Abstract
Oocyte activation is a fundamental event in mammalian fertilization and is initiated by a cascade of calcium signaling and oscillation pathways. Phospholipase C zeta (PLCζ) is involved in modulating cortical granule exocytosis, releasing oocyte meiotic arrest, regulating gene expression, and early embryogenesis. These processes are considered to be initiated and controlled by PLCζ activity via the inositol-1,4,5-triphosphate pathway. The decrease or absence of functional PLCζ due to mutational defects in protein expression or maintenance can impair male fertility. In this literature review, we highlight the significance of PLCζ as a sperm factor involved in oocyte activation, its mechanism of action, the signaling pathway involved, and its close association with oocyte activation. Finally, we discuss the relationship between male infertility and PLCζ deficiency.
The role of the male partner in infertility has been the subject of several studies. Male infertility occurs when there is a deficit in the quantity, mobility, morphology, or function of spermatozoa, and the etiology of infertility can be identified in 50-60% of cases [1]. It can also occur due to testicular and post-testicular disorders along with the presence of endocrine disruptors and consanguinity [2-4].
During mammalian fertilization, the spermatozoon activates a series of biochemical events that initiate embryonic development called “egg activation” [5,6]. In all species, the first event following oocyte activation is an increase in cytosolic free Ca2+ concentration [6]. An acute increase in cytosolic Ca2+ concentration in mammals occurs due to long-lasting calcium oscillations that begin directly after gamete fusion and persists for several hours after meiotic completion, leading to egg activation and stimulation of the early stages of embryonic development [6,7]. Several recent studies have highlighted the presence of sperm-derived molecules in soluble spermatozoa as potential factors responsible for generating Ca2+ oscillations during mammalian fertilization [8,9]; the testis-specific phospholipase C called PLC zeta (PLCζ), discovered in 2002, is the primary candidate. Accumulating experimental evidence suggests that PLCζ meets all the characteristics of the soluble sperm factor responsible for oocyte activation by inducing Ca2+ increase during mammalian fertilization [10,11]. During the fusion of male and female gametes, sperm proteins are released from the fertilizing sperm inside the oocyte that subsequently trigger Ca2+ oscillations via the inositol 1,4,5-trisphosphate (IP3) signaling pathway [12]. Several studies have highlighted the causal link between sperm-specific PLCζ deficiency and male infertility [13-15]. Human spermatozoa without PLCζ cannot induce calcium release and initiate the early stages of embryonic development [16].
In this review, we discuss the importance of PLCζ as a sperm factor inducing oocyte activation, different signaling pathways involved, and the clinical importance of the spermatic factor.
PLCζ exhibits a unique biochemical structure and is currently known as the smallest mammalian PLC isoform with a molecular mass of ~70 kDa in humans and ∼74 kDa in mice [16]. The domain structure of PLCζ comprises four tandem EF hand domains at the N-terminus, the catalytic X and Y domains at the center of the molecule that form the active site common to all PLCs, followed by a single C2 domain at the C-terminus [17] (Fig. 1). Each domain of the PLC protein plays an important role in calcium release. The catalytic X and Y domains are separated by a short segment, the XY-linker, which, due to its net positive charge, plays an important role in targeting PLCζ to intracellular membranes through direct electrostatic interactions with its negatively charged substrate [18]. The EF domain contains a cluster of basic amino acid residues and plays a vital role given its high Ca2+ sensitivity compared to other somatic PLCs, allowing PLCζ activation after sperm-egg fusion and Ca2+ release into the egg cytosol [19]. The C-terminal domain of PLCζ comprising 120 amino acid residues and is essential for PLCζ function; deletion of the C-terminal domain abolishes the Ca2+ oscillatory activity of PLCζ at the oocyte level without altering its enzymatic activity or Ca2+ sensitivity. Unlike other PLC isoforms, PLCζ lack a pleckstrin homology domain at the N-terminus [16].
PLCζ is located in the subcellular part of the spermatozoon. During fertilization, the mobility of spermatozoa enables them to cross the mature oocyte through the acrosome reaction, allowing gamete fusion and the subsequent release of male gamete contents, including soluble sperm proteins [20]. The soluble sperm factor reside in a compartment called the perinuclear theca (PT), which is a condensed layer of cytosolic proteins surrounding the sperm nucleus that enters the oocyte during or after gamete fusion [21].
Notably, PLCζ was identified in the fraction of sperm extracts that was able to induce calcium release. A significant amount of this protein is expressed in the acrosomal, equatorial, and post-acrosomal regions of the human sperm head, and in the main part of the flagellum [22,23]. An analysis of sperm samples showed that ~88% of PLCζ was expressed in the equatorial region, while ~35% and ~21% of the protein was expressed in the acrosomal and post-acrosomal region, respectively [23].
This enzyme triggers the signaling cascade, thereby enabling the blockage of polyspermy through exocytosis of cortical granules, resumption of meiosis, and appearance of the two pronuclei, via calcium variations within the oocyte cytoplasm [24].
Many theories have attempted to explain the close relationship between Ca2+ concentration and oocyte activation in mammals [14]. There is considerable evidence to support the “sperm factor” hypothesis as the most appropriate model for oocyte activation in mammals. This theory assumes that semen contains a soluble factor capable of stimulating calcium release within the oocyte [25,26]. The term “soluble” implies that the factor used by the sperm can diffuse throughout the cytosol of the egg to initiate Ca2+ release [25].
The diffusion of soluble PLCζ hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to IP3 and diacylglycerol (DAG). Subsequently, IP3 binds to specific receptors that induce Ca2+ oscillations, leading to oocyte activation [26,27]. The elevation of Ca2+ leads to an increase in PLCζ activity, which in turn stimulates further increase in Ca2+ and IP3 through a positive feedback loop [16].
Testis-specific PLCζ has a significant influence on oocyte activation in mammals in general and in humans in particular [23,28,29]. Injection of complementary RNA (cRNA) or recombinant PLCζ proteins resulted in calcium oscillations similar to those observed during fertilization and supported embryonic development up to the blastocyst stage in a mouse model; this provides evidence of the role of PLCζ as a sperm-specific factor that induces oocyte activation [28]. Indeed, sperm extracts and PLCζ cRNA microinjected into the female gametes of another species can induce Ca2+ release [30]. Regarding the involvement of RNA, existing theories are debatable, given that the total amount of PLCζ RNA presented in the sperm influences the concentration of calcium that is released [31]. Mutation in the catalytic domain of PLCζ, which is required for PIP2 hydrolysis, completely inhibits calcium release in oocytes, indicating that PLCζ mediates this action through the production of IP3 [32].
Many studies support the idea that PLCζ is the primary physiological stimulus that triggers the required specific pattern of calcium oscillations, thus ensuring monozoospermia and eventually successful egg activation and early embryonic development.
Fertilization is characterized by the fusion of normal sperm and a metaphase two mature oocyte to form a diploid zygote. Before fertilization, a series of molecular and biochemical changes occur during the fusion of the spermatozoa and oocyte, such as the release of Ca2+ that activates the secretion of cortical granules and the extrusion of the second polar body [35]. It is clear that a sperm might have genetic defects that result in subsequent failure of the fertilization process, which could affect embryonic development. Over the years, studies on PLCζ have formed the basis by which we understand the correlation between oocyte activation and the spermatic factor PLCζ, coded by the PLCζ1 gene [36].
The absence or abnormal expression of PLCζ1 gene is known to cause fertilization failure (FF) due to sperm dysfunction. Kashir et al. [17] revealed that in morphologically normal sperms, a mutation in PLCζ1 is associated with an FF phenotype. Yan et al. [37] investigated different PLCζ1 mutations in 14 samples obtained from patients with primary infertility exhibiting total or poor FF. After extracting genomic DNA from the peripheral blood and sequencing the whole exons of PLCζ1 using Sanger sequencing, five of the 14 patients were found to have biallelic PLCζ1 mutations in the Y and X domains, including four missense mutations, an in-frame deletion, and a splicing mutation; however, no mutations were detected in the C2 domain [38]. As shown using western blotting, no expression of PLCζ1 protein was detected in the five patients [39,40]. These mutations can affect protein expression, structure, and stability. The injection of wild-type PLCζ1 cRNA efficiently induced pronucleus formation, reaching an 86% (6/7) formation rate, whereas the injection of mutant cRNA significantly decreased the pronucleus formation rate [41].
Dai et al. [41] studied the correlation between PLCζ1 mutations and the localization of PLCζ on the spermatozoa. Ten Chinese men who exhibited poor fertilization following intracytoplasmic sperm injection (ICSI), with a fertilization rate (FR) <20%, were included in this study [41]. Three novel homozygous mutations in the PLCζ1 gene have been identified as causing FF: a nonsense variation, c.C588A (p. C196X) (National Center for Biotechnology Information, Bethesda, MD, USA), and two missense variants, c. T1048C (p. S350P) (Broad Institute, Cambridge, MA, USA) and c. C736T (p. L246F) (Wellcome Sanger Institute, Hinxton, UK) [42,43]. In normal sperm, PLCζ is located in the three regions of the perinuclear theca: acrosomal and equatorial region, equatorial region alone, and equatorial and post-acrosomal region. No PLCζ expression was detected in sperms with the homozygous nonsense variation p. C196X (National Center for Biotechnology Information). Moreover, 93.5% of sperms with p. S350P (Genetic Research Labs, Houston, TX, USA) showed diffuse signals in the post-acrosomal region, and 92.6% of sperms with p. L246F (Genetic Research Labs) showed signals in the equatorial region [44]. Collectively, these data suggest that PLCζ1 variations led to abnormal localization patterns of PLCζ in sperms [15,45-47]. Decreased PLCζ expression leads to abnormal Ca2+ oscillations and FF [48].
Another study investigated 37 patients presenting total or partial FF (FR, ≤25%) after ICSI [13]. Thirteen affected patients carried at least one mutation in each coding region of the PLCζ1 gene. Five mutations were single: p. I120M, p. R197H, p. L224P, p. H233L, and p. S500 L; the sixth mutation was caused by a deletion of two nucleotides (p. V326K fs*25). These mutations were located all over the gene [48-50]: p. I120M was located at the c-terminus of the EFhand domain, which regulates calcium sensitivity; the three mutations p.R197H, p.L224P, and p.H233L were located at the X catalytic domain, which enables PLCζ to release Ca2+; the mutation p.S500 L was found at the C2 N-terminal domain; and the last mutation p.V326K fs*25 lacked both the Y- and C2, thereby controlling PLCζ function and it’s degree of sensitivity [36].
Thus, functional analysis ofPLCζ1 mutations in humans confirms PLCζ/PLCζ1 altered activity and their involvement in impaired oocyte activation. PLCζ1 gene sequencing has been proposed as a useful diagnostic tool and should be recommended for couples presenting with FF after ICSI due to oocyte activation failure (Table 1).
Stimulation of the phosphoinositide signaling pathway is an essential component of the Ca2+ oscillations observed during mammalian fertilization, where intracellular IP3 and DAG are generated by the hydrolysis of PIP2 (Fig. 2). The generated IP3 then binds to IP3R in the endoplasmic reticulum, resulting in the release of Ca2+ [18,51]. The produced DAG activates the protein kinase C pathway, which is thought to translate Ca2+ signals into cellular responses [15]. These oscillations within the oocyte are linked to many processes responsible for its activation, such as the exocytosis of cortical granules, release of meiotic arrest, regulation of gene expression, recruitment of maternal mRNA, pronuclear formation, and initiation of embryogenesis [52].
Many studies have reported that overexpression of PLCζ in mouse oocytes leads to increased DAG production, subsequently resulting in abnormal secondary Ca2+ oscillations [53]. These secondary Ca2+ oscillations are undesirable for mouse oocytes, as all failed to reach the blastocyst stage after injection of increased concentrations of PLCζ [43].
Therefore, an optimal range of PLCζ introduced into the oocyte is vital during fertilization, as this would keep the subsequent production of secondary messengers at a minimal physiological level so as not to disrupt the Ca2+ homeostasis of the oocytes [54]. Along with these relevant findings, recent studies focusing on aspects of PLCζ and its strong association with fertility, suggest that male infertility is due to deficiencies in PLCζ expression, its structure, and thus its function [55].
Total FF can occur in ICSI cycles, leading to considerable disappointment and confusion in infertile patients [56]. The main cause of infertility in this case is due to an alteration of the signaling pathways that induce oocyte activation [57]. Sperm abnormalities are primarily associated with a spermspecific PLCζ protein involved in the mechanisms of infertility [58]. In addition, infertile human spermatozoa with altered quantity and quality of the enzyme do not induce Ca2+ oscillations. Recent studies have shown that ICSI failure in infertile patients is associated with oocyte activation deficiency (OAD) [59].
Clearly, PLCζ deficiency is intimately related to OAD and is likely to be the predominant causal factor. Sperms obtained from patients with ICSI failure were unable to induce calcium oscillations [60,61]. In addition, spermatozoa from patients diagnosed with partial or complete globozoospermia were unable to fertilize oocytes naturally due to reduced levels or total absence of PLCζ [62].
Conversely, the presence of abnormalities in the gene encoding PLCζ further emphasized the link with oocyte activation deficiency, and exon screening revealed new mutations in patients with infertility [63]. These variants generate abnormal protein structures that disrupt calcium activity. However, it turned out to be more complicated than expected in that patients with infertility have reduced total levels of PLCζ than that in fertile patients [64].
The acrosome of the spermatozoa plays an essential role in the interaction of gametes, precisely the perinuclear theca, which is a unique structure within the sperm head containing proteins such as PLCζ and the post-acrosomal sheath WW domain-binding protein (PAWP). Maturation of the acrosome is crucial for successful fertilization and oocyte activation. While PLCζ is a key factor, it has been suggested that other factors like DPY19-like 2 (DPY19L2) also contribute to fertilization. DPY19L2 is a key causative factor related to human globozoospermia, a severe male infertility disorder diagnosed by the presence of 100% round-headed spermatozoa lacking an acrosome [65,66]. Mutation of this gene disrupts the maturation of the acrosome and the expression of izumo sperm-oocyte fusion 1, a protein which promotes the interaction of the spermatozoa with the oocyte, PLCζ, and PAWP [67]. Further studies are required to elucidate the clinical importance and potential expression of these proteins [67].
Accumulating data suggest that PLCζ mutation or a relative absence of this protein within the sperm, may explain some cases of male infertility [64]. There is an important connection between the levels of the spermatic factor (PLCζ), Ca2+ release, and the early development of embryo. Currently, cases of ICSI failure are clinically resolved through assisted oocyte activation (AOA) involving artificial induction of Ca2+ release using the Ca2+ ionophore A23187 [68,69].
The second most common ionophore used in oocyte activation is ionomycin, which is important for the activation of Ca2+ or calmodulin-dependent kinases and phosphatases to stimulate gene expression [49]. Indeed, the only Ca2+ ionophore reported to produce oscillations instead of single transients is strontium chloride (Sr2+Cl2-), which in mice has led to Ca2+ oscillations, oocyte activation, and efficient parthenogenesis [65]. However, the efficiency of Sr2+Cl2- in human oocytes remains debatable because no Ca2+ oscillations have been observed [66]. ICSI combined with AOA has been reported to increase fertilization and pregnancy rates. Finally, ionophores, such as A23187, have multiple effects on cellular homeostasis, including genetic, epigenetic, biochemical, and physiological effects, which remain to be examined in oocytes [69].
The production and injection of the purified protein PLCζ is indeed a viable method to clinically treat cases of OAD [70]. Kashir et al. [25] described the generation of purified and highly active recombinant PLCζ, which induced Ca2+ oscillations after injection into mouse and human oocytes. Notably, other data demonstrate that the effects of mutant PLCζ in mouse and humans can be effectively solved using purified recombinant PLCζ; the success rates following PLCζ injection were comparable to control sperm injections [25,71,72].
Heterologous and homologous ICSI assays, such as the mouse oocyte activating test (MOAT), mouse oocyte calcium analysis (MOCA), and human oocyte calcium analysis (HOCA), are used to improve the oocyte activation rate and to analyze the capacity of human sperm after injection into mammalian oocytes [73]. The mouse model is the preferred option for heterologous ICSI tests because a high yield of oocytes can be easily obtained, and the housing and handling of this species is easier compared to other mammals. Mouse heterologous ICSI tests are valuable for predicting the response to ICSI-AOA treatment [74]. The MOAT assay involved injecting the sperm of a patient into mouse oocytes and assessing the activation rate 24 hours after ICSI [75]. Depending on the percentage of activation rate, the patients were subdivided into three groups: MOAT group 1 (0-20% activation rate) included patients diagnosed with sperm-related OAD; MOAT group 2 (20-84% activation rate) included patients with reduced capacity of oocyte activation; and MOAT group 3 correlated with the activation range of fertile control spermatozoa (85-100% activation rate). The high rate of activation in MOAT group 3 suggests that OAD may be related to oocytes [76].
The MOCA is a more sensitive diagnostic test than MOAT, which examines the ability of human sperm to induce Ca2+ oscillation after injection into mouse oocytes [75]. After ICSI, Ca2+ expression was analyzed using an inverted epifluorescence microscope at different wavelengths. The fluorescence emitted by each oocyte was recorded every 2 hours, which indicated an increase in calcium oscillations. To determine whether this pattern is normal, the average product of the mean frequency (F) and mean amplitude (A) of all oocytes injected with the patients’ sperm was calculated and compared to oocytes injected with control sperm. Factor A×F ≤9 indicates a decreased capacity of the patients’ sperm to activate the oocyte, while A×F ≥9 indicates normal Ca2+ release and suggests oocyte-related activating deficiency. Indeed, the sensibility of the MOCA test can approve those patients from MOAT group 2 suffers from reduced activating capacity [77]. AOA reportedly increased FR in patients in MOAT groups 1 and 2 (70% and 63%, respectively). However, for MOAT group 3, the increase in FR was significantly lower than that for MOAT groups 1 and 2, implying that AOA treatment is not helpful for patients with oocyte-related deficiency [56].
The HOCA test can assess the Ca2+ oscillatory capacity of human sperm after injection into human oocytes (prophase I: germinal vesicle, metaphase I, or in vivo matured metaphase II oocytes) with smooth endoplasmic reticulum aggregate. This assay is different from the MOCA test because the Ca2+ measurements were acquired every 30 seconds for 10 hours to ensure the recording of all Ca2+ peaks produced, and because of prolonged Ca2+ release during human fertilization compared to mouse fertilization. For HOCA test, the factor A×F ≤0.6 indicates a sperm-related problem and favorable response to ICSI-AOA, and A×F ≥0.6 indicates an oocyte-related activation deficiency and unfavorable response to ICSI-AOA [78].
Recent studies have suggested that PLCζ is not the only sperm factor essential for oocyte activation during fertilization [70,79,80]. In 2007, Wu et al. [21] described a novel alkaline protein in the sperm head that resides precisely in the PT [81]. The protein shares sequence homology with the N-terminal half of the WW domain-binding protein 2 (WBP2). The C-terminal half of the protein is unique and proline-rich, and is called PAWP, also known as WW domain binding protein 2-novel like. During fertilization, PAWP is retained on the sperm head after the acrosomal reaction and binding and penetration of the zona pellucida. PAWP is among the first components dispersed from the sperm head to the oocyte cytoplasm at the time of gamete fusion [74,82] (Fig. 3).
PAWP is involved in the activation of oocyte during fertilization in humans and other mammals, and has been validated as a biomarker of sperm quality and fertility in humans. The discovery of PAWP was based on the post-acrosomal sheath region of the PT, a cytoskeletal capsule located at the head of the spermatozoon that protects the sperm nuclear material. This protein has been detected in the cytoplasmic lobe of spermatids in several species, including mice, rabbits, pigs, cows, rhesus monkeys, and humans [83,84].
Several hypotheses suggest that PAWP plays a crucial role as a sperm factor. Microinjection of recombinant human PAWP or an alkaline extract of PT into oocytes showed that PAWP could be a candidate sperm factor for oocyte activation. Co-injection of PAWP and a competitive inhibitor derived from the WWI domain-binding motif of PAWP suppressed the induction of pronuclear formation, implying the requirement of sperm PAWP and an oocyte-derived WWI domain protein substrate of PAWP for successful fertilization. During fertilization, pronucleus formation can be observed by injection of PAWP with a mutated peptide containing the proline tyrosine motif [85]. Although the injection of PAWP or cRNA has been observed to induce calcium oscillations, the mechanism remains unclear. Aarabi et al. [86,87] hypothesized that PAWP is involved in oocyte activation via interactions with other proteins. Recent studies have stated that infertile patients with ICSI failure associated with OAD have under-expressed PLCζ and PAWP, which suggests that these proteins can serve as a biomarker of oocyte activation [88,89].
ICSI is a significant improvement for couples with severe male factors [90]. PLCζ plays a major role in oocyte activation as the sperm factor, but oocyte activation is not necessarily due to PLCζ alone, as the induction of oocyte activation may be due to multiple factors and pathways. Spermatic proteins are physiological agents that trigger calcium oscillations and initiate embryogenesis. Data demonstrate that absence of functional PLCζ, as a spermatic factor, decreases the FR. In the absence of PLCζ expression, a “rescue pathway” can induce egg activation through PAWP, suggesting its role as a complementary factor of PLCζ enabling the release of calcium. There is clearly a need to completely understand the precise mechanism of PLCζ in the activation of phosphoinositide signaling pathways and calcium release in the oocyte. PLCζ is now widely accepted as the physiological “sperm factor” that plays an essential role in mammalian fertilization. Numerous clinical associations have also reported the direct link of male infertility and OAD with reduced or absent expression levels and mutated forms of PLCζ. Collectively, such findings require further research into the underlying molecular mechanisms of PLCζ during fertilization. As many clinical reports emerge, it is also becoming clear that many potential cases of male infertility could benefit from the application of PLCζ for therapy and diagnosis. Therefore, PLCζ could be considered as a prognostic or diagnostic molecular marker to identify male patients who could benefit from assisted reproductive technology. Moreover, although calcium oscillations are the main agents for artificial activation of oocytes, PLCζ could potentially be a safer therapeutic agent.
References
1. Bach PV, Schlegel PN. Male infertility. In : Strauss JF, Barbieri RL, editors. Yen and Jaffe’s reproductive endocrinology. 8th ed. Philadelphia: Elsevier;2019. p. 582–93.
2. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018; 62:2–10.

3. McElreavey K, Krausz C, Patrat C, Fellous M. Male infertility and microdeletions of the Y chromosome. Gynecol Obstet Fertil. 2022; 30:405–12.
4. Khatun A, Rahman MS, Pang MG. Clinical assessment of the male fertility. Obstet Gynecol Sci. 2018; 61:179–91.

5. Saleh A, Kashir J, Thanassoulas A, Safieh-Garabedian B, Lai FA, Nomikos M. Essential role of sperm-specific PLCzeta in egg activation and male factor infertility: an update. Front Cell Dev Biol. 2020; 8:28.

6. Ramadan WM, Kashir J, Jones C, Coward K. Oocyte activation and phospholipase C zeta (PLCζ): diagnostic and therapeutic implications for assisted reproductive technology. Cell Commun Signal. 2012; 10:12.

7. Kashir J, Deguchi R, Jones C, Coward K, Stricker SA. Comparative biology of sperm factors and fertilizationinduced calcium signals across the animal kingdom. Mol Reprod Dev. 2013; 80:787–815.

8. Nomikos M, Swann K, Lai FA. Starting a new life: sperm PLC-zeta mobilizes the Ca2+ signal that induces egg activation and embryo development: an essential phospholipase C with implications for male infertility. Bioessays. 2012; 34:126–34.
9. Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002; 124:611–23.

10. Nomikos M. Novel signalling mechanism and clinical applications of sperm-specific PLCζ. Biochem Soc Trans. 2015; 43:371–6.

12. Ravel C, Kazdar N, Drapier H, Duros S, Viard P. Assisted oocyte activation: a new tool for severe male factor infertility treatment. Med Sci (Paris). 2016; 32:198–203.
13. Torra-Massana M, Cornet-Bartolomé D, Barragán M, Durban M, Ferrer-Vaquer A, Zambelli F, et al. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum Reprod. 2019; 34:1494–504.
14. Swann K, Saunders CM, Rogers NT, Lai FA. PLCzeta (zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol. 2006; 17:264–73.
15. Yelumalai S, Yeste M, Jones C, Amdani SN, Kashir J, Mounce G, et al. Total levels, localization patterns, and proportions of sperm exhibiting phospholipase C zeta are significantly correlated with fertilization rates after intracytoplasmic sperm injection. Fertil Steril. 2015; 104:561–8.e4.

16. Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, et al. PLC zeta: a sperm-specific trigger of Ca (2+) oscillations in eggs and embryo development. Development. 2002; 129:3533–44.
17. Kashir J, Nomikos M, Lai FA. Phospholipase C zeta and calcium oscillations at fertilisation: the evidence, applications, and further questions. Adv Biol Regul. 2018; 67:148–62.

18. Nomikos M, Elgmati K, Theodoridou M, Calver BL, Nounesis G, Swann K, et al. Phospholipase Cζ binding to PtdIns (4,5)P2 requires the XY-linker region. J Cell Sci. 2011; 124:2582–90.
19. Nomikos M, Yu Y, Elgmati K, Theodoridou M, Campbell K, Vassilakopoulou V, et al. Phospholipase Cζ rescues failed oocyte activation in a prototype of male factor infertility. Fertil Steril. 2013; 99:76–85.

20. Yanagimachi R. Fertility of mammalian spermatozoa: its development and relativity. Zygote. 1994; 2:371–2.

21. Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, et al. PAWP, a sperm-specific WW domainbinding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem. 2007; 282:12164–75.

22. Tavalaee M, Nasr-Esfahani MH. Expression profile of PLCζ, PAWP, and TR-KIT in association with fertilization potential, embryo development, and pregnancy outcomes in globozoospermic candidates for intra-cytoplasmic sperm injection and artificial oocyte activation. Andrology. 2016; 4:850–6.

23. PGrasa P, Coward K, Young C, Parrington J. The pattern of localization of the putative oocyte activation factor, phospholipase Czeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod. 2008; 23:2513–22.
24. Sutovsky P. New approaches to boar semen evaluation, processing and improvement. Reprod Domest Anim. 2015; 50 Suppl 2:11–9.

25. Kashir J, Nomikos M, Lai FA, Swann K. Sperm-induced Ca2+ release during egg activation in mammals. Biochem Biophys Res Commun. 2014; 450:1204–11.
26. Anifandis G, Michopoulos A, Daponte A, Chatzimeletiou K, Simopoulou M, Messini CI, et al. Artificial oocyte activation: physiological, pathophysiological and ethical aspects. Syst Biol Reprod Med. 2018; 65:3–11.

27. Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010; 16:690–703.

28. Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H, et al. Ca2+ oscillation-inducing phospholipase C zeta expressed in mouse eggs is accumulated to the pronucleus during egg activation. Dev Biol. 2004; 268:245–57.
29. Kouchi Z, Shikano T, Nakamura Y, Shirakawa H, Fukami K, Miyazaki S. The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Czeta. J Biol Chem. 2005; 280:21015–21.
30. Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest. 2008; 118:3671–81.

31. Jones KT. Mammalian sperm contain two factors for calcium release and egg activation: phospholipase C zeta and a cryptic activating factor. Mol Hum Reprod. 2018; 24:465–8.

32. Nozawa K, Satouh Y, Fujimoto T, Oji A, Ikawa M. Spermborne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci Rep. 2018; 8:1315.

33. Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, et al. Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem. 2005; 280:31011–8.
34. Hachem A, Godwin J, Ruas M, Lee HC, Ferrer Buitrago M, Ardestani G, et al. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development. 2017; 144:2914–24.
35. Jiménez-Movilla M, Hamze JG, Romar R. Oolemma receptors in mammalian molecular fertilization: function and new methods of study. Front Cell Dev Biol. 2021; 9:662032.

36. Escoffier J, Lee HC, Yassine S, Zouari R, Martinez G, Karaouzène T, et al. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum Mol Genet. 2016; 25:878–91.

37. Yan Z, Fan Y, Wang F, Yan Z, Li M, Ouyang J, et al. Novel mutations in PLCZ1 cause male infertility due to fertilization failure or poor fertilization. Hum Reprod. 2020; 35:472–81.

38. Bunney TD, Katan M. PLC regulation: emerging pictures for molecular mechanisms. Trends Biochem Sci. 2011; 36:88–96.
39. Mu J, Zhang Z, Wu L, Fu J, Chen B, Yan Z, et al. The identification of novel mutations in PLCZ1 responsible for human fertilization failure and a therapeutic intervention by artificial oocyte activation. Mol Hum Reprod. 2020; 26:80–7.

40. Nomikos M, Sanders JR, Kashir J, Sanusi R, Buntwal L, Love D, et al. Functional disparity between human PAWP and PLCζ in the generation of Ca2+ oscillations for oocyte activation. Mol Hum Reprod. 2015; 21:702–10.
41. Dai J, Dai C, Guo J, Zheng W, Zhang T, Li Y, et al. Novel homozygous variations in PLCZ1 lead to poor or failed fertilization characterized by abnormal localization patterns of PLCζ in sperm. Clin Genet. 2020; 97:347–51.
42. Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2010; 94:520–6.

43. Palermo GD, Neri QV, Schlegel PN, Rosenwaks Z. Intracytoplasmic sperm injection (ICSI) in extreme cases of male infertility. PLoS One. 2014; 9:e113671.
44. Aras-Tosun D, Cakar Z, Can A, Ozkavukcu S, Kaplanoglu I, Cinar O. Phospholipase C-zeta levels are not correlated with fertilisation rates in infertile couples. Andrologia. 2022; 54:e14269.

45. Ferrer-Vaquer A, Barragan M, Freour T, Vernaeve V, Vassena R. PLCζ sequence, protein levels, and distribution in human sperm do not correlate with semen characteristics and fertilization rates after ICSI. J Assist Reprod Genet. 2016; 33:747–56.

46. Kashir J, Konstantinidis M, Jones C, Heindryckx B, De Sutter P, Parrington J, et al. Characterization of two heterozygous mutations of the oocyte activation factor phospholipase C zeta (PLCζ) from an infertile man by use of minisequencing of individual sperm and expression in somatic cells. Fertil Steril. 2012; 98:423–31.

47. Kashir J, Konstantinidis M, Jones C, Lemmon B, Lee HC, Hamer R, et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod. 2012; 27:222–31.

48. Wang F, Zhang J, Kong S, Li C, Zhang Z, He X, et al. A homozygous nonsense mutation of PLCZ1 cause male infertility with oocyte activation deficiency. J Assist Reprod Genet. 2020; 37:821–8.
49. Taylor SL, Yoon SY, Morshedi MS, Lacey DR, Jellerette T, Fissore RA, et al. Complete globozoospermia associated with PLCζ deficiency treated with calcium ionophore and ICSI results in pregnancy. Reprod Biomed Online. 2010; 20:559–64.

50. Ferrer-Vaquer A, Barragan M, Freour T, Vernaeve V, Vassena R. PLCζ sequence, protein levels, and distribution in human sperm do not correlate with semen characteristics and fertilization rates after ICSI. J Assist Reprod Genet. 2016; 33:747–56.

51. Shimada K, Ono T, Mizushima S. Application of intracytoplasmic sperm injection (ICSI) for fertilization and development in birds. Gen Comp Endocrinol. 2014; 196:100–5.

52. Dale B, Wilding M, Coppola G, Tosti E. How do spermatozoa activate oocytes? Reprod Biomed Online. 2010; 21:1–3.

53. Fukami K, Inanobe S, Kanemaru K, Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res. 2010; 49:429–37.
54. Meng X, Melo P, Jones C, Ross C, Mounce G, Turner K, et al. Use of phospholipase C zeta analysis to identify candidates for artificial oocyte activation: a case series of clinical pregnancies and a proposed algorithm for patient management. Fertil Steril. 2020; 114:163–74.

55. Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium. 2014; 55:24–37.

56. Vanden Meerschaut F, Leybaert L, Nikiforaki D, Qian C, Heindryckx B, De Sutter P. Diagnostic and prognostic value of calcium oscillatory pattern analysis for patients with ICSI fertilization failure. Hum Reprod. 2013; 28:87–98.

57. Ratti S, Follo MY, Ramazzotti G, Faenza I, Fiume R, Suh PG, et al. Nuclear phospholipase C isoenzyme imbalance leads to pathologies in brain, hematologic, neuromuscular, and fertility disorders. J Lipid Res. 2019; 60:312–7.

58. Yeste M, Jones C, Amdani SN, Yelumalai S, Mounce G, da Silva SJ, et al. Does advancing male age influence the expression levels and localisation patterns of phospholipase C zeta (PLCζ) in human sperm? Sci Rep. 2016; 6:27543.
59. Amdani SN, Yeste M, Jones C, Coward K. Sperm factors and oocyte activation: current controversies and considerations. Biol Reprod. 2015; 93:50.
60. Chithiwala ZH, Lee HC, Hill DL, Jellerette-Nolan T, Fissore R, Grow D, et al. Phospholipase C-zeta deficiency as a cause for repetitive oocyte fertilization failure during ovarian stimulation for in vitro fertilization with ICSI: a case report. J Assist Reprod Genet. 2015; 32:1415–9.

61. Alshahrani RA. Evaluation and characterization of PLCzeta in the male reproductive tract [Internet]. Riyadh: Alfaisal University;c2022. [cited 2024 Jul 1]. Available from: https://www.proquest.com/openview/50116e91037b1b3b0ad52c9b6f8afe85/1?pq-origsite=gscholar&cbl=2026366&diss=y.
62. Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod. 2009; 24:2417–28.
63. Jones C, Meng X, Coward K. Sperm factors and egg activation: phospholipase C zeta (PLCZ1) and the clinical diagnosis of oocyte activation deficiency. Reproduction. 2022; 164:F53–66.
64. Guo Y, Jiang J, Zhang H, Wen Y, Zhang H, Cui Y, et al. Proteomic analysis of dpy19l2-deficient human globozoospermia reveals multiple molecular defects. Proteomics Clin Appl. 2019; 13:e1900007.

65. Abdelhedi F, Chalas C, Petit JM, Abid N, Mokadem E, Hizem S, et al. Altered three-dimensional organization of sperm genome in DPY19L2-deficient globozoospermic patients. J Assist Reprod Genet. 2019; 36:69–77.

66. Koscinski I, Elinati E, Fossard C, Redin C, Muller J, Velez de la Calle J, et al. DPY19L2 deletion as a major cause of globozoospermia. Am J Hum Genet. 2011; 88:344–50.

67. Granados-Gonzalez V, Aknin-Seifer I, Touraine RL, Chouteau J, Wolf JP, Levy R. Preliminary study on the role of the human IZUMO gene in oocyte-spermatozoa fusion failure. Fertil Steril. 2008; 90:1246–8.

68. Cocco L, Follo MY, Manzoli L, Suh PG. Phosphoinositide-specific phospholipase C in health and disease. J Lipid Res. 2015; 56:1853–60.
69. Xu Z, Yao G, Niu W, Fan H, Ma X, Shi S, et al. Calcium Ionophore (A23187) rescues the activation of unfertilized oocytes after intracytoplasmic sperm injection and chromosome analysis of blastocyst after activation. Front Endocrinol (Lausanne). 2021; 12:692082.

70. Amdani SN, Yeste M, Jones C, Coward K. Phospholipase C zeta (PLCζ) and male infertility: clinical update and topical developments. Adv Biol Regul. 2016; 61:58–67.

71. Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod Biomed Online. 2008; 17:662–8.

72. Bonte D, Ferrer-Buitrago M, Dhaenens L, Popovic M, Thys V, De Croo I, et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril. 2019; 112:266–74.

73. Cardona Barberán A, Boel A, Vanden Meerschaut F, Stoop D, Heindryckx B. Diagnosis and treatment of male infertility-related fertilization failure. J Clin Med. 2020; 9:3899.
74. Javadian-Elyaderani S, Ghaedi K, Tavalaee M, Rabiee F, Deemeh MR, Nasr-Esfahani MH. Diagnosis of genetic defects through parallel assessment of PLCζ and CAPZA3 in infertile men with history of failed oocyte activation. Iran J Basic Med Sci. 2016; 19:281–9.
75. Campos G, Sciorio R, Esteves SC. Total fertilization failure after ICSI: insights into pathophysiology, diagnosis, and management through artificial oocyte activation. Hum Reprod Update. 2023; 29:369–94.

76. Ferrer-Buitrago M, Dhaenens L, Lu Y, Bonte D, Vanden Meerschaut F, De Sutter P, et al. Human oocyte calcium analysis predicts the response to assisted oocyte activation in patients experiencing fertilization failure after ICSI. Hum Reprod. 2018; 33:416–25.

77. Ebner T, Köster M, Shebl O, Moser M, Van der Ven H, Tews G, et al. Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Fertil Steril. 2012; 98:1432–7.
78. Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod Biomed Online. 2014; 28:560–71.
79. Azad N, Nazarian H, Nazari L, Ghaffari Novin M, Piryaei A, Heidari MH, et al. Evaluation of PAWP and PLC? Expression in infertile men with previous ICSI fertilization failure. Urol J. 2018; 15:116–21.
80. Satouh Y, Nozawa K, Ikawa M. Sperm postacrosomal WW domain-binding protein is not required for mouse egg activation. Biol Reprod. 2015; 93:94.
81. Zafar MI, Lu S, Li H. Sperm-oocyte interplay: an overview of spermatozoon’s role in oocyte activation and current perspectives in diagnosis and fertility treatment. Cell Biosci. 2021; 11:4.

82. Tanhaei S, Abdali-Mashhadi S, Tavalaee M, JavadianElyaderani S, Ghaedi K, Seifati SM, et al. Assessment of sperm PAWP expression in infertile men. Urol J. 2019; 16:488–94.
83. Kamali-Dolat Abadi M, Tavalaee M, Shahverdi A, NasrEsfahani MH. Evaluation of PLCζ and PAWP expression in globozoospermic individuals. Cell J. 2016; 18:438–45.
84. Sutovsky P, Manandhar G, Wu A, Oko R. Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc Res Tech. 2003; 61:362–78.

85. Nakai M, Ito J, Suyama A, Kageyama A, Tobari Y, Kashiwazaki N. Phospholipase Cζ (PLCζ) versus postacrosomal sheath WW domain-binding protein (PAWP): which molecule will survive as a sperm factor? Anim Sci J. 2020; 91:e13345.

86. Aarabi M, Qin Z, Xu W, Mewburn J, Oko R. Spermborne protein, PAWP, initiates zygotic development in Xenopus laevis by eliciting intracellular calcium release. Mol Reprod Dev. 2010; 77:249–56.
87. Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, et al. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril. 2014; 102:440–7.

88. Kashir J, Nomikos M, Swann K, Lai FA. PLCζ or PAWP: revisiting the putative mammalian sperm factor that triggers egg activation and embryogenesis. Mol Hum Reprod. 2015; 21:383–8.
89. Gonzalez-Castro RA, Amoroso-Sanches F, Stokes JE, Graham JK, Carnevale EM. Localisation of phospholipase Cζ1 (PLCZ1) and postacrosomal WW-binding protein (WBP2 N-terminal like) on equine spermatozoa and flow cytometry quantification of PLCZ1 and association with cleavage in vitro. Reprod Fertil Dev. 2019; 31:1778–92.

Fig. 1.
Human phospholipase C zeta (PLCζ) structure. (A) Modeling of human PLCζ homology (3D ribbon representation) [8]. The sequence of the protein is predicted using a model from the National Center for Biotechnology Information and the structure is predicted with the sequence AF-Q86YW0-F1 (National Center for Biotechnology Information, Bethesda, MD, USA) using the AlphaFold structure modeling and prediction tool. AlphaFold produces a confidence score per residue between 0 and 100 in the predicted local distance difference test (pLDDT). Some regions below 50 pLDDT may be unstructured in isolation. The PLCζ domain structure consists of four tandem EF hand domains at the N-terminus, the X and Y catalytic domains at the center of the molecule, and the C2 domain at the C-terminus. (B) Schematic representation of the distribution of different domains constituting PLCζ; the four tandem EF-hand domains at the N-terminus regulate the sensitivity of the protein to calcium levels, the second domain has the catalytic domains X and Y that cause changes in the functional ability of PLCζ to release Ca2+, and are separated by a short segment, the XY-linker, followed by a single C2 domain at the C-terminus that controls PLCζ function and it’s degree of sensitivity. 3D, three-dimensional.
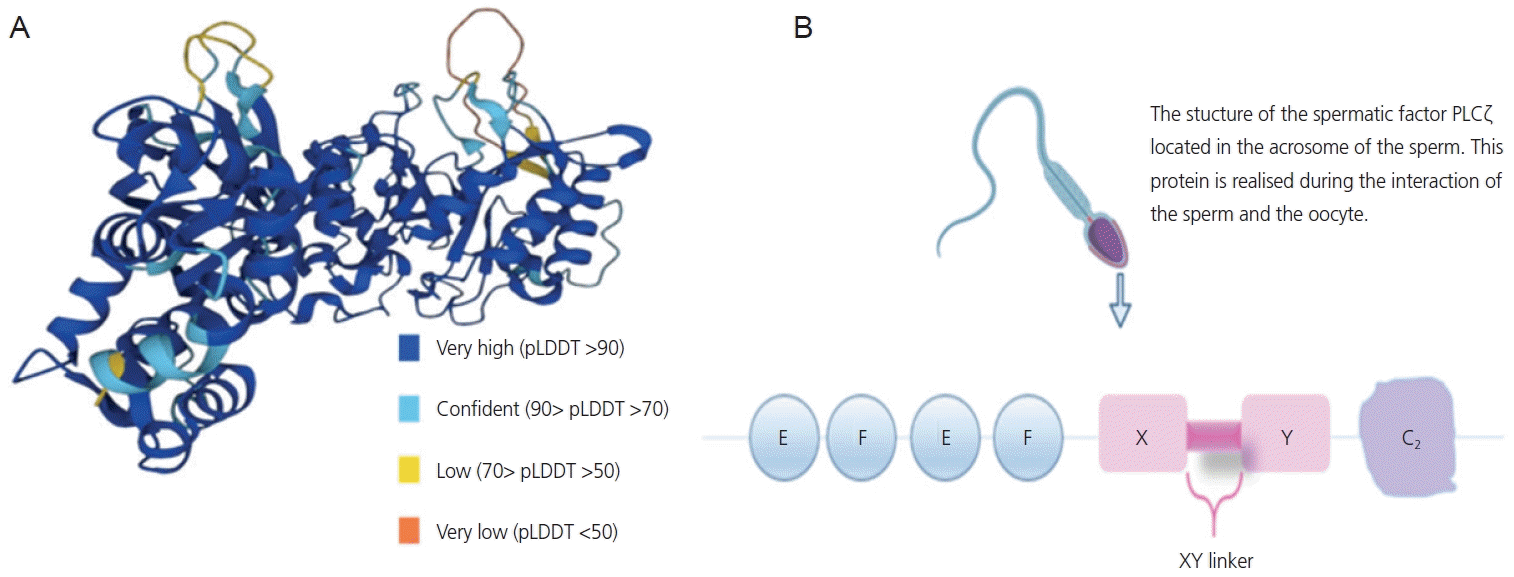
Fig. 2.
Schematic representation of the mechanisms of action of PAWP and PLCζ [10]. PAWP (left side) binds to the WW1 domain of the YAP protein; the activation of PLC gamma subsequently hydrolyzes PIP2 into a second messenger IP3; PLCζ (right side) induces the generation of IP3 through the hydrolysis of PIP2. The generated IP3 binds to IP3R on the ER and increases the levels of Ca2+ resulting in oocyte activation. GRAM, glucosyltransferases; PAWP, post-acrosomal WW1 domain binding protein; SF, sperm factor; PLCζ, phospholipase C zeta; YAP, yes-associated protein; PLCγ, phospholipase C gamma; IP3, inositol 1,4,5-triphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3R, inositol 1,4,5-trisphosphate receptor; ER, endoplasmic reticulum.
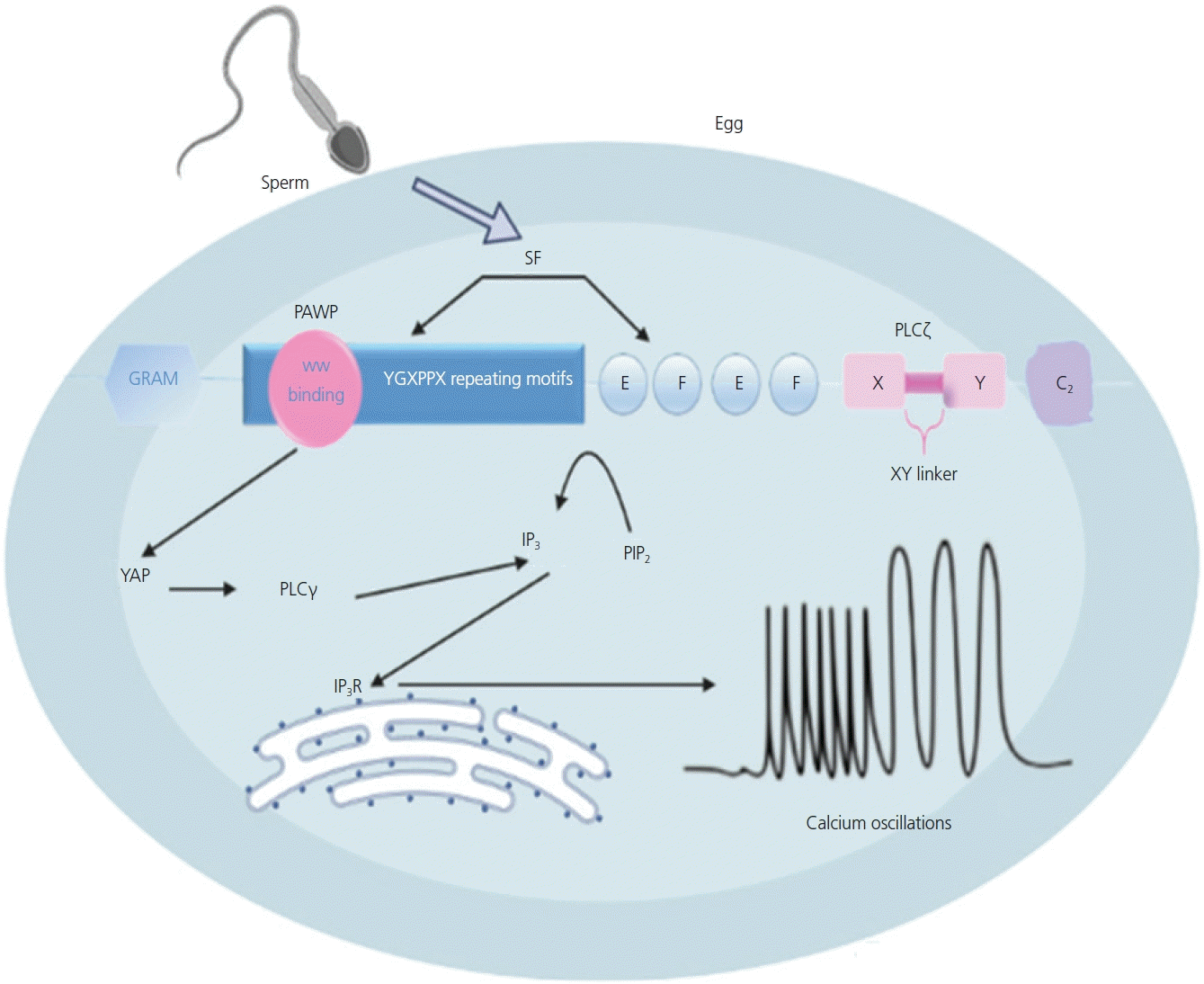
Fig. 3.
Schematic representation of domains constituting PAWP [88]. SOAF, GRAM, Rab-like GTPase activators, and myotubularins domain. The post-acrosomal sheath WW domain-binding protein located in the perinuclear matrix of the sperm head shares a homology with the N-terminal half of the WW domain-binding protein 2; PY motifs with WW binding to the YGXPPX repeating motifs consensus binding site for group-1 WW domain-containing proteins followed by the N-terminal GRAM. PAWP, post-acrosomal sheath WW domain-binding protein; SOAF, sperm oocyte activating factor; GRAM, glucosyltransferases; PY, proline-tyrosine; GTPase, guanosine triphosphatase.
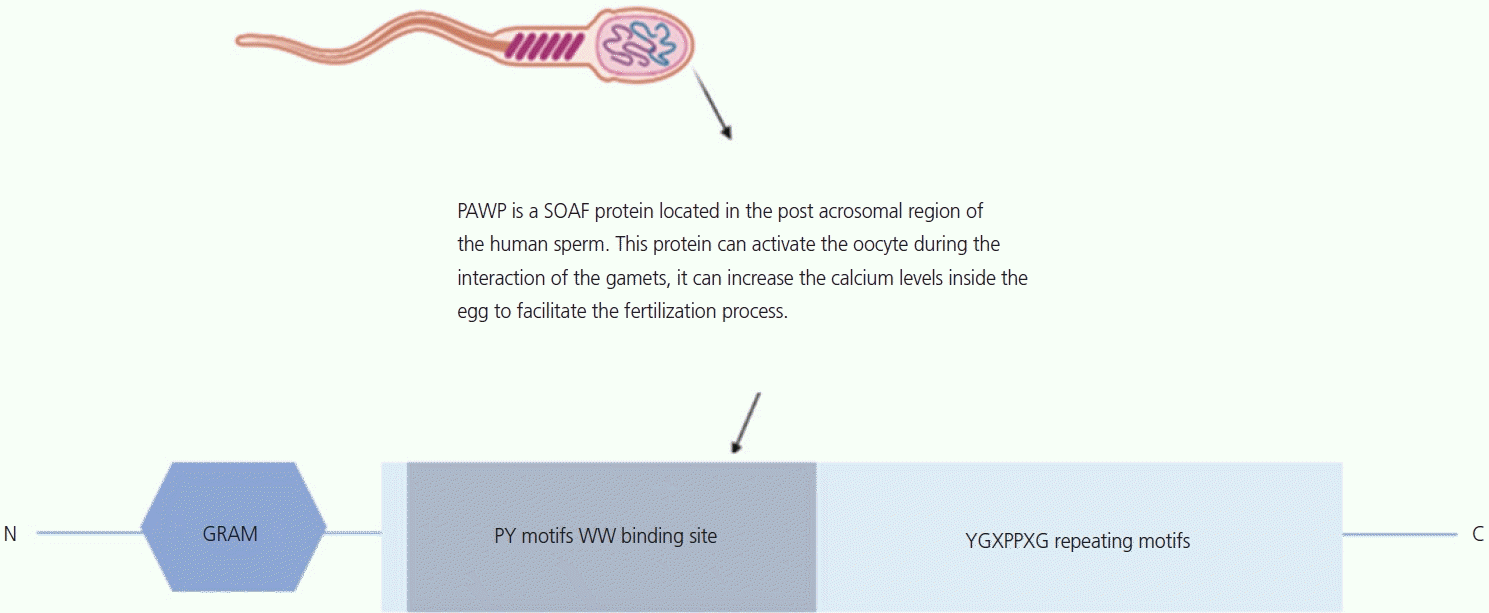
Table 1.
Descriptive summary of some studies reporting different mutations of the spermatic protein in each domain and its effect on oocyte activation and fertilization rate
| Domain affected | In vitro phenotype | In vivo phenotype | Associated study |
|---|---|---|---|
| X | |||
| C196X | Predicted alteration of local protein fold | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Nomikos et al. [18] (2011), Shimada et al. [51] (2014), and Dale et al. [52] (2010) |
| R197H | Predicted alteration of local protein fold | OAD; low fertilization success | Fukami et al. [53] (2010) |
| L224P | Predicted alteration of local protein fold | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Meng et al. [54] (2020) |
| H233L | Reduced expression in mammalian cells; reduced/absent oscillations following cRNA injections in mouse oocytes; reduced embryogenesis in mouse; predicted alteration of local protein fold | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Neri et al. [55] (2014), Vanden Meerschaut et al. [56] (2013), and Ratti et al. [57] (2019) |
| L246F | Predicted alteration of local protein fold | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Shimada et al. [51] (2014) |
| L277P | Predicted alteration of local protein fold; reduced activation success following cRNA injection in human oocytes | OAD; reduced/absent PLCζ in patient sperm; low fertilization success | Dale et al. [52] (2010) |
| C588C | Predicted to induce a loss of function | Complete fertilization failure in an ICSI cycle/absent expression of the spermatic protein (PLCζ) | Yeste et al. [58] (2016) |
| Y | |||
| N377del/A384V | Alteration of local protein fold; no activation success following cRNA injection in human oocytes/predicted alteration of local protein fold; no activation success following cRNA injection in human oocytes | OAD; reduced/absent PLCζ in patient sperm; low fertilization success | Dale et al. [52] (2010) |
| H398P | Reduced expression in mammalian cells; reduced/absent oscillations following cRNA injections in mouse oocytes; predicted alteration of local protein fold | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Neri et al. [55] (2014), Vanden Meerschaut et al. [56] (2013), Ratti et al. [57] (2019), and Amdani et al. [59] (2015) |
| R412fs/P420L | Truncated recombinant protein produced by mammalian cells; reduced activation success following cRNA injection in mouse oocytes/reduced recombinant protein produced by mammalian cells; reduced activation success following cRNA injection in mouse oocytes | OAD; low fertilization success | Nomikos et al. [18] (2011) |
| K448N | Alteration of local protein fold; reduced activation success following cRNA injection in human oocytes | OAD; reduced/absent PLCζ in patient sperm; low fertilization success | Dale et al. [52] (2010) |
| S350P | Predicted alteration of local protein fold | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Shimada et al. [51] (2014) |
| C2 | |||
| R553P | Reduced/absent fertilization following cRNA injections in mouse oocytes; predicted alteration of local protein fold; mouse fertilization and embryogenesis comparable following injection of higher levels of mutant cRNA | Comparable levels of PLCζ in patient sperm | Chithiwala et al. [60] (2015) |
| M578T | Predicted alteration of local protein fold; no activation success following cRNA injection in human oocytes | OAD; reduced/absent PLCζ in patient sperm; low fertilization success | Dale et al. [52] (2010) |
| I489F/S500L | Reduced/absent oscillations following cRNA injections in mouse oocytes; reduced embryogenesis in mouse; predicted alteration of local protein fold; similar enzymatic properties, but dramatically reduced substrate binding/predicted alteration of local protein fold | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Meng et al. [54] (2020), Alshahrani [61] (2022), and Heytens et al. [62] (2009) |
| X-Y linker | |||
| V326fs*25 | Predicted frameshift truncation of protein | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Meng et al. [54] (2020) |
| T324fs | Truncated recombinant protein produced by mammalian cells; reduced activation success following cRNA injection in mouse oocytes | OAD; low fertilization success | Nomikos et al. [18] (2011) |
| EF-X linker | |||
| I120M | Predicted alteration of local protein fold | OAD; reduced/absent PLCζ in patient sperm; abnormal PLCζ localization | Meng et al. [54] (2020) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download