Abstract
Trisomy 9 is a rare chromosomal abnormality that occurs in both mosaic and non-mosaic states. The present study reports a case of mosaic trisomy 9 detected during pregnancy in a 41-year-old woman in the second trimester screening. Maternal serum screening results were used to diagnose a chromosomal abnormality in utero. The results were validated by karyotyping. High levels of alpha-fetoprotein and low levels of unconjugated estriol (uE3), human chorionic gonadotropin (hCG), and inhibin A indicate a high risk for chromosomal abnormalities, including trisomy 18. Amniotic fluid karyotyping revealed 47, XX, +9 (30)/46, XX (20) in the fetus. Because a high level (60%) of mosaicism for trisomy 9 in the fetus can affect many parts of the body, the pregnancy was terminated. It seems that a significant reduction in the levels of hCG and uE3 is an informative marker for the detection of chromosomal abnormalities such as trisomy 9.
Trisomy 9 is an uncommon chromosomal abnormality that was first described by Feingold and Atkins in 1973 [1]. Trisomy 9 is reported to be in a mosaic and complete state. There are both differences and similarities between the manifestations of the two states [2]. The first patient reported to have trisomy 9 was diagnosed with severe clinical manifestations, including low-set malformed ears, microcephaly, low hairline, enophthalmos, prominent nose, micrognathia, skeletal abnormalities, and a congenital heart defect. The patient survived for only 28 days [1]. Trisomy 9 is a lethal condition, and only 25% of patients with complete trisomy 9 live for more than 1 week [3]. There are different survival rates between various forms of trisomy 9, and patients with the mosaic form survive longer than those with the complete form [2]. Prenatal diagnosis of trisomy 9 is based on maternal serum screening results and sonographic findings, with results subsequently confirmed by karyotyping [4]. Here, we describe a case of mosaic trisomy 9 that was recognized in the second trimester of pregnancy based on abnormal maternal serum screening results and confirmed by karyotyping.
A pregnant woman, 41 years old, was referred to Shams Medical Center, Neyshabur, Razavi Khorasan, for the first time at 15 weeks of gestation because of abnormal maternal serum screening results in the second trimester. Non-invasive prenatal testing (NIPT) and first-trimester screening, which is a combination of ultrasound evaluation of the fetus and maternal blood testing, had not been performed. Second-trimester screening (STS), which considers maternal age and serum markers including alpha-fetoprotein (AFP), unconjugated estriol (uE3), human chorionic gonadotropin (hCG), and inhibin A, and had been performed. For non-medical reasons, first-trimester screening had not been done. There was a history of four pregnancies, two healthy children, and one spontaneous abortion. The woman had no consanguineous marriage or history of chromosomal abnormalities in her children or relatives. The aborted fetus was not examined for any chromosomal abnormalities. Her partner was 43 years old and had no family history of inherited diseases. Chromosomal abnormalities were not screened during the first trimester, and the screening results in the second trimester revealed a high risk for trisomy 18 (50%) and Smith-Lemli-Opitz syndrome (SLOS) (12.5%) based on biochemical markers and maternal age at 15 weeks of gestation. The clinical data of the patient in the STS are shown in Table 1. To confirm these screening results, amniotic fluid karyotyping was performed at 16 weeks of gestation. The study was approved by the Ethics Committee of the Department of Medical Biotechnology, Neyshabour University of Medical Sciences (IR.NUMS.MEDICINE.REC.1399.199).
Genetic amniocentesis revealed trisomy 9 mosaicism, mos 47, XX, +9 (30)/46, XX (20) (Fig. 1). The level of trisomy 9 mosaicism was 60%, which can strongly affect fetal health. After counseling, with the family, the pregnancy was terminated at 16 weeks of gestation. A female fetus was delivered with malformations, including a bulbous nose, low-set ears, hypertelorism, short neck, and micrognathia.
Trisomy 9 is a rare chromosomal abnormality that occurs in a mosaic or complete state. Non-mosaic trisomy 9 is a lethal condition with an incidence of 2.2-2.7% in first-trimester spontaneous abortions [2]. Mosaic trisomy 9 is compatible with life and characterized by micrognathia, skeletal malformations, cardiac malformations, low-set ears, microphthalmia, microcephaly, kidney problems, and respiratory difficulties [5]. Since it was first reported, more than 100 cases of mosaic trisomy 9 have been reported worldwide [2]. STS, which considers maternal age and serum markers including AFP, uE3, hCG, and inhibin A, is a routine part of screening for chromosomal abnormalities [6].
Screening for serum markers in the first and second trimesters of pregnancy improves the prenatal diagnosis of chromosomal abnormalities. We describe a case of trisomy 9 mosaicism in which STS results indicated a high risk of trisomy 18 and SLOS. STS was done using biochemical markers including AFP, uE3, hCG, and inhibin A. A mild increase and decrease in the level of AFP and inhibin A (1.3 multiples of the median [MoM] and 0.7 MoM, respectively), and a significant decrease in the level of hCG and uE3 (0.1 MoM and 0.32 MoM, respectively) was measured.
Younesi et al. [7] showed that the rate of chromosomal abnormalities increases with increasing maternal age. When maternal age is more than 40 years, 1 in 28.8 pregnancies will be diagnosed with a chromosomal abnormality. Additionally, 1 in 22.8 pregnancies will be identified with chromosomal abnormalities of any sort when second-trimester trisomy 18 findings are positive [7]. Lam et al. [8] described a 41-year-old Chinese woman with abnormalities observed on ultrasonography. In this patient, hCG levels were significantly reduced at 15 weeks of gestation (0.1 MoM), and trisomy 9 was detected in the fetus [8]. Chen et al. [4] detected a complete trisomy 9 by a high level of AFP and a low level of free beta-hCG at 16 weeks of gestation.
The pregnant woman in our study was 41 years old, and maternal age over 40 years is significantly associated with different types of chromosomal abnormalities [7]. Based on studies of high-risk STS using biochemical markers, maternal age can be helpful in the detection of chromosomal abnormalities [8].
The most effective and irreplaceable prenatal test for detecting chromosomal abnormalities is amniocentesis, which involves the collection of amniotic fluid from the uterus surrounding the embryo. Despite the potential for abortion, amniocentesis is highly recommended for expectant women with genetic indications owing to its reliable results [9]. Amniotic fluid karyotyping in pregnant women with indications for amniocentesis as a diagnostic method is the gold standard for cytogenetic diagnosis [7]. To decrease the detection time, techniques such as the BACs-on-Beads assay, fluorescence in situ hybridization (FISH), quantitative fluorescence polymerase chain reaction, and chromosomal microarray analysis (CMA) have been used [9,10].
In the prenatal setting, CMA is recommended as the best rapid diagnostic test for cases with one or more fetal structural abnormalities by the American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. In addition, CMA or karyotyping is recommended for pregnant women who want prenatal screening and whose fetuses do not have any structural anomalies [11]. Both karyotyping and CMA have advantages and disadvantages in the detection of chromosomal aneuploidy. Karyotyping has low resolution and a lengthy diagnosis period, whereas CMA is unable to detect balanced structural abnormalities. CMA can detect cytogenetic alterations and offers certain advantages over karyotyping, including the ability to detect microdeletions and duplications. Karyotyping in prenatal diagnosis results in some fetuses with chromosomal abnormalities remaining undiagnosed. Hence, to improve the diagnostic rate of genetic diseases in prenatal diagnosis, the combined application of CMA and karyotyping is recommended [11,12].
Both CMA and karyotyping can identify chromosomal aneuploid mosaicism, but the differences between these methods lead to different results in the detection of mosaicism. In a study conducted by Hao et al. [13], CMA calculated a higher level of mosaicism than karyotyping in cases of trisomic mosaicism. Moreover, three of seven mosaic trisomies were not detected by karyotyping. Conversely, CMA demonstrated monosomic mosaicism in two cases that exhibited a combination of monosomic and trisomic mosaicism. This may result in the reporting of normal results in the event of equivalent numbers of monosomic and trisomic cells [13].
Trisomy 9 is a rare chromosomal abnormality that can be identified in both mosaic and non-mosaic states. As shown in Table 2, the mosaic form of trisomy 9 is diagnosed in different medical centers worldwide using several tests, such as karyotyping, CMA, and FISH. Because each method has advantages and disadvantages, using a combination of these tests can boost diagnostic yield.
Trisomy 9 mosaicism is diagnosed in the first trimester based on a nuchal translucency of >3 mm and abnormal reverse ductus venosus wave. In the second and third trimesters, detection is based on abnormalities in serum markers and in the central nervous system, heart, and skeletal and craniofacial regions on ultrasonography. NIPT has been used to identify trisomy 9 mosaicism in the first and second trimesters [2,14]. Partial trisomy 9 may occur in children when their parents have reciprocal balanced translocations. Accurate diagnosis and genetic counseling are essential in cases of partial trisomy 9 because of the increased likelihood of recurrence in subsequent pregnancies. In most cases of trisomy 9, both parents have a normal karyotype and the likelihood of recurrence is very unlikely. Although there is a minor chance that mosaic and non-mosaic trisomy 9 may recur, women with a history of pregnancy with trisomy 9 would benefit from diagnostic tests such as karyotyping and CMA in addition to screening for chromosomal aneuploidy using serum markers, nuchal translucency, and NIPT.
Our findings support the idea that second-trimester screening using maternal serum markers and maternal age is informative for detecting chromosomal abnormalities, especially in women who did not undergo first-trimester screening. Based on our findings and those of other studies, a significant reduction in hCG and uE3 levels, as well as a high AFP level, are potential markers for the diagnosis of chromosomal abnormalities.
Notes
References
2. Ma N, Zhu Z, Hu J, Pang J, Yang S, Liu J, et al. Case report: detection of fetal trisomy 9 mosaicism by multiple genetic testing methods: report of two cases. Front Genet. 2023; 14:1121121.

3. McDuffie RS Jr. Complete trisomy 9: case report with ultrasound findings. Am J Perinatol. 1994; 11:80–4.
4. Chen CP, Chern SR, Cheng SJ, Chang TY, Yeh LF, Lee CC, et al. Second-trimester diagnosis of complete trisomy 9 associated with abnormal maternal serum screen results, open sacral spina bifida and congenital diaphragmatic hernia, and review of the literature. Prenat Diagn. 2004; 24:455–62.
5. Bruns D. Presenting physical characteristics, medical conditions, and developmental status of long-term survivors with trisomy 9 mosaicism. Am J Med Genet A. 2011; 155A:1033–9.

7. Younesi S, Taheri Amin MM, Hantoushzadeh S, Saadati P, Jamali S, Modarressi MH, et al. Karyotype analysis of amniotic fluid cells and report of chromosomal abnormalities in 15,401 cases of Iranian women. Sci Rep. 2021; 11:19402.

8. Lam YH, Lee CP, Tang MH. Low second-trimester maternal serum human chorionic gonadotrophin in a trisomy 9 pregnancy. Prenat Diagn. 1998; 18:1212.
9. Liu Y, Sun XC, Lv GJ, Liu JH, Sun C, Mu K. Amniotic fluid karyotype analysis and prenatal diagnosis strategy of 3117 pregnant women with amniocentesis indication. J Comp Eff Res. 2023; 12:e220168.

10. Lu S, Kakongoma N, Hu WS, Zhang YZ, Yang NN, Zhang W, et al. Detection rates of abnormalities in over 10,000 amniotic fluid samples at a single laboratory. BMC Pregnancy Childbirth. 2023; 23:102.

11. Hay SB, Sahoo T, Travis MK, Hovanes K, Dzidic N, Doherty C, et al. ACOG and SMFM guidelines for prenatal diagnosis: is karyotyping really sufficient? Prenat Diagn. 2018; 38:184–9.

12. Qian G, Cai L, Yao H, Dong X. Chromosome microarray analysis combined with karyotype analysis is a powerful tool for the detection in pregnant women with high-risk indicators. BMC Pregnancy Childbirth. 2023; 23:784.

13. Hao M, Li L, Zhang H, Li L, Liu R, Yu Y. The difference between karyotype analysis and chromosome microarray for mosaicism of aneuploid chromosomes in prenatal diagnosis. J Clin Lab Anal. 2020; 34:e23514.
14. Stipoljev F, Kos M, Kos M, Miskovi B, Matijevic R, Hafner T, et al. Antenatal detection of mosaic trisomy 9 by ultrasound: a case report and literature review. J Matern Fetal Neonatal Med. 2003; 14:65–9.

15. Takahashi H, Hayashi S, Miura Y, Tsukamoto K, Kosaki R, Itoh Y, et al. Trisomy 9 mosaicism diagnosed in utero. Obstet Gynecol Int. 2010; 2010:379534.
16. Chen CP, Lin HM, Su YN, Chern SR, Tsai FJ, Wu PC, et al. Mosaic trisomy 9 at amniocentesis: prenatal diagnosis and molecular genetic analyses. Taiwan J Obstet Gynecol. 2010; 49:341–50.

17. Chen CP, Hung FY, Su YN, Chern SR, Su JW, Lee CC, et al. Prenatal diagnosis of mosaic trisomy 9. Taiwan J Obstet Gynecol. 2011; 50:549–53.

18. Merino A, De Perdigo A, Nombalais F, Yvinec M, Le Roux MG, Bellec V. Prenatal diagnosis of trisomy 9 mosaicism: two new cases. Prenat Diagn. 1993; 13:1001–7.
Fig. 1.
Karyotype of amniotic fluid cells. Genetic amniocentesis analysis revealed the existence of trisomy 9 mosaicism, mos 47, XX, +9 (30)/46, XX (20). Amniocentesis showed that 20 cells (40%) were normal (46, XX) and 30 cells (60%) were 47, XX, +9 (red arrow).
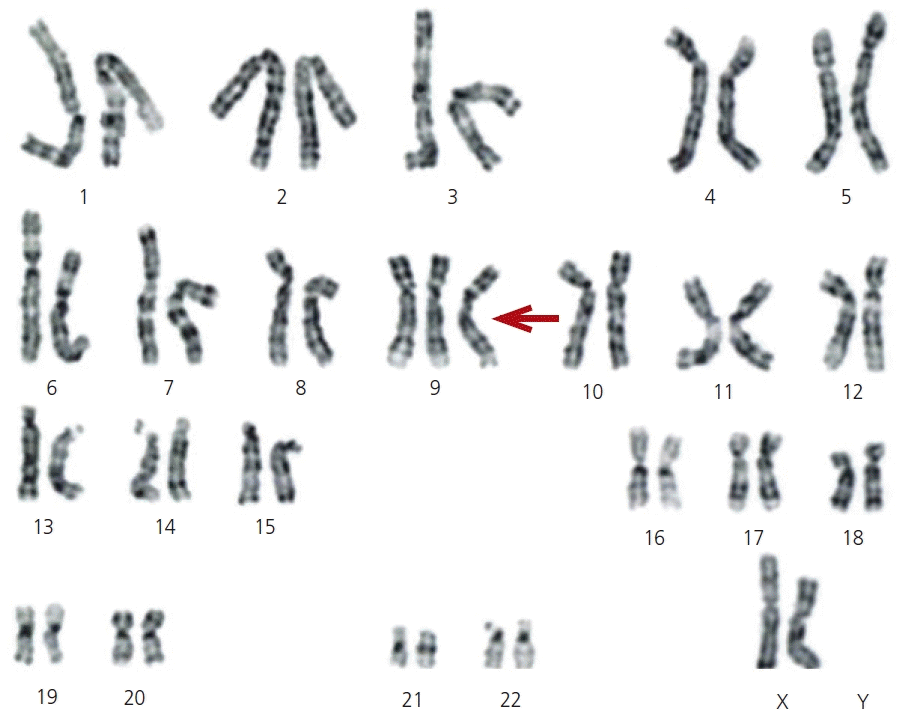
Table 1.
Clinical data of patient in the second-trimester screening
| Age (yr) | Gestation age |
Serum markers (MoM) |
Reference (MoM) |
Results |
||||
|---|---|---|---|---|---|---|---|---|
| AFP | hCG | uE3 | Inhibin A | Trisomy 18 | SLOS | |||
| 41 | 15 weeks 0 day | 1.3 | 0.1 | 0.32 | 0.7 | 0.5-1.5 | 1/2 | 1/8 |
Table 2.
Prenatal detection of mosaic trisomy 9 by different methods
| Study | Maternal age (yr) | Gestational age | Tanique | Sample | Mosaicism (%) |
|---|---|---|---|---|---|
| Ma et al. [2] (2023) | |||||
| Case 1 | 26 | 22 weeks | Karyotype | Amniotic fluid cells | 50 |
| 27 weeks | FISH | Umbilical blood | 22.4 | ||
| 27 weeks | CMA | Umbilical blood | 34 | ||
| Case 2 | 31 | 18 weeks | Karyotype | Amniotic fluid cells | 42 |
| Takahashi et al. [15] (2010) | |||||
| Case 1 | 36 | 29 weeks | Karyotype | Amniotic fluid cells | 10 |
| Case 2 | 36 | 31 weeks | Karyotype | Amniotic fluid cells | 29 |
| Stipoljev et al. [14] (2003) | 25 | 25 weeks | Karyotype | Chorionic villus sampling | 31 |
| Chen et al. [16] (2010) | 35 | 17 weeks | Karyotype | Amniotic fluid cells | 33.3 |
| 19 weeks | Karyotype | Amniotic fluid cells | 24 | ||
| 22 weeks | FISH | Amniotic fluid cells | 18 | ||
| Chen et al. [17] (2011) | 42 | 18 weeks | Karyotype | Amniotic fluid cells | 40 |
| 20 weeks | Karyotype | Amniotic fluid cells | 25 | ||
| 20 weeks | FISH | Amniotic fluid cells | 48 | ||
| Merino et al. [18] (1993) | |||||
| Case 1 | 20 | 23 weeks | Karyotype | Amniotic fluid cells | 65 |
| Case 2 | 39 | 23 weeks | Karyotype | Amniotic fluid cells | 12 |
| Zhang et al. [19] (2023) | |||||
| Case 1 | 32 | 22 weeks | Karyotype | Amniotic fluid cells | 10 (9p duplication |
| 22 weeks | CMA | Amniotic fluid cells | 21.5 (9p duplication | ||
| 22 weeks | FISH | Amniotic fluid cells | 25 (9p duplication | ||
| Case 2 | 22+4 weeks | Karyotype | Amniotic fluid cells | 82 (trisomy 9)/14 | |
| 22+4 weeks | CMA | Amniotic fluid cells | 50 (trisomy 9)/50 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download