This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To assess the effect of endometrial thickness (EMT) on live birth rates (LBR) in women with endometrial lining between 7.0–9.9 mm.
Methods
This retrospective cohort study included women who underwent fresh and frozen embryo transfers between 2008 and 2018, grouped according to their maximum EMT; group 1, 7.0–7.9 mm; group 2, 8.0–8.9 mm; and group 3, 9.0–9.9 mm and underwent blastocyst transfer.
Results
The study included 7,091 in-vitro fertilization cycles: 1,385 in group 1, 3,000 in group 2, and 2,706 in group 3. The combined LBR was 22.2%. The mean age of women at oocyte retrieval day was 36.7±4.5 years. There was no difference in female age at oocyte retrieval or in the quality of embryos transferred between the three groups. Group 1 had more diagnoses of diminished ovarian reserve (25.8% vs. 19.5% and 19.1%; P<0.001) and less male factor infertility compared with group 2 and 3, respectively (25.0% vs. 28.8% and 28.5%; P=0.024). LBR was higher with increasing endometrial thickness, group 2 vs. group 1 (22.0% vs. 17.4%; P=0.0004), group 3 vs. group 1 (25.0% vs. 17.2%; P<0.001), and group 3 vs. group 2 (25.0% vs. 22.0%; P=0.008). After controlling for confounding factors, these three groups did not differ in LBR (group 1 vs. group 2, odds ratio [OR], 1.08; 95% confidence interval [CI], 0.83–1.4; P=0.54 and group 1 vs. group 3, OR, 1.16; 95% CI, 0.90–1.51; P=0.24).
Conclusion
Live birth rates in women with endometrial thickness between 7.0–9.9 mm were not affected by different cut-offs when blastocyst transfer was performed.
Go to :

Keywords: Endometrium thicknes, Live birth, Embryo transfer
Introduction
The most common method to assess the potential of the endometrium before embryo transfer in
in vitro fertilization (IVF) is to measure endometrial thickness. However, a recent retrospective study demonstrated that a very low endometrial thickness before embryo transfer is not a predictive factor for failure to have a live birth [
1]. Other studies have shown that endometrial thickness plays a role in pregnancy outcomes [
2–
8]. Currently, the data suffer from wide groupings of endometrial thickness, making it difficult to interpret the ideal cutoff for endometrial thickness that will result in the highest pregnancy outcomes in fresh and frozen cycles [
3,
9–
11]. Two large studies on fresh embryo transfers found that an endometrial thickness >15 mm was associated with significantly higher clinical pregnancy rates and live birth rates (LBR) than thinner linings [
3,
9]. Regarding frozen embryo transfer (FET) cycles, it was reported that endometrial thickness below 9 mm was associated with lower live birth rates than those with thickness >9 mm, and there was no significant difference between outcomes at 9–13 mm and those above 14 mm [
12]. A recent large study from the Canadian Assisted Reproductive Technology Registry Plus database, where they grouped the cycles into 2-millimeter differences in endometrial thickness, reported that in fresh cycles, LBR increased significantly until an endometrial thickness of 10–12 mm, whereas in FET cycles, the LBR plateaued after 7–10 mm [
13].
Currently, fertility centers select individual cut-offs for maximum endometrial thickness to achieve the best pregnancy outcomes, usually ranging between 7 and 9 mm [
14]. Consequently, in this study, we assessed the effect of endometrial thickness on LBR in narrow groups of 1-mm intervals between 7.0–9.9 mm, among women with fresh and frozen cycles of blastocyst transfer.
Go to :

Materials and methods
This was a retrospective cohort study conducted at a University Reproductive Center. This study included women who underwent fresh and frozen embryo transfers between January 2008 and December 2018. This study was approved by the McGill University Health Center Research Ethics Board #2023-9133 and complies with the 1964 Helsinki Declaration, its later amendments, and all comparable ethical standards.
Women were grouped according to their maximum endometrial thickness with group 1: 7.0–7.9 mm, group 2: 8.0–8.9 mm, and group 3: 9.0–9.9 mm, having measurements as listed. All subjects underwent blastocyst transfer. Endometrial thickness was measured using a transvaginal ultrasound probe (Voluson S8; General Electric Corporation, Milwaukee, WI, USA) with an anterior-posterior diameter and an endometrial stripe running along the length of the cervix and uterine cavity. The maximum anteroposterior thickness was recorded. All transfers were performed only if the endometrial pattern was a triple-line pattern, comprising a central hyperechoic line surrounded by two hypoechoic layers.
Endometrial thickness in fresh IVF-embryo transfer cycles was measured on the day of the trigger, whereas for FET cycles, it was measured before progesterone administration, documentation of luteinizing hormone surge, or human chorionic gonadotropin (hCG) administration. Women with uterine anomalies, untreated uterine polyps, submucosal fibroids, intramural fibroids at least 4 cm in diameter, untreated thyroid disease, hyperprolactinemia, or cleavage-stage embryo transfers were excluded from the study. Moreover, women with recurrent pregnancy loss were defined as those with a history of at least three prior pregnancy losses from conception to 20 weeks of gestational age. Repeated implantation failure was defined as the absence of intrauterine pregnancy after ≥3 high-quality single blastocyst transfer. Patients from both groups were excluded from the study. Only blastocyst transfers (single- or double-embryo) were included. None of the women underwent transfer of three or more blastocysts per clinical policy. Preimplantation genetic testing was not performed on the embryos.
The primary outcome of the study was the live birth rate, defined as the delivery of a live fetus after 24 weeks of gestation. Secondary outcomes were clinical pregnancy, ectopic pregnancy, and miscarriage rates. Clinical pregnancy was defined as a fetal heartbeat on transvaginal ultrasound at 6–8 weeks of gestation (Voluson S8; General Electric Corporation). The clinical pregnancy and live birth rates were calculated for embryo transfer. For fresh cycles, the peak estradiol levels and the number of oocytes retrieved were also recorded. Adjustments were made for known potential confounders, including female age at oocyte retrieval, embryo grade, cause of infertility, number of oocytes retrieved, number of embryos transferred, and protocol type (fresh vs. frozen). To overcome any potential bias during the study period, we adjusted for the year of transfer. When two blastocysts were transferred, the grade of the better embryo was used for adjustment.
In fresh cycles, the ovaries are stimulated with different types of gonadotropins [
15], and the endogenous luteinizing hormone surge is suppressed using a gonadotropin-releasing hormone agonist or antagonist. In the FET cycles, the protocols included were natural cycles, artificial cycles with hormone replacement therapy (HRT), and modified natural cycles with hCG triggering. In the natural cycle, no medication was administered. Embryo transfer timing was determined based on urinary and serum luteinising hormone surges. In the modified natural cycle, hCG was administered when the lead follicle reached a mean diameter of 18 mm, and the endometrium was at least 7 mm in thickness. In an artificial cycle, endometrial preparation was achieved by the administration of 6–12 mg estradiol valerate tablets for 10–14 days, with the dose based on the endometrial response. After the endometrial thickness reached 7 mm or more, progesterone was introduced, and embryo transfer was planned. Luteal phase support was provided by progesterone administration (vaginally 200 mg or intramuscular progesterone 50 mg intramuscular progesterone daily) for 12 weeks of gestation.
Blastocysts were graded according to the Gardner classification system [
16]. Good (Expanded AA; Hatching AA, AB, BA; Hatched AA, AB, BA; Mid AA; Expanded AB, BA; Early AA, AB, BA; Mid AB, BA), moderate (Hatching BB; Hatched BB; Early BB; Mid BB; Expanded BB), and poor (Early AC, BC, CA, CB, CC; Mid AC, BC, CA, CB, CC; Expanded AC, CA, BC, CB, CC; Hatching BC, CC, CB, CB, CC).
The protocols used for ovarian stimulation for IVF were long gonadotropin-releasing hormone (GnRH) agonists (21%), GnRH antagonists (46%), and long GnRH microdose flares (32%). Different types of gonadotropins, including follitropin alpha, follitropin beta, and highly purified urinary menopausal gonadotropins, have been used for stimulation. The trigger was either recombinant hCG 250 mcg (Merck Serono, Montreal, Canada) or gonadotropin-releasing hormone agonist trigger 1,000 international units (IU) Buserelin (Xediton Pharmaceutical Inc, Mississauga, Canada) in antagonist cycles when there was a risk of ovarian hyperstimulation syndrome.
Descriptive statistics in terms of means, standard deviation, and percentages were determined for all parameters. The normal distribution of continuous parameters was tested using the Kolmogorov-Smirnov test. Analysis of variance with multiple comparisons was performed using Scheffe’s test between the three groups based on maximum endometrial thickness, and differences between categorical parameters were tested using chi-square and Fisher’s exact tests. Multivariate logistic regression was performed to explore the effect of endometrial thickness on pregnancy outcomes after adjusting for potential confounders including female age at oocyte retrieval, number of oocytes retrieved, number of transferred embryos, transferred embryo grade, infertility diagnosis, endometrial preparation protocol, and the year of transfer. Statistical significance was set at P<0.05. Receiver operating characteristic curve analysis was performed to evaluate the predictive value of endometrial thickness for live births. A power calculation found that at least 1,349 subjects were needed for an alpha of 0.05 and a power of 80% to detect a 10% difference in live birth outcomes. SPSS version 27 (IBM Corporation, Chicago, IL, USA) was used for all statistical analyses.
Go to :

Results
The study included 7,091 IVF cycles performed in 4,037 women undergoing embryo transfer at our fertility center. Group 1 (endometrial thickness of 7.0–7.9 mm) included 1,385 cycles. Group 2 (endometrial thickness of 8.0–8.9 mm) included 3,000 cycles, and group 3 (endometrial thickness of 9.0–9.9 mm) included 2,706 cycles. During these 10 years, the annual percentage of FET cycles to total transfer cycles increased from 36.4–64.9%.
The LBR for the entire study population was 22.2% per embryo transfer. The mean age of women at oocyte retrieval day was 36.7±4.5 years. The baseline characteristics of the three study groups are presented in
Table 1.
Table 1
Baseline characteristics of the three groups based on maximum endometrial thickness
|
Characteristic |
Group 1 (7.0–7.9 mm) |
Group 2 (8.0–8.9 mm) |
Group 3 (9.0–9.9 mm) |
P-value |
|
Female age (yr) |
36.7±4.5 |
36.0±4.6 |
36.1±4.5 |
P1=0.001, P2=0.004, P3=0.89 |
|
Embryo grade |
|
|
|
0.13 |
|
Good |
559 (87.8) |
1,527 (85.8) |
1,311 (88.9) |
|
|
Moderate |
62 (9.7) |
202 (11.4) |
129 (8.7) |
|
|
Poor |
16 (2.5) |
50 (2.8) |
35 (2.4) |
|
|
Number of embryos transferred |
|
|
|
<0.001 |
|
1 |
919 (66.4) |
2,144 (71.5) |
1,836 (67.9) |
|
|
2 |
464 (33.6) |
855 (28.5) |
866 (32.1) |
|
|
Fresh vs. frozen ET |
825 (59.6) and 560 (40.4) |
1,413 (47.1) and 1,587 (52.9) |
1,553 (57.4) and 1,153 (42.6) |
<0.001 |
|
Infertility cause: female infertility |
|
Unexplained infertility |
312 (22.5) |
675 (22.5) |
634 (23.4) |
0.67 |
|
Ovulation disorder |
141 (10.2) |
316 (10.5) |
275 (10.2) |
0.88 |
|
Endometriosis |
52 (3.8) |
142 (4.7) |
119 (4.4) |
0.34 |
|
Diminished ovarian reserve |
357 (25.8) |
586 (19.5) |
517 (19.1) |
<0.001 |
|
Tubal factor |
109 (7.9) |
252 (8.4) |
264 (9.8) |
0.076 |
|
Male factor infertility |
346 (25.0) |
864 (28.8) |
770 (28.5) |
0.024 |

There was no clinical difference in female age at oocyte retrieval (although there was a statistical difference, all groups averaged 36 years of age) and no difference in the quality of embryos transferred (
P=0.13) between the three groups (
Table 1). Group 2 had more single embryo transfers than groups 1 and 3, respectively (71.5% vs. 66.4%;
P<0.001 and 67.9%;
P<0.001). Group 2 had fewer fresh embryo transfers compared to groups 1 or 3, respectively (47.1% vs. 59.6%;
P<0.001 and 57.4%;
P<0.001). Group 1 had more diagnoses of diminished ovarian reserve (25.8% vs. 19.5% and 19.1%;
P<0.001) and less male factor infertility compared to groups 2 and 3, respectively (25.0% vs. 28.8% and 28.5%;
P=0.024)
Table 1. There were no differences in the rates of other causes of infertility among the three groups. In fresh cycles, the protocols were: long agonist (21%), antagonist (46%), and microdose flare (32%). Different types of gonadotropins have been used, including follitropin-alpha, follitropin-beta, and highly purified urinary menopausal gonadotropins. The trigger was recombinant hCG 250 mcg or gonadotropin-releasing hormone agonist trigger 1,000 IU in antagonist cycles. Increased endometrial thickness in fresh cycles was associated with higher peak estradiol levels and a higher number of oocytes retrieved (
P=0.001 and
P=0.002, respectively).
The pregnancy outcomes of the three groups are presented in
Table 2. In univariate analysis without controlling for confounding effects, in fresh cycles, the LBR was higher with increasing endometrial thickness: group 2 vs. group 1 (22.1% vs. 15.6%;
P<0.001), group 3 vs. group 1 (26.0% vs. 15.6%;
P<0.001), and group 3 vs. group 2 (26.0% vs. 22.1%;
P<0.001). Moreover, the clinical pregnancy rate was higher with increasing endometrial thickness: group 2 vs. group 1 (29.7% vs. 22.9%;
P<0.001), group 3 vs. group 1 (34.1% vs. 22.9%;
P<0.001), and group 3 vs. group 2 (34.1% vs. 29.7%;
P<0.001). However, there were no differences in the rates of live births and clinical pregnancies between frozen cycles (
Table 2). Moreover, there were no differences in the rates of miscarriages and ectopic pregnancies among the three groups in both fresh and frozen cycles (
Table 2). The increase in LBR as the endometrium thickened was true also in the sub-analysis of different fresh transfer protocols (agonist: 8.1% in group 1, 35.8% in group 2, and 56.1% in group 3) (antagonist: 18.5% in group 1, 34.0% in group 2, and 47.5% in group 3) and (microdose flare: 14.8% in group 1, 40.8% in group 2, and 44.4% in group 3). In frozen cycles, the highest LBR was observed in group 2 compared with group 1 and group 3 (HRT: 12.3% in group 1, 48.2% in group 2, and 39.5% in group 3) (naturally modified natural cycles: 23.8% in group 1, 44.4% in group 2, and 31.7% in group 3).
Table 2
Pregnancy outcomes based on maximum endometrial thickness in the three groups in fresh and frozen cycles
|
Pregnancy outcome |
Fresh cycles |
FET cycles |
|
|
|
Group 1 (7.0–7.9 mm) |
Group 2 (8.0–8.9 mm) |
Group 3 (9.0–9.9 mm) |
P-value |
Group 1 (7.0–7.9 mm) |
Group 2 (8.0–8.9 mm) |
Group 3 (9.0–9.9 mm) |
P-value |
|
Live birth rates |
129 (15.6) |
312 (22.1) |
404 (26.0) |
P1,2,3<0.001 |
110 (19.6) |
348 (21.9) |
271 (23.5) |
P1,2,3=0.19 |
|
|
Clinical pregnancy rates |
187 (22.9) |
416 (29.7) |
528 (34.4) |
P1,2,3<0.001 |
160 (28.7) |
509 (32.1) |
395 (34.4) |
P1,2,3=0.058 |
|
|
Miscarriage rates |
51 (6.2) |
94 (6.7) |
109 (7.0) |
0.74 |
45 (8.0) |
142 (8.9) |
111 (9.6) |
0.55 |
|
|
Ectopic pregnancy rates |
3 (0.4) |
5 (0.4) |
5 (0.3) |
0.98 |
3 (0.5) |
3 (0.2) |
1 (0.1) |
0.16 |

Since increasing endometrial thickness was associated with an increased number of retrieved oocytes, we analyzed the relationship between endometrial thickness and LBR in fresh cycles within different groups according to the number of oocytes retrieved (<4 oocytes, 4–8 oocytes, 9–12 oocytes, and 13 or more oocytes) (
Fig. 1). After stratifying for the number of oocytes at retrieval, in women with 4–8 oocytes retrieved, the LBR was significantly higher in group 3 and group 2 compared with group 1 (
P=0.006). In women with 9–12 oocytes, the LBR was significantly higher in groups 2 and 3 than in group 1 (
P=0.01). However, in women with fewer than 4 oocytes retrieved or with 13 oocytes or more collected, endometrial thickness did not affect LBR (
P=0.1).
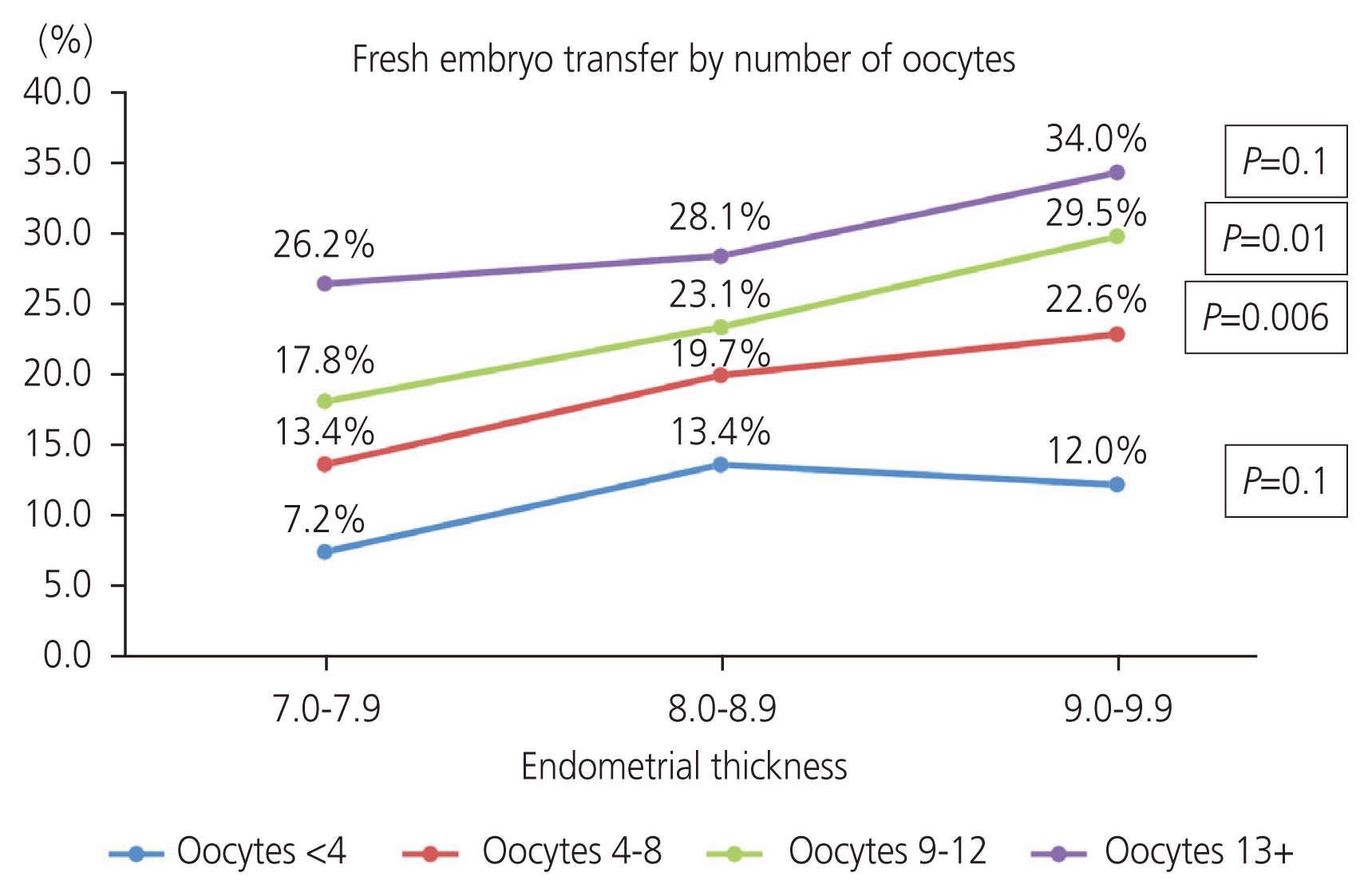 | Fig. 1Live birth rates in fresh embryo transfer. 
|
In the multivariate regression model, after controlling for confounding effects including female age at oocyte retrieval, embryo grade, number of embryos transferred, protocol type (fresh vs. frozen), year of transfer, infertility diagnosis, number of oocytes retrieved, and these three groups did not differ in LBRs (group 1 vs. group 2: odds ratio [OR], 1.08; 95% contidence interval [CI], 0.84–1.41;
P=0.54 or group 1 vs. group 3: OR, 1.16; 95% CI, 0.90–1.51;
P=0.24)
Table 3.
Table 3
Adjusted relative risk for live birth according to various endometrial thicknesses, stratified by female age, number of embryos, embryo grade, infertility cause, treatment protocol (fresh vs. frozen), year of treatment, and number of oocytes retrieved
|
Live birth |
Odds ratio |
95% confidence interval (upper-lower) |
P-value |
|
Female age at oocyte retrieval |
0.95 |
0.93–0.97 |
<0.001 |
|
Endometrial thickness |
|
Group 1, reference |
|
|
|
|
Group 2, 8.0–8.9 |
1.08 |
0.84–1.41 |
0.54 |
|
Group 3, 9.0–9.9 |
1.16 |
0.90–1.51 |
0.24 |
|
Male factor |
0.995 |
0.81–1.22 |
0.96 |
|
PCOS |
1.21 |
0.93–1.59 |
0.16 |
|
DOR |
1.015 |
0.75–1.38 |
0.93 |
|
Protocol type: fresh vs. frozen |
1.81 |
1.07–3.09 |
0.027 |
|
Number of oocytes retrieved |
|
<4 |
1.31 |
0.76–2.26 |
0.33 |
|
4–8 |
1.37 |
0.78–2.42 |
0.27 |
|
9–12 |
1.37 |
0.76–2.46 |
0.29 |
|
13 and above, reference |
|
|
|
|
Number of embryos 2 vs. 1 |
0.96 |
0.71–1.29 |
0.77 |
|
Embryo grade |
|
Good quality, reference |
|
|
|
|
Moderate |
1.39 |
0.74–2.63 |
0.30 |
|
Poor |
1.79 |
0.97–3.22 |
0.06 |

Go to :

Discussion
The main finding of our study is that, after controlling for relevant confounders, live birth rates in women with endometrial linings between 7.0–9.9 mm were similar.
Many studies have addressed the effect of endometrial thickness on pregnancy outcomes in fresh and frozen embryo transfer cycles with confounding results, depending on the ranges selected in each study [
9–
14,
17–
20]. Moreover, there has been a debate in the literature regarding whether the ideal minimal endometrial thickness for embryo transfer is 7, 8, or 9 mm [
2,
7,
14]. Despite the clear relationship between endometrial thickness and pregnancy, it was previously demonstrated that relatively high pregnancy rates of approximately 50% can be achieved even with an endometrial thickness of 7 mm [
7]. A previous study found that there were no differences in pregnancy rates between 7.0–7.9 mm compared to 8.0–8.9 mm [
2]. Currently, fertility centers select individual cutoffs for endometrial thickness, usually between 7 and 9 mm, to achieve the best pregnancy outcomes [
14].
The novelty of our study is that we selected women based on narrow ranges of endometrial thickness, knowing that in each fertility center and even between different physicians in the same fertility clinic, there are different attitudes regarding the ideal endometrial thickness to maximize pregnancy outcomes. In the current study, no cancellations occurred in the study group because the thicknesses were within an acceptable range for transfer at our institution.
Two large studies revealed a strong association between increased endometrial thickness and higher live birth rates [
17,
18], even after controlling for confounders. A large prospective observational study by Vaegter et al. [
17], which included 8,451 IVF/intracytoplasmic sperm injection treatments, demonstrated that LBR with an endometrium <7 mm, measured on the day of oocyte retrieval, was 15.2%. While the live birth rate was 29.2% with an endometrial thickness of at least 10 mm. In the study by Vaegter et al. [
17], endometrial thicknesses of 7.0–9.0 mm were combined into a single group. Another study by Simeonov et al. [
18], which included a total of 5,133 cycles, demonstrated that LBRs were 17.98% (380/2,114) in cycles with endometrial thickness of 7.0–9.0 mm and 23.44% (476/2,031) in cycles with endometrial thickness of 10–12 mm. In this study, an endometrial thickness of 7–9 mm was considered as a single group. Both studies included only cleavage-stage embryos [
17,
18]. Moreover, a previous large Canadian study found that only in fresh and non-frozen cycles, the LBR declined as the endometrial thickness decreased below 8 mm compared to 7 mm [
2]. However, this Canadian study lacked information regarding patient and cycle characteristics, including the reason for infertility and the number of oocytes retrieved, which could mask undetected bias [
2]. In addition, this was a very large study that puts it at risk of type one error, accepting a difference when one does not exist.
According to the current study, the rates of miscarriage were similar among the different endometrial thickness groups. So far, studies have found conflicting results regarding the association between endometrial thickness before embryo transfer and miscarriage rates [
1,
2,
8,
13,
21]. Some studies have reported a strong association between endometrial thickness and miscarriage rate. These studies reported that an optimal endometrial thickness threshold of 10 mm minimized pregnancy losses [
8]. Another study found that an endometrial thickness >14 mm was associated with a higher rate of pregnancy loss [
21]. However, two recent studies found no association with pregnancy loss, even within the endometrial thickness ranges listed [
1,
2], which is consistent with our results.
The main limitations of our study are its retrospective nature and the lack of data, including body mass index, gravidity, and parity. However, this study included a large population from a single reproductive center. Another limitation is that sonographic imaging can vary between performers. However, this reflects the actual conditions that occur in all clinics in this endometrial thickness range, and as such, supports the validity of the results.
One strength of this study is that the range of endometrial thicknesses included was small. The use of such small ranges prevented the inclusion of what some clinicians might consider thin linings being combined with acceptable lining thicknesses for the analysis. Given the conflicting results in the medical literature, prospective studies are recommended. The inclusion of frozen and fresh cycles can be considered as both a strength and a weakness. This may be considered a weakness in the inclusion of different cycle types. This may be considered a strength as it demonstrated similar results in a diverse population of patients with different IVF and frozen embryo protocols.
In conclusion, live birth rates in women with maximum endometrial thicknesses between 7.0–9.9 mm were similar when blastocyst transfer was performed and after controlling for confounding effects. Further large-scale prospective studies are needed to confirm these results.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download