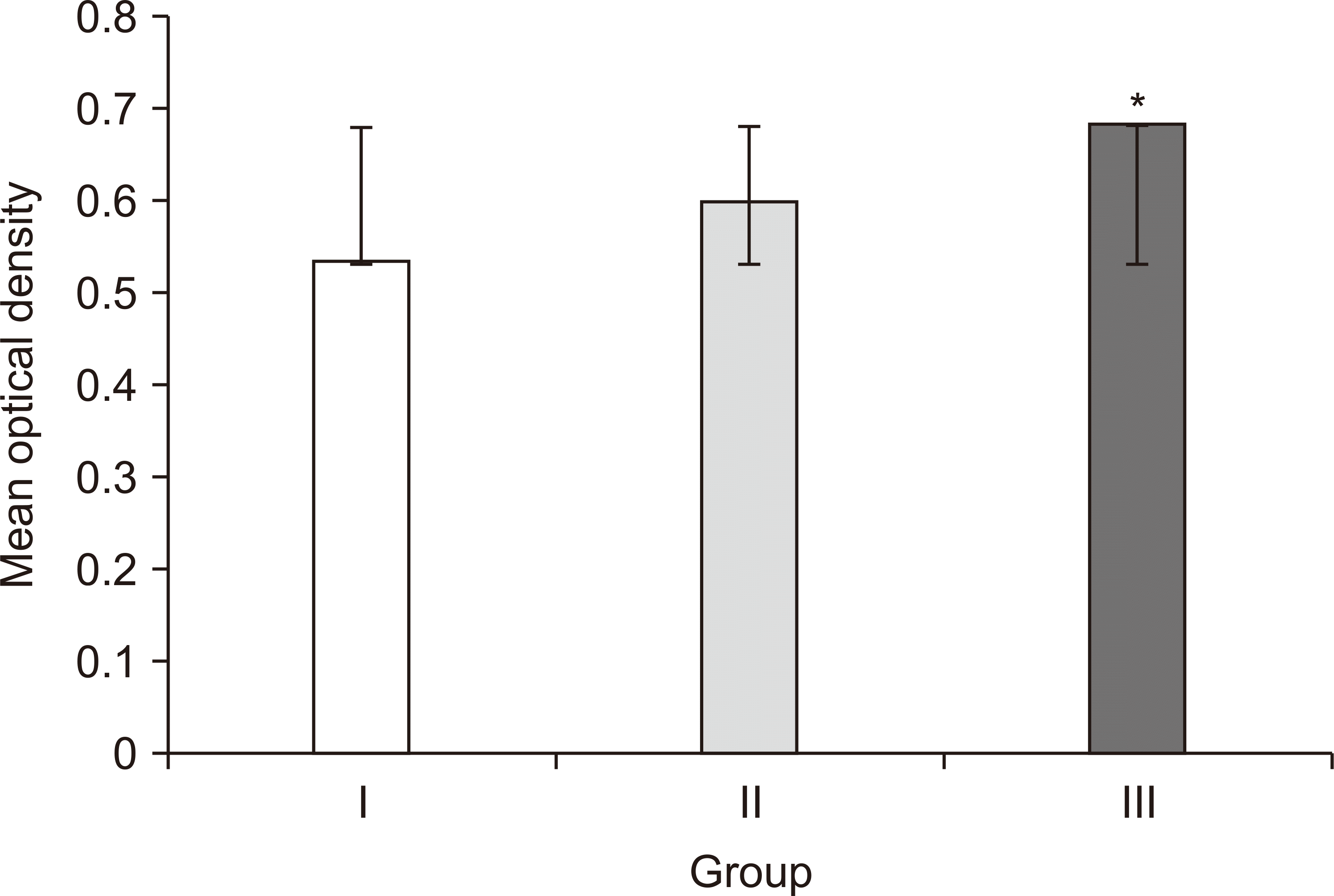






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download